Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vijay Devra | -- | 2786 | 2023-02-28 12:02:27 | | | |
| 2 | Dean Liu | Meta information modification | 2786 | 2023-03-01 01:38:02 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Bairwa, P.; Kumar, N.; Devra, V.; Abd-Elsalam, K.A. Biogenic Synthesis of Nanomaterials as Nanofertilizers. Encyclopedia. Available online: https://encyclopedia.pub/entry/41736 (accessed on 06 March 2026).
Bairwa P, Kumar N, Devra V, Abd-Elsalam KA. Biogenic Synthesis of Nanomaterials as Nanofertilizers. Encyclopedia. Available at: https://encyclopedia.pub/entry/41736. Accessed March 06, 2026.
Bairwa, Preeti, Nimish Kumar, Vijay Devra, Kamel A. Abd-Elsalam. "Biogenic Synthesis of Nanomaterials as Nanofertilizers" Encyclopedia, https://encyclopedia.pub/entry/41736 (accessed March 06, 2026).
Bairwa, P., Kumar, N., Devra, V., & Abd-Elsalam, K.A. (2023, February 28). Biogenic Synthesis of Nanomaterials as Nanofertilizers. In Encyclopedia. https://encyclopedia.pub/entry/41736
Bairwa, Preeti, et al. "Biogenic Synthesis of Nanomaterials as Nanofertilizers." Encyclopedia. Web. 28 February, 2023.
Copy Citation
Nanotechnology is critically dependent on the usage of biosynthetic or “green” technologies, which are inexpensive, environmentally friendly, and produce minimal contamination.
green nanotechnology
nanomaterials
biosynthesis
1. Microbe-Mediated Synthesis
Microorganisms as nanofactories represent enormous promise as energy-efficient, low-cost, and non-toxic technologies that can produce MNPs more quickly than physicochemical processes [1]. The nanomaterials are obtained from the microbes via two different methods including intracellular and extracellular. Intracellular biosynthesis involves unique transport systems in microorganisms in which the cell wall plays an important role due to its negative charge—positively charged metal ions are deposited in negatively charged cell walls through electrostatic interactions. After transport into the cells of the microorganism, ions are reduced using metabolic reactions mediated by enzymes such as nitrate reductase to form MNPs [2]. While in the extracellular mechanism, the metal ions are converted to their respective NPs via the nitrate-reductase synthesis method [3].
Extracellular synthesis is one of the different green MNP production processes, and it is particularly interesting since it avoids the requirement for expensive and time-consuming subsequent processing steps to recover intracellular nanoparticles [4][5]. Microorganisms hold a unique place among the numerous biological sources for the synthesis of MNPs because of their rapid growth rate, ease of cultivation, and ability to develop under circumstances of ambient temperature, pH, and pressure. This is known as “green synthesis,” which is mediated by microorganisms. [6]. It has been proven that a variety of microorganisms, including bacteria, fungi, yeasts, and microalgae, can create MNPs either intracellularly or extracellularly (Table 1).
A wide range of microorganisms can be used as potential biofactories to create several MNPs that contain metals such as silver, gold, copper, zinc, titanium, palladium, and nickel in an environmentally benign and affordable manner. This allows MNPs with a specific form, size, composition, and particle mono-dispersity to be generated [7][8]. In general, microorganisms occurring in metal-rich habitats are very resistant to these metals due to the uptake and chelation of the metals by intracellular and extracellular proteins. As a result, this technique, which imitates the natural bio-mineralization process, may be advantageous for the synthesis of MNPs [9][10].
Microorganisms contain a variety of different components, including proteins, enzymes, and other biological substances, which are all crucial to the reduction of MNPs. Since bacteria have a remarkable capacity to reduce heavy metals, they have been widely exploited as nano factories to produce various metal nanoparticles. To resist pressures such as the toxicity of nanomaterials, several bacterial species have evolved defense mechanisms [11][12]. These bacteria are suitable options for nanoparticle synthesis due to their abundance in the environment and adaptability to harsh circumstances [13]. França Bettencourt et al. [14] used 12 bacterium supernatants containing auxin complex (indole-3-acetic, IAA) to synthesize iron and manganese mono- and bimetallic nanoparticles (NPs) and evaluated them as plant nanofertilizers. The generated NPs confirmed their suitability as micronutrient fertilizers for agricultural growth. The use of bimetallic NPs in particular exhibited positive benefits on maize seedling growth by enhancing seed germination, root growth, and fresh and dry weights.
Nanoparticles can be produced by microalgae either intracellularly or extracellularly. Developing the required microalgae, allowing them to interact with the precursor solution, and separating and purifying them are all steps in intracellular synthesis [15].
It has been discovered that the fungal system is a flexible biological system that can generate metal nanoparticles both intracellularly and extracellularly. Numerous fungi have been studied for the production of different metal nanoparticles of varied shapes and sizes due to their broad dispersion in nature and the fact that they are preferred to other biological systems [16]. Ganoderma lucidum extract was used by Sedefoglu et al. [17] to physiologically generate ZnO nanoparticles. Lepidium sativum, or garden cress, has been the subject of research into the properties of nanofertilizers. It was discovered that the pure wurtzite phase with p63 mc space group characterizes the structural features of ZnO NPs. The effect of nanofertilizers on garden cress, Lepidium sativum, was investigated. ZnO nanoparticles were produced at 250 ppm using a 25 mL extract concentration, and the contents of the radicle, plumule lengths, fresh weights, and dry weights of L. sativum increased at rates of 45%, 41%, 16%, and 33%, respectively. This study was the first to report the use of green-generated ZnO with G. lucidum extracts as a nanonutrient.
Table 1. Green synthesis of nanofertilizers using different microbes.
| S.N. | Bio-Extract | Nanoparticles | Characterization Results | Applications | Ref |
|---|---|---|---|---|---|
| 1 | Twelve bacteria supernatants containing auxin complex (indole-3-acetic, IAA) | Iron and manganese mono- and bimetallic nanoparticles | λmax: 250–300 nm. Spherical shape with size 26.65 nm of FeOx NPs, MnOx NPs at around 22.32 nm, and MnOx/FeOx NPs at around 23.42 nm | Plant growth, especially in germination, root growth, and fresh weight in maize plantlets. Used as micronutrient nanofertilizer. | [14] |
| 2 | Ganoderma lucidum extract | Zinc Oxide nanoparticle | Three hexagonal shapes (discs, rods, and pyramidals) | Nanofertilizers properties on Lepidium sativum | [17] |
| 3 | Calcium phosphate (CaP) biological hard tissues | CaP nanoparticles | Round-shaped with a size of 10–25 nm | Multinutrient nanofertilizers | [18] |
| 4 | Using microalgal algal extract | Iron-oxide nanoparticles | Spherical biofabricated Fe3O4-NPs particle size was 76.5 nm | Plant growth stimulant | [19] |
| 5 | Using Lactobacillus casei Subsp. Casei | Copper-oxide nanoparticles | Spherical in shape with 30 nm to 75 nm size range | Plant micronutrient | [20] |
| 6 | Acidophilus, Lactobacillus casei, and Bifdobacterium sp. | Se nanoparticles | Nanoparticle size ranged from 100–500 nm | Plant disease enhancer and nanofertilizer | [21] |
| 7 | Microalgae | Silver nanoparticles | Nanoparticle size ranged from 13 to 31 nm | Act as nanofertilizer, have antioxidative properties | [22] |
2. Plant-Mediated Synthesis
In comparison to microbes, plants are easily available natural sources that produce greater quantities of bimolecular reducing agents. Extracts from a wide range of plant materials, such as stalks, leaflets, roots, blooms, and fruits, have been used to synthesize metallic NPs [4] (Table 2). To prepare plant extract, plant matter is cleansed, dried, boiled, and then filtered. The plant filtrate is then combined with a metallic salt solution and further incubated to produce metallic nanoparticles [23]. Terpenoids, flavones, quinones, ketones, and aldehydes are some of the phytochemicals found in plant extracts that act as electron donors to change metal ions into nanoparticles (NPs) in aqueous solutions (Figure 1).
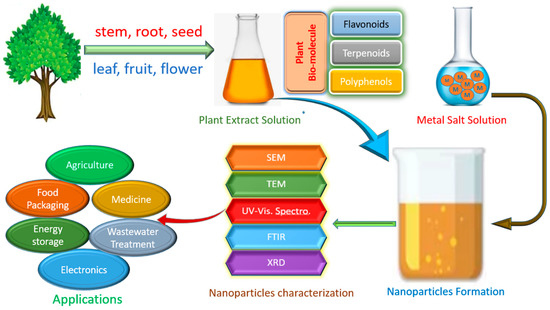
Figure 1. Plant-mediated synthesis of nanoparticles from various plant extracts.
Plant extracts contain several reducing agents that also serve as capping agents to stabilize metallic NPs when they are produced [24][25].
Elham Rostamizadeh et al. [26] recently created Fe2O3 nanoparticles utilizing the fruit extract of C. mas. Compared to equivalent quantities of bulk counterparts, the growth of barley seedlings was improved by synthetic Fe2O3 nanoparticles. The nano-form stimulated root biomass by an average of 42%, compared to the bulk counterparts’ average of 18%. Lower concentrations of Fe2O3 nanoparticles boosted the transfer of iron from the root to the shoot, according to research by Rui et al. [27]. Additionally, it was noticed that the adsorption of Fe2O3 nanoparticles to soil particles could decrease nutrient loss and improve the fertilizer’s cost-effectiveness. Ahmed Shebl et al. have developed a successful green chemistry approach for the hydrothermal synthesis of Zn, Mn, and Fe nano oxides [28]. Throughout two seasons in 2017 and 2018, they examined the impact of foliar treatments of micronutrient-oxide nanoparticles of zinc, iron, and manganese as well as combinations of these oxides on the development, performance, and quality of squash plants. The observed results demonstrated that spraying the plants with manganese-oxide nanoparticles produced the best fruit and vegetative development characteristics, yield, and photosynthetic pigment content.
Manpreet Singh et al. [29] reported the green synthesis of zinc-oxide nanoparticles (ZnO NPs) with spherical morphology and an average size of 50 nm, using an aqueous extract of Azadirachta indica leaves. The peak of UV-visible absorbance was found near 363 nm. Mung bean seeds were used to test the synthetic nanoparticles’ ability to stimulate seed germination. In comparison to untreated seeds, a significant improvement in seed germination was seen after the plant was treated with ZnO nanoparticles. Overall, compared to untreated seeds, seedlings treated with green ZnO NPs displayed longer roots and shoots by 237 and 168%, respectively.
The extract from the leaves of the Calotropis plant was used in the green production of zinc-oxide nanoparticles. The maximum UV-Vis absorption maxima were spherical, with a peak near 350 nm. When ZnO nanoparticles were applied topically to nursery-stage tree seedlings of the neem (Azadirachta indica), karanj (Pongamia pinnata), and milkwood-pine (Alstonia scholaris), growth was noticeably accelerated. Alstonia scholaris, one of the three treated seedlings, developed to its maximum height [30]. Varada V. Ukidave and Lalit T. Ingale did work using Coriandrum sativum leaf extract to green synthesize zinc-oxide nanoparticles [31]. The effects of zinc-oxide nanoparticles as fertilizer on the Bengal gram, Turkish gram, and green gram plants were studied in vitro. For an evaluation of the growth-stimulating effects of zinc-oxide nanoparticles, various media’s protein and chlorophyll contents, seed germination rates, root and shoot lengths, fresh weights, and dried weights were assessed. The green manufacturing of 100 nm-sized zinc-oxide nanoparticles was verified using the transmission microscopy technique. Plants successfully germinated in MS media and MS media Plus nanoparticles with a 100% success rate. Following MS media, MS media only with nanoparticles, and MS medium without zinc in the current investigation, it was shown that MS medium + nanoparticles had the maximum root length, shoot length, and weight. It has been demonstrated that zinc-oxide nanoparticles promote plant growth, aid in seed germination, and increase plant protein and chlorophyll levels. In media treated with zinc-oxide nanoparticles, green gram and Turkish gram showed significantly improved growth and development when compared to Bengal gram. According to the findings of the protein examination, the protein content in green gram (1.26 mg/mL), Turkish gram (1.19 mg/mL), and Bengal gram (1.23 mg/mL) plants were higher after 7 days than that in the roots and shoots. In prior applications, the plant had seedlings with a chlorophyll content of 12.6 mg/L, according to the results of the application of MS media + ZnO nanoparticles. On the other side, a zinc deficiency inhibits plant development and the production of chlorophyll and proteins. This research provides credence to the idea that zinc-oxide nanoparticles derived from Coriandrum sativum leaves can be produced sustainably and function as biofertilizers.
In a green synthesis of TiO2 nanoparticles for crop-farming yield enhancement, Citrus medica L. fruit peel extract was used as a reduction and capping media, according to Prakashraj et al. [32]. This revealed their crystalline structure and spherical form with 20–30 nm dimensions. Synthesized TiO2 nanoparticles were found to be effective at improving Capsicum annuum seed germination and other growth traits. As a result, the agro-food industry can use TiO2 nanoparticles generated from fruit peel extract of C. medica L as a catalyst and nutrition. Jaspreet et al. [33] created green ZnO NPs by extracting the leaves of Aloe barbadensis Mill. The average particle size of green ZnO NPs was 35 nm, a far smaller value than that produced using traditional chemical techniques (e.g., 48 nm). The optimal ZnO NP concentrations for wheat (Triticum aestivum L.) seedling emergence and germination were then investigated at various NP levels. Compared to the control seeds, the seeds treated with green ZnO NPs grew better. Additionally, the root and shoot length of the wheat-seed samples that had been exposed to moderate amounts of green ZnO NPs (e.g., 62 mg/L) were significantly increased (p = 0.005) when compared with other concentrations or those created chemically (by 50% and 105%, respectively). This has led to the identification of the potential for green ZnO NPs as a nano-based nutrition source for agricultural applications.
The development of the nanofertilizer nanoparticles ZnO MnO-NPs and FeO ZnO-NPs and evaluation of their effectiveness in promoting the growth of Andean lupin (Lupinus mutabilis sweet) and cabbage (Brassica oleracea var. capitata) crops was carried out by Murgueitio-Herrera et al. [34] using Andean blueberry extract. Both plants contained zinc, which has advantageous properties for plant growth. Foliar NP sprays were applied at the phenological stage of the vegetative growth of the Andean lupin or cabbage plants growing in greenhouses. The sizes of the NPs, which are 9.5 nm for ZnO, 7.8 nm for FeO, and 10.5 nm for MnO, make it easier for plant stomata to bind to them. In Andean lupin, treatment with 270 ppm of iron and zinc increased height by 6%, root size by 19%, chlorophyll-content index by 3.5%, and leaf area by 300%; however, treatment with 540 ppm of iron and zinc did not appear to increase any of the variables. At a concentration of 270 ppm, the ZnO MnO-NPs in cabbage exhibited increases of 10.3% in root size, 55.1% in dry biomass, 7.1% in chlorophyll content, and 25.6% in leaf area. Cabbage plants treated at a dose of 540 ppm produced increases in root size of 1.3% and chlorophyll content of 1.8%, when compared to the control, which was sprayed with distilled water. Consequently, the spraying of nanofertilizers at 270 ppm produced a significant improvement in the development of both plants.
One of the essential elements needed to support plant development and metabolism is zinc. Ahmed, et al. [35] reported on the green production of zinc nanoparticles (Zn NPs) using clove buds (plant material) and examined how the Zn NPs affected the growth and yield of Pisum sativum L. In comparison to the control and plants treated with zinc sulfate, the greatest growth and yield were obtained at 400 and 600 ppm. In conclusion, green-produced zinc nanoparticles can improve the productivity and growth of crop pea plants. Reshma et al. [36] examined the effects of zinc nanoparticles (Zn NPs) produced biologically using moringa-leaf extract on amaranth seed germination, growth characteristics, and zinc content Plant metabolites such as amino acids, alkaloids, flavonoids, sugars, and fatty acids are abundant in moringa leaves. The maximum plant height and fresh weight were obtained with a foliar treatment of 10 ppm biosynthesized zinc NPs. Zinc NPs at a concentration of 10 ppm resulted in better nutrient recovery and improved yield and productivity relative to the nutrient input, according to the nutrient-use efficiency indices, even though increasing the concentration of zinc applied via the foliar route led to higher zinc content in the plant biomass.
Mathew et al. [37] used Cuminum cyminum seed extract for the synthesis of Titanium dioxide nanoparticles (TiO2 NPs) for seed germination and germination indices of mung bean to promote sustainable and biocompatible nano agriculture (Vigna radiata) and analyze plant growth parameters such as root length, shoot length, germination percentage, the germination rate. The results indicated significantly enhanced values of germination indices. Six potential TiO2 NP concentrations (25, 50, 100, 150, 200, and 250 g mL−1) were examined alongside the absolute control. The mung bean seeds’ ability to germinate and thrive was affected by the TiO2 NPs absorption.
Table 2. Green synthesis of nanofertilizers using different plant extracts.
| S.N. | Bio-Extract | Nanoparticles | Characterization Results | Applications | Ref |
|---|---|---|---|---|---|
| 1 | Pomegranate peel (PPE) and coffee ground (CE) extracts | Phosphorous-containing hydroxyapatite nanoparticles (nHAP) | Average diameters were 167.5 nm, 153 nm (nHAPs CE), and 229.6 nm, 120.6 nm (nHAPs PPE), respectively | Investigating improvements in Punica granatum L., metabolites, photosynthetic activity, carbohydrate levels, and biocompatibility. | [38] |
| 2 | Using Bamboo | Silicon nanoparticles | - | Soil application, foliar application | [39] |
| using rice husk | |||||
| using sugar beet bagasse | |||||
| 3 | Fruit extracts of Cornelian cherry | Iron-oxide nanoparticles | Spherical shape with size 20 to 40 nm | Stimulation in both root and shoot biomass | [26] |
| 4 | Leaf extract of Aloe barbadensis Mill | Zinc-oxide nanoparticles | Spherical shape with a size of 35 nm | Nutrient source for plant growth | [33] |
| 5 | Flower extract of Elaeagnus Angustifolia | Zinc-oxide nanoparticles | λmax: 330–340 nm, irregular to nearly spherical shape | Germination and metabolic activities of the plant | [40] |
| 6 | Berberis pachyacantha leaf extract (BPL) | Nickel-oxide nanoparticles | Rhombohedral structure with a size of 22.53 nm | Seed germination | [41] |
| 7 | Coriandrum sativum leaves extract | Zinc-oxide nanoparticles | Rod-shaped and polycrystalline with a size of 100 nm | Growing effects of fertilizer on green grain, Turkish gram, and Bengal gram plants | [31] |
| 8 | Clove buds (plant material) | Zinc nanoparticles | - | Growth and yield of Pisum sativum L. | [35] |
| 9 | Leaves of Zataria multiflora Boiss | Zinc nanostructure | - | Foliar application on pomace extract of Punica granatum | [42] |
| 10 | Citrus medica peel extract | Zinc oxide nanoparticles | λmax: 375 nm, the average crystallite size of 20−30 nm | ZnO nanofertilizer improves the growth and yield of Abelmoschus esculentus | [43] |
| 11 | Leaf extract of Parthenium hysterophorus | Zinc oxide nanoparticles | Spherical with a size of 10 nm | Germination of seeds and vegetative growth of Sesamum indicum h | [44] |
| 12 | The seed extract of black seeds (Nigella sativa L.) | Zinc-oxide nanoparticles | Spherical shape with a size of 24 nm | Nano-supplement to improve the production of B. oleracea var. Italicaa | [45] |
| 13 | Vegetable peel extract | Zinc-oxide nanoparticles | - | Boost the value of cluster bean (Cyamopsis tetragonoloba) seeds as well as the higher yield of cluster bean pods | [46] |
| 14 | Cassia occidentalis L. flower extract | Iron-oxide nanoparticles | Irregular surfaces with size 20–50 nm | Enhance germination and overall seedling growth | [47] |
| 15 | Leaf extract of Cassia fistula | Copper-oxide nanoparticles) | λmax: 320 nm, spherical-shaped with size 12–38 nm | Root and foliar application | [48] |
| 16 | Panicum sumatrense grains aqueous extract | Copper-oxide nanoparticles | λmax: 305 nm, crystallite with size 25 nm | Enhance plant growth | [49] |
| 17 | Microalgal algal extract | Iron-oxide nanoparticles | Spherical shape with size 76.5 nm | Plant growth stimulant | [50] |
| 18 | Leaf extracts of Moringa oleifera L | Bimetallic Ag/ZnO nanomaterials | λmax: 366 to 379 nm, spherical shape with sizes from 46 nm to 66 nm | Nitrogen-based fertilizers on biochemical and yield attributes of two wheat varieties | [51] |
| 19 | Peel extract of Citrus reticulate. | Zinc-oxide nanoparticles | Spherical shape with 23–90 nm size Hexagonal structure with 8.89 to 8.62 nm size | Boosting seed germination of Brassica nigra seeds | [52] |
| 20 | Seeds of Juniperus procera | Ag-containing nanoparticles | λmax: 400 and 262 nm, spherical with average size 100 nm | Seed germination | [53] |
References
- Zulfiqar, F.; Navarro, M.; Ashraf, M.; Akram, N.A. Plant Science Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci. 2019, 289, 110270.
- Hulkoti, N.I.; Taranath, T.C. Biosynthesis of nanoparticles using microbes—A review. Colloids Surf. B Biointerfaces 2014, 121, 474–483.
- Aslam, A.A.; Aslam, A.A.; Aslam, M.S.; Quazi, S. An Overview on Green Synthesis of Nanomaterials and Their Advanced Applications in Sustainable Agriculture. Preprints 2022, 2022020315.
- Bahrulolum, H.; Nooraei, S.; Javanshir, N.; Tarrahimofrad, H.; Mirbagheri, V.S.; Easton, A.J.; Ahmadian, G. Green synthesis of metal nanoparticles using microorganisms and their application in the agrifood sector. J. Nanobiotechnol. 2021, 19, 1–26.
- Singh, P.; Kim, Y.-J.; Zhang, D.; Yang, D.-C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 588–599.
- Ali, A.; Ahmed, T.; Wu, W.; Hossain, A.; Hafeez, R.; Masum, M.I.; Wang, Y.; An, Q.; Sun, G.; Li, B. Advancements in Plant and Microbe-Based Synthesis of Metallic Nanoparticles and Their Antimicrobial Activity against Plant Pathogens. Nanomaterials 2020, 10, 1146.
- Mandal, D.; Bolander, M.E.; Mukhopadhyay, D.; Sarkar, G.; Mukherjee, P. The use of microorganisms for the formation of metal nanoparticles and their application. Appl. Microbiol. Biotechnol. 2005, 69, 485–492.
- Khan, T.; Abbas, S.; Fariq, A.; Yasmin, A. Microbes: Nature’s cell factories of nanoparticles synthesis. In Exploring the Realms of Nature for Nanosynthesis; Prasad, R., Jha, A.K., Prasad, K., Eds.; Springer: Cham, Switzerland, 2018; pp. 25–50.
- Kato, Y.; Suzuki, M. Synthesis of Metal Nanoparticles by Microorganisms. Crystals 2020, 10, 589.
- Burketová, L.; Martinec, J.; Siegel, J.; Macůrková, A.; Maryška, L.; Valentová, O. Noble metal nanoparticles in agriculture: Impacts on plants, associated microorganisms, and biotechnological practices. Biotechnol. Adv. 2022, 58, 107929.
- Chhipa, H. Mycosynthesis of nanoparticles for smart agricultural practice: A green and eco-friendly approach. In Green Synthesis, Characterization and Applications of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 87–109.
- Yusof, H.M.; Mohamad, R.; Zaidan, U.H.; Rahman, N.A.A. Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: A review. J. Anim. Sci. Biotechnol. 2019, 10, 57.
- Asmathunisha, N.; Kathiresan, K. A review on biosynthesis of nanoparticles by marine organisms. Colloids Surfaces B Biointerfaces 2013, 103, 283–287.
- Bettencourt, G.M.D.F.; Degenhardt, J.; Torres, L.A.Z.; de Andrade Tanobe, V.O.; Soccol, C.R. Green biosynthesis of single and bimetallic nanoparticles of iron and manganese using bacterial auxin complex to act as plant bio-fertilizer. Biocatal. Agric. Biotechnol. 2020, 30, 101822.
- Delilah, D.; Mathew, N.M.; Joseph, A.; Jose, S.; Kumar, S. Plant Micronutrient Nanoparticles from Microalgae-Biosynthesis and their Applications–A Review. Int. J. Bot. Stud. 2022, 7, 135–139.
- Rai, M.; Bonde, S.; Golinska, P.; Trzcińska-Wencel, J.; Gade, A.; Abd-Elsalam, K.; Shende, S.; Gaikwad, S.; Ingle, A. Fusarium as a Novel Fungus for the Synthesis of Nanoparticles: Mechanism and Applications. J. Fungi 2021, 7, 139.
- Sedefoglu, N.; Zalaoglu, Y.; Bozok, F. Green synthesized ZnO nanoparticles using Ganoderma lucidum: Characterization and In Vitro Nanofertilizer effects. J. Alloys Compd. 2022, 918, 165695.
- Ramírez-Rodríguez, G.B.; Dal Sasso, G.; Carmona, F.J.; Miguel-Rojas, C.; Pérez-De-Luque, A.; Masciocchi, N.; Guagliardi, A.; Delgado-López, J.M. Engineering Biomimetic Calcium Phosphate Nanoparticles: A Green Synthesis of Slow-Release Multinutrient (NPK) Nanofertilizers. ACS Appl. Bio Mater. 2020, 3, 1344–1353.
- Win, T.T.; Khan, S.; Bo, B.; Zada, S.; Fu, P. Green synthesis and characterization of Fe3O4 nanoparticles using Chlorella-K01 extract for potential enhancement of plant growth stimulating and antifungal activity. Sci. Rep. 2021, 11, 1–11.
- Kouhkan, M.; Ahangar, P.; Babaganjeh, L.A.; Allahyari-Devin, M. Biosynthesis of Copper Oxide Nanoparticles Using Lactobacillus casei Subsp. Casei and its Anticancer and Antibacterial Activities. Curr. Nanosci. 2020, 16, 101–111.
- Eszenyi, P.; Sztrik, A.; Babka, B.; Prokisch, J. Elemental, Nano-Sized (100–500 nm) Selenium Production by Probiotic Lactic Acid Bacteria. Int. J. Biosci. Biochem. Bioinform. 2011, 1, 148–152.
- Terra, A.L.M.; Kosinski, R.D.C.; Moreira, J.B.; Costa, J.A.V.; De Morais, M.G. Microalgae biosynthesis of silver nanoparticles for application in the control of agricultural pathogens. J. Environ. Sci. Health Part B 2019, 54, 709–716.
- Rajeshkumar, S.; Bharath, L.V. Mechanism of plant-mediated synthesis of silver nanoparticles–a review on biomolecules involved, characterisation and antibacterial activity. Chem. -Biol. Interact. 2017, 273, 219–227.
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure–activity relationship: An update review. Phytother. Res. 2019, 33, 13–40.
- Sarker, U.; Oba, S. Drought stress enhances nutritional and bioactive compounds, phenolic acids and antioxidant capacity of Amaranthus leafy vegetable. BMC Plant Biol. 2018, 18, 258.
- Rostamizadeh, E.; Iranbakhsh, A.; Majd, A.; Arbabian, S.; Mehregan, I. Green synthesis of Fe2O3 nanoparticles using fruit extract of Cornus mas L. and its growth-promoting roles in Barley. J. Nanostructure Chem. 2020, 10, 125–130.
- Rui, M.; Ma, C.; Hao, Y.; Guo, J.; Rui, Y.; Tang, X.; Zhao, Q.; Fan, X.; Zhang, Z.; Hou, T.; et al. Iron Oxide Nanoparticles as a Potential Iron Fertilizer for Peanut (Arachis hypogaea). Front. Plant Sci. 2016, 7, 815.
- Shebl, A.; Hassan, A.A.; Salama, D.M.; El-Aziz, M.E.A.; Elwahed, M.S.A.A. Green Synthesis of Nanofertilizers and Their Application as a Foliar for Cucurbita pepo L. J. Nanomater. 2019, 2019, 3476347.
- Singh, M.; Singh, J.; Sharma, D.; Kaur, B.; Rawat, M. Plant leaves mediated synthesis of semiconductor ZnO nanoparticles and its application for seed germination. In Proceedings of the Recent Advances in Experimental and Theoretical Physics (RAETP-201), Jammu, India, 17–18 April 2018; AIP Publishing LLC.: Melville, NY, USA, 2018; Volume 2006, p. 030031.
- Chaudhuri, S.K.; Malodia, L. Biosynthesis of zinc oxide nanoparticles using leaf extract of Calotropis gigantea: Characterization and its evaluation on tree seedling growth in nursery stage. Appl. Nanosci. 2017, 7, 501–512.
- Ukidave, V.V.; Ingale, L.T. Green Synthesis of Zinc Oxide Nanoparticles from Coriandrum sativum and Their Use as Fertilizer on Bengal Gram, Turkish Gram, and Green Gram Plant Growth. Int. J. Agron. 2022, 2022, 8310038.
- Prakashraj, R.; Vijayakumar, S.; Punitha, V.N.; Vidhya, E.; Nilavukkarasi, M.; Praseetha, P.K. Fabricated TiO2 Nanofertilizers for Foliar Assimilation to Enhance Yield and Disease Resistance in Capsicum annuum L. J. Plant Growth Regul. 2021, 41, 3387–3394.
- Singh, J.; Kumar, S.; Alok, A.; Upadhyay, S.K.; Rawat, M.; Tsang, D.; Bolan, N.; Kim, K.-H. The potential of green synthesized zinc oxide nanoparticles as nutrient source for plant growth. J. Clean. Prod. 2019, 214, 1061–1070.
- Murgueitio-Herrera, E.; Falconí, C.E.; Cumbal, L.; Gómez, J.; Yanchatipán, K.; Tapia, A.; Martínez, K.; Sinde-Gonzalez, I.; Toulkeridis, T. Synthesis of Iron, Zinc, and Manganese Nanofertilizers, Using Andean Blueberry Extract, and Their Effect in the Growth of Cabbage and Lupin Plants. Nanomaterials 2022, 12, 1921.
- Ahmed, S.; Qasim, S.; Ansari, M.; Shah, A.A.; Rehman, H.U.; Shah, M.N.; Ghafoor, U.; Naqvi, S.A.H.; Hassan, M.Z.; Rehman, S.U.; et al. Green synthesis of zinc nanoparticles and their effects on growth and yield of Pisum sativum. J. King Saud Univ. Sci. 2022, 34, 102132.
- Reshma, Z.; Meenal, K. Foliar application of biosynthesised zinc nanoparticles as a strategy for ferti-fortification by improving yield, zinc content and zinc use efficiency in amaranth. Heliyon 2022, 8, e10912.
- Mathew, S.S.; Sunny, N.E.; Shanmugam, V. Green synthesis of anatase titanium dioxide nanoparticles using Cuminum cyminum seed extract; effect on Mung bean (Vigna radiata) seed germination. Inorg. Chem. Commun. 2021, 126, 108485.
- Abdelmigid, H.M.; Morsi, M.M.; Hussien, N.A.; Alyamani, A.A.; Alhuthal, N.A.; Albukhaty, S. Green Synthesis of Phosphorous-Containing Hydroxyapatite Nanoparticles (nHAP) as a Novel Nano-Fertilizer: Preliminary Assessment on Pomegranate (Punica granatum L.). Nanomaterials 2022, 12, 1527.
- Snehal, S.; Lohani, P. Silica nanoparticles: Its green synthesis and importance in agriculture. J. Pharmacogn. Phytochem. 2018, 7, 3383–3393.
- Singh, A.; Singh, N.; Hussain, I.; Singh, H.; Yadav, V.; Singh, S. Green synthesis of nano zinc oxide and evaluation of its impact on germination and metabolic activity of Solanum lycopersicum. J. Biotechnol. 2016, 233, 84–94.
- Uddin, S.; Iqbal, J.; Safdar, L.B.; Ahmad, S.; Abbasi, B.A.; Capasso, R.; Kazi, M.; Quraihi, U.M. Green synthesis of BPL-NiONPs using leaf extract of Berberis pachyacantha: Characterization and multiple in vitro biological applications. Molecules 2022, 27, 2064.
- Bahmanzadegan, A.; Tavallali, H.; Tavallali, V.; Karimi, M.A. Variations in biochemical characteristics of Zataria multiflora in response to foliar application of zinc nano complex formed on pomace extract of Punica granatum. Ind. Crop. Prod. 2022, 187, 115369.
- Keerthana, P.; Vijayakumar, S.; Vidhya, E.; Punitha, V.N.; Nilavukkarasi, M.; Praseetha, P.K. Biogenesis of ZnO nanoparticles for revolutionizing agriculture: A step towards anti-infection and growth promotion in plants. Ind. Crops Prod. 2021, 170, 113762.
- Sharma, P.; Urfan, M.; Anand, R.; Sangral, M.; Hakla, H.R.; Sharma, S.; Das, R.; Pal, S.; Bhagat, M. Green synthesis of zinc oxide nanoparticles using Eucalyptus lanceolata leaf litter: Characterization, antimicrobial and agricultural efficacy in maize. Physiol. Mol. Biol. Plants 2022, 28, 363–381.
- Awan, S.; Shahzadi, K.; Javad, S.; Tariq, A.; Ahmad, A.; Ilyas, S. A preliminary study of influence of zinc oxide nanoparticles on growth parameters of Brassica oleracea var italic. J. Saudi Soc. Agric. Sci. 2020, 20, 18–24.
- Rexlin, J.; Vijayakumar, S.; Nilavukkarasi, M.; Vidhya, E.; Alharthi, N.S.; Sajjad, M.; Punitha, V.N.; Praseetha, P.K. Bioengineered ZnO nanoparticles as a nano priming agent in Cyamopsis tetragonoloba (L).Taub. to improve yield and disease resistance. Appl. Nanosci. 2022, 1–9.
- Afzal, S.; Sharma, D.; Singh, N.K. Eco-friendly synthesis of phytochemical-capped iron oxide nanoparticles as nano-priming agent for boosting seed germination in rice (Oryza sativa L.). Environ. Sci. Pollut. Res. 2021, 28, 40275–40287.
- Ashraf, H.; Anjum, T.; Riaz, S.; Ahmad, I.S.; Irudayaraj, J.; Javed, S.; Qaiser, U.; Naseem, S. Inhibition mechanism of green-synthesized copper oxide nanoparticles from Cassia fistula towards Fusarium oxysporum by boosting growth and defense response in tomatoes. Environ. Sci. Nano 2021, 8, 1729–1748.
- Velsankar, K.; Parvathy, G.; Mohandoss, S.; Kumar, R.M.; Sudhahar, S. Green synthesis and characterization of CuO nanoparticles using Panicum sumatrense grains extract for biological applications. Appl. Nanosci. 2022, 12, 1993–2021.
- Ehsan, M.; Raja, N.I.; Mashwani, Z.U.R.; Zohra, E.; Abasi, F.; Ikram, M.; Mustafa, N.; Wattoo, F.H.; Proćków, J.; de la Lastra, J.M.P. Effects of Phytogenically Synthesized Bimetallic Ag/ZnO Nanomaterials and Nitrogen-Based Fertilizers on Biochemical and Yield Attributes of Two Wheat Varieties. Nanomaterials 2022, 12, 2894.
- Rafique, M.; Sohaib, M.; Tahir, R.; Tahir, M.B.; Rizwan, M. Plant-Mediated Green Synthesis of Zinc Oxide Nanoparticles Using Peel Extract of Citrus reticulate for Boosting Seed Germination of Brassica nigra Seeds. J. Nanosci. Nanotechnol. 2021, 21, 3573–3579.
- Salih, A.M.; Qahtan, A.A.; Al-Qurainy, F.; Al-Munqedhi, B.M. Impact of Biogenic Ag-Containing Nanoparticles on Germination Rate, Growth, Physiological, Biochemical Parameters, and Antioxidants System of Tomato (Solanum tuberosum L.) In Vitro. Processes 2022, 10, 825.
- Kumar, R.; Kumar, R.; Prakash, O. Chapter-5 the Impact of Chemical Fertilizers on Our Environment and Ecosystem. In Research Trends in Environmental Science, 2nd ed.; AkiNik: Delhi, India, 2019; Volume 35, p. 69.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
02 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





