Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Viviana Frantellizzi | -- | 2298 | 2023-02-27 16:43:39 | | | |
| 2 | Dean Liu | -12 word(s) | 2286 | 2023-02-28 02:23:51 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pontico, M.; Conte, M.; Petronella, F.; Frantellizzi, V.; De Feo, M.S.; Di Luzio, D.; Pani, R.; De Vincentis, G.; De Sio, L. 18F-fluorodeoxyglucose Functionalized Gold Nanoparticles. Encyclopedia. Available online: https://encyclopedia.pub/entry/41706 (accessed on 07 February 2026).
Pontico M, Conte M, Petronella F, Frantellizzi V, De Feo MS, Di Luzio D, et al. 18F-fluorodeoxyglucose Functionalized Gold Nanoparticles. Encyclopedia. Available at: https://encyclopedia.pub/entry/41706. Accessed February 07, 2026.
Pontico, Mariano, Miriam Conte, Francesca Petronella, Viviana Frantellizzi, Maria Silvia De Feo, Dario Di Luzio, Roberto Pani, Giuseppe De Vincentis, Luciano De Sio. "18F-fluorodeoxyglucose Functionalized Gold Nanoparticles" Encyclopedia, https://encyclopedia.pub/entry/41706 (accessed February 07, 2026).
Pontico, M., Conte, M., Petronella, F., Frantellizzi, V., De Feo, M.S., Di Luzio, D., Pani, R., De Vincentis, G., & De Sio, L. (2023, February 27). 18F-fluorodeoxyglucose Functionalized Gold Nanoparticles. In Encyclopedia. https://encyclopedia.pub/entry/41706
Pontico, Mariano, et al. "18F-fluorodeoxyglucose Functionalized Gold Nanoparticles." Encyclopedia. Web. 27 February, 2023.
Copy Citation
The glucose analogue 2-(18F)fluoro-2-deoxy-D-glucose (18F-2-FDG) is a well-known positron emission tomography (PET) imaging radiotracer.
18F-FDG
gold nanoparticles
plasmonic photothermal ablation
1. 18F-FDG: Synergistic Targeting Agent and PET Imaging Nanoprobe
Fluorodeoxyglucose has been utilized successfully in cancer imaging by taking advantage of cancer cells’ glucose avidity. Because cancer cells reproduce quickly, they utilize glucose at a greater rate compared to healthy cells. The Warburg effect is the name given to this phenomenon [1]. The glucose analogue 2-(18F)fluoro-2-deoxy-D-glucose (18F-2-FDG) is a well-known positron emission tomography (PET) imaging radiotracer (Figure 1).
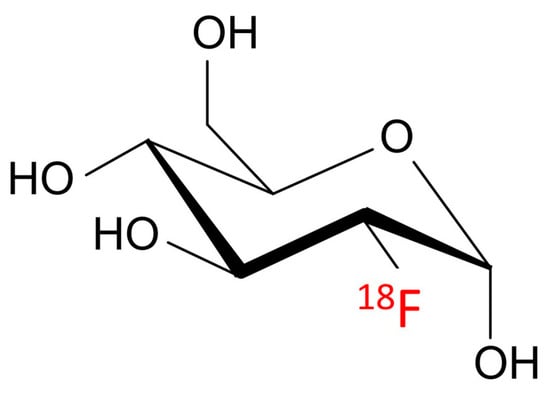
Figure 1. Chemical structure of the glucose analogue 2-(18F)fluoro-2-deoxy-D-glucose.
The most extensively documented metabolic inhibitor for targeting glucose metabolism is the glucose analogue 2-deoxy-D-glucose (2DG), which inhibits glycolytic ATP generation and glucose transport. Furthermore, 2DG can cause oxidative stress, block N-linked glycosylation, and cause apoptosis through endoplasmic reticulum stress [2]. Some earlier studies described the Warburg effect and discussed the mechanisms underlying 2DG and its potential application in cancer treatment [3][4]. Thus, glucose labeling could be an effective method of facilitating GNP internalization into cells. Recently, 2-deoxy-D-glucose modified PEG-coated nanomaterials were created as a possible dual-targeted drug delivery strategy for boosting drug accumulation in glioma via GLUT-mediated endocytosis and enhancing blood-brain barrier passage by Glut-mediated transcytosis [5]. Suvarna et al. described the synthesis of various capped GNPs, as well as the characterization of the nanoparticles, using various approaches. The study was designed to evaluate the potential biomedical application of gold nanomaterials capped with 2DG and citrate using different reducing agents, portraying 2DG-capped GNPs as better candidates for theranostic application [6]. Unak et al. [7] provided a novel architecture by conjugation of 18F-FDG, GNPs, and anti-metadherin (anti-MTDH) antibody (see Figure 2). This antibody is specific for MTDH, which is a surface protein overexpressed in breast cancer cells. In detail, researchers proposes a route for the preparation of a nano-bioconjugate that merges the radiopharmaceutical with GNPs and the antibody as an active-targeting agent. The preparation strategy consists first of the synthesis of 18F-FDG functionalized through a mannose triflate molecule as a precursor for 18F-FDG by nucleophilic fluorination. The obtained radiopharmaceutical carries a thiol group introduced using cysteamine in the synthesis step. The thiol group promotes the in situ generation of GNRs by the reduction of HAuCl4. The final step of the preparation procedure is the bioconjugation of 18F-FDG functionalized GNPs by generating a covalent bond between the 18F-FDG and the amine groups of the antibody. Roa et al. [8] synthesized a multi-functional, radiosensitizing agent based on thiol-6-fluoro-6-deoxy-d-glucose GNPs (thiol-6-FDG–GNPs). Remarkably, the group of Roa et al. was the first to report the biodistribution, pharmacokinetic evaluation, and toxicological safety of 6-FDG–GNPs both in vitro and in vivo. Moreover, thiol-6-FDG–GNPs were developed with the capability of being labeled by the radioisotope 18F for eventual PET imaging. In comparison with 18F-2-FDG which is poorly absorbed by the intestines and kidneys, the analogue 18F-labeled 6-FDG (18F-6-FDG) is actively transported by the kidneys and intestines. After 2 h of intravenous injection of 6-FDG-GNPs into the murine model, approximately 30% of GNPs were detected in the liver, spleen, and kidney. PEGylation of 6-FDG-GNPs was reported to drastically ameliorate 6-FDG-GNP biodistribution by minimizing inadvertent uptake into these organs, while simultaneously tripling the cellular uptake of gold nanoparticle GNPs in implanted breast MCF-7 cancer. This research intends to replace the 18F atom in the 6-FDG molecule in the future so that it can be used as a PET radiotracer to facilitate cancer detection and image-guided radiation therapy planning. Increased uptake of Glu-GNPs by cancer cells compared to unbound GNPs leads to improved radiotherapeutic cytotoxicity in vitro [8] and contrast enhancement of tumors in computed tomography (CT) imaging [9]. Some investigators have outlined how gold nanomaterials show significantly higher X-ray absorption than iodinated CT agents, thus providing the opportunity of using GNPs to improve the resolution of functional CT and molecular imaging until the micrometer scale. Feng et al. [10] discussed how they employed FDG-coated GNPs as multimodality CT and PET imaging contrast agents. They demonstrated in vitro and in vivo that cancer cells consume PEG-Glu-GNPs at 10 to 100 times higher concentrations than healthy cells and verified that this nano-compound has much better targeting ability than gold nanoparticles without surface modification. By analogy, Hu et al. [11] investigated whether there is any significant difference in targeted treatment due to glucose conjugation, again in GNPs designed as radiosensitizers; they found that Glu-GNPs are better radiosensitizers and enhance the cancer-killing of specific cancer cells 20% more than X-ray irradiation alone. In Table 1, a summary of the main studies is reported.
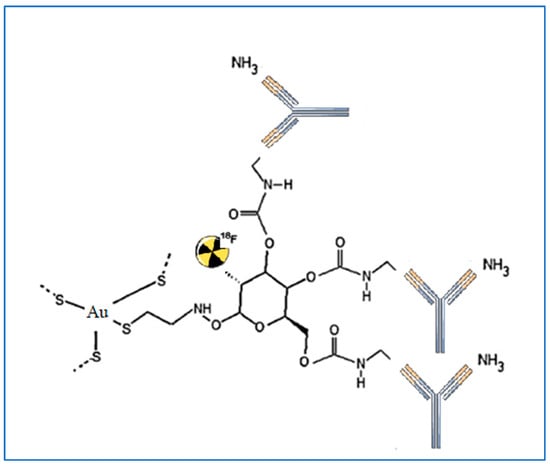
Figure 2. An example of GNP conjugated with the anti-MTDH antibody and labeled with 18F-FDG described in the study of Unak et al. [7].
Table 1. GNPs modified with FDG-related molecules for biomedical applications.
| Author | GNPs Surface Agent | Target | Biomedical Application |
|---|---|---|---|
| Roa et al. (2012) [8] | 6-fluoro-6-deoxy-D-glucose | Breast adenocarcinoma | Radiosensitizer |
| Suvarna et al. (2017) [6] | 2-deoxy-D-glucose | cancer cell lines such as HepG2, HeLa and HCT 116 | Theragnostic |
| Hu et al. (2015) [11] | Glucose-coating | Breast adenocarcinoma | Radiosensitizer |
| Jiang et al. (2014) [5] | PEGylation + 2-deoxy-D-glucose | Brain glioma | Drug-delivery |
| Unak et al. (2012) [7] | 18F-2-fluoro-2-deoxy-D-glucose + Ab anti-metadherin (MTDH) | Breast adenocarcinoma | Theragnostic |
| Feng et al. (2014) [10] | PEGylation + glucose-coating | BALB/c nude mice | CT imaging contrast agent |
| Hu et al. (2015) [11] | Glucose-coating | Leukemic stem cell line THP-1 and breast cell line MCF-7 | Radiosensitizer |
2. GNP-Mediated PTT for Effective Cancer Ablation
Traditional hyperthermia procedures (photodynamic therapy, radiofrequency hyperthermia, microwave hyperthermia) are still not optimum, since they are not minimally invasive and result in non-specific heat release across the body, which causes unpleasant side effects [12][13]. However, the ability to finely modulate heating around the tumoral zone, which is achieved by photothermal therapy, is highly sought in order to safely optimize hyperthermia. Photothermal therapy is one of the most effective adjuvants to radiotherapy and is a promising approach to eradicate radioresistant cancer cells. Indeed, hyperthermia and PTT, in addition to their direct toxic effect on tumors, can destroy radioresistant malignant cells, such as cancerous cells in hypoxic, low pH areas, and cells in the S-phase, which are primarily responsible for cancer recurrence and malignant dissemination after radiation RT [14][15]. It is worth pointing out that, from a practical point of view, light is an ideal external stimulus, as it can be easily regulated, focused, and remotely controlled to provide better-targeted treatments that lead to less damage to healthy tissues [16][17].
2.1. GNPs for Thermoablation of Cancer Cells
Several investigations have established the efficiency of plasmonic GNPs for the thermal ablation of different cellular types by PTT, as summarized by outstanding reviews [18][19][20][21]. Pitsillides et al. established the usefulness of gold nanoparticle GNPs for thermally induced cellular death: anti-CD8-labeled GNPs were used for the selective targeting and killing of T-cells. However an efficient in vivo application of GNP-mediated PTT requires GNPs with plasmon bands lying in the “biological window [22]. The coupled LSPR of nanospheres can be exploited by employing aggregated or assembled systems, as suggested by El-Sayed et al. [23]. According to the study, 30 nm gold nanospheres coated with anti-EGFR may be assembled on cell membranes with upregulated EGFR at high concentrations. When compared to the initial state, the formed nanospheres’ absorption signal was redshifted. (non-assembled nanoparticles). Anisotropic nanoparticles, such as GNRs [24] or nanobypyramids [25] can absorb light in the biological water window, which is extremely advantageous for PTT-related applications. Zhang et al. investigated in vitro NIR plasmonic photothermal therapy using anti-epidermal growth factor receptor (EFGR)-conjugated GNRs [26]. The GNRs bind selectively to the cytoplasmic membranes of malignant-type cells with EGFR hyperexpression. As a consequence, the malignant cells were killed at roughly half the laser strength required to kill non-malignant cells. Later, the researchers proved the viability of in vivo PTT employing polyethylene glycol (PEG)ylated GNRs, observing a significant size decrease in human squamous carcinoma tumors treated with gold nanorods and photothermal therapy (PTT). By altering the surface of popcorn-shaped GNPs with anti-prostate-specific membrane antigen (PSMA) antibodies, Lu et al. created a targeted PTT agent [27] (Figure 3).
Figure 3. Popcorn-shaped GNPs with anti-prostate-specific membrane antigen (PSMA) antibodies taken by Lu et al. [27].
After 30 min of irradiation (785 nm cw laser, 12.5 W/cm2), LNCaP human prostate cancer cells were treated with anti-prostate-specific membrane antigen GNPs and displayed substantial mortality. Furthermore, Black et al. created polydopamine (PD)-coated GNRs [28]. Anti-EGFR antibodies were attached to the PD-coated GNRs, and the anti-EGFR-conjugated, PD-coated GNRs were effectively employed for targeted PTT. Gold nanoparticle GNPs with more complex morphologies were also studied and evaluated for tumor eradication through PTT. Hirsch et al., for example, described the GNS-mediated NIR PTT of tumors using magnetic resonance guidance [29]. They incubated human breast cancer cells with PEGylated GNSs and found that the cells became photothermally ablated after NIR laser irradiation. Furthermore, Au et al. [30] demonstrated that gold nanocage GNCs have a photothermal effect on SK-BR-3 breast cancer cells (Figure 4).
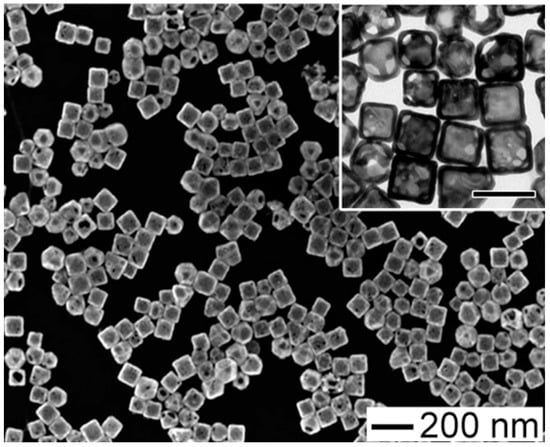
Figure 4. Gold nanocages developed by Au et al. [30] for PPT on SK-BR-3 breast cancer.
Gold nanocages conjugated with anti-HER-2 antibodies showed selective photothermal destruction of breast cancer cells. Gold nanostars conjugated to drugs have been applied in tumor treatment. When administered intravenously to mice, PEGylated gold nanostars accumulated for 48 h, extravasation was demonstrated, and localized photothermal ablation was reported within 10 min of irradiation [31]. Chen et al. conjugated GNSTs with cyclic peptide-RGD and an anticancer drug (DOX) to show the synergistic effects of PTT and chemotherapy [32]. Relative to GNRs and GNCs, PEGylated nanohexapods demonstrated the greatest tumor uptake and photothermal conversion efficiency. Simón et al. used 800 nm Resonant BioPureTM Gold NS, which is a silica core surrounded by a thin gold shell, for the murine subcutaneous colorectal tumor model [33]. They applied two fractionated PTT protocols, one with two laser treatments and one with four laser treatments. Since in previous studies an unspecific temperature increase of ΔT ~10 °C determined by laser attenuation in the dermal layer was observed [34][35], they swabbed into the tumor glycerol to enhance transdermal laser penetration, obtaining a significant reduction of maximum temperatures reached. The results demonstrated no substantial differences in tumor growth and survival between mice receiving single doses and mice treated with fractionated PTT. Only a few animals receiving fractionated PTT showed the complete disappearance of cancer. Recently, doxorubicin-encapsulated iron-gallic acid (FeGA-DOX) nanoparticles (NPs) were linked with agarose hydrogels (AG) for PTT in osteosarcoma [36], providing an innovative therapeutic tool, while a smart deoxyribose nucleic acid nanogel-coated polydopamine nanosphere hybrid was developed for chemo-PTT for oncological applications [37].
Breast cancer is a flourishing field of research for nanomaterials and PTT. Different studies have been conducted. An innovative advance was the application of a sub-10 nm supramolecular nano assembly of aluminum and indocyanine green with lignosulfonate (LS-Al-ICG) for breast cancer PTT in a murine model [38]. Firstly, it was observed the capacity of aggregation in the tumor site confirmed the high photothermal-conversion effect of LS-Al-ICG. Since PTT can elicit ICD through the release of tumor-associated antigens (TAAs) and damage-associated molecular patterns (DAMPs) liberated by dying tumor cells [39][40], dendritic cell maturation and cytotoxic T-cell responses were stimulated; therefore, the activation of immune responses was seen. A considerable inhibition of tumor growth was evident, but the immunomodulation of the microenvironment improved efficacy for distant tumors as well. In fact, when the volume of distant tumors was measured, a significant reduction resulted, implying that an abscopal effect occurred. In addition, while in previous studies it was demonstrated to have limited effects on CD8+T cell populations using aluminum hydroxide and aluminum phosphate [41], providing an aluminum adjuvant with ICG elicited better cellular immunity. Another application of PTT in breast cancer is the encapsulation of 6-mercaptopurine (6MP) with chitosan nanoparticles (CNPs) loaded with gold nanomaterials. In particular, the effect on cellular proliferation of the human breast carcinoma cell line MCF7 was demonstrated, comparing free 6MP, which produces maximum inhibition of 39% at 100 μM, 6MP-CNPs with an IC50 at 9.3 μM, and 6MP-CNPs reaching 8.7 μM. The encapsulation of 6MP guarantees higher anti-proliferation activity, an augmented intracellular uptake, and thus a reduction of side effects [42]. Hyaluronic acid (HA)-Au@SiO2@Au NPs were proposed for the treatment of bladder and prostate cancers in mice. The tumors in mice disappeared at 15 and 18 days without signs of recurrence, highlighting the excellent targeted photothermal ablation and high biocompatibility [43].
GNRs surrounded by a polymeric shell constructed employing the layer-by-layer approach in which doxorubicin (DOXO) was incorporated and linked to HA (PSS/DOXO/PLL/HA-coated AuNRs) were proposed. The compound provoked cell death mainly through apoptosis in HeLa cells, even in the presence of NIR light irradiation [44]. Polypyrrole, cystine dihydrochloride, and hyaluronan nanoparticles loaded were tested in MDA-MB-231 breast cancer cells and breast tumor-bearing mice. Good photothermal effects, in vitro efficiency-induced apoptosis, and the inhibitory effect on cancer growth in mice models showed the great potential of chemo-photothermal therapy combination [45].
2.2. Lasers in PTT
The distribution of laser flux is an essential element in producing efficient ablative efficiency. Some characteristics, such as laser time and intensity, have been demonstrated to impact cell damage in PTT [46]. Furthermore, lasers in pulsed mode, particularly those with high pulse energy, are commonly associated with significant heating effects, which can have a wide range of implications. Compared to continuous-wave lasers, a pulsed laser with a brief pulse duration delivers more energy to the tissue. Furthermore, because they transfer heat to fluids, pulsed lasers produce a phase change in the water, resulting in the formation of bubbles in the biological system [47]. GNPs treated with a pulsed laser in the cytoplasm were found to create large bubbles. As a result, using pulsed lasers in the presence of GNPs provides the advantage of lowering the pulse laser intensity while maintaining the same effects. The wavelength of the incident light must be chosen carefully for the PTT. Regarding practical uses, the NIR region (650 nm 900 nm) is typically used because it exhibits negligible absorption and scattering by water, hemoglobin, skin, and other biomolecules of tissue components. NIR light penetrates tissue deeply, allowing it to reach deep within the body.
References
- Kritikou, E. Warburg effect revisited. Nat. Rev. Cancer 2008, 8, 247.
- Xi, H.; Kurtoglu, M.; Liu, H.; Wangpaichitr, M.; You, M.; Liu, X.; Savaraj, N.; Lampidis, T.J. 2-Deoxy-D-glucose activates autophagy via endoplasmic reticulum stress rather than ATP depletion. Cancer Chemother Pharm. 2011, 67, 899–910.
- Maximchik, P.; Abdrakhmanov, A.; Inozemtseva, E.; Tyurin-Kuzmin, P.A.; Zhivotovsky, B.; Gogvadze, V. 2-Deoxy-D-glucose has distinct and cell line-specific effects on the survival of different cancer cells upon antitumor drug treatment. FEBS J. 2018, 285, 4590–4601.
- Pajak, B.; Siwiak, E.; Sołtyka, M.; Priebe, A.; Zieliński, R.; Fokt, I.; Ziemniak, M.; Jaśkiewicz, A.; Borowski, R.; Domoradzki, T.; et al. 2-Deoxy-d-Glucose and Its Analogs: From Diagnostic to Therapeutic Agents. Int. J. Mol. Sci. 2019, 21, 234.
- Jiang, X.; Xin, H.; Ren, Q.; Gu, J.; Zhu, L.; Du, F.; Feng, C.; Xie, Y.; Sha, X.; Fang, X. Nanoparticles of 2-deoxy-d-glucose functionalized poly(ethylene glycol)-co-poly(trimethylene carbonate) for dual-targeted drug delivery in glioma treatment. Biomaterials 2014, 35, 518–529.
- Suvarna, S.; Das, U.; Kc, S.; Mishra, S.; Sudarshan, M.; Saha, K.D.; Dey, S.; Chakraborty, A.; Narayana, Y. Synthesis of a novel glucose capped gold nanoparticle as a better theranostic candidate. PLoS ONE 2017, 12, e0178202.
- Unak, G.; Ozkaya, F.; Medine, E.I.; Kozgus, O.; Sakarya, S.; Bekis, R.; Unak, P.; Timur, S. Gold nanoparticle probes: Design and in vitro applications in cancer cell culture. Colloids Surf. B Biointerfaces 2012, 90, 217–226.
- Roa, W.; Xiong, Y.; Chen, J.; Yang, X.; Song, K.; Yang, X.; Kong, B.; Wilson, J.; Xing, J.Z. Pharmacokinetic and toxicological evaluation of multi-functional thiol-6-fluoro-6-deoxy-D-glucose gold nanoparticles in vivo. Nanotechnology 2012, 23, 375101.
- Aydogan, B.; Li, J.; Rajh, T.; Chaudhary, A.; Chmura, S.J.; Pelizzari, C.; Wietholt, C.; Kurtoglu, M.; Redmond, P. AuNP-DG: Deoxyglucose-labeled gold nanoparticles as X-ray computed tomography contrast agents for cancer imaging. Mol. Imaging Biol. 2010, 12, 463–467.
- Feng, G.; Kong, B.; Xing, J.; Chen, J. Enhancing multimodality functional and molecular imaging using glucose-coated gold nanoparticles. Clin. Radiol. 2014, 69, 1105–1111.
- Hu, C.; Niestroj, M.; Yuan, D.; Chang, S.; Chen, J. Treating cancer stem cells and cancer metastasis using glucose-coated gold nanoparticles. Int. J. Nanomed. 2015, 10, 2065–2077.
- Yi, X.; Duan, Q.Y.; Wu, F.G. Low-Temperature Photothermal Therapy: Strategies and Applications. Research 2021, 2021, 9816594.
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108.
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2017, 12, 908–931.
- Zharov, V.P.; Galitovsky, V.; Viegas, M. Photothermal detection of local thermal effects during selective nanophotothermolysis. Appl. Phys. Lett. 2003, 83, 4897.
- Terentyuk, G.S.; Maslyakova, G.N.; Suleymanova, L.V.; Khlebtsov, N.G.; Khlebtsov, B.N.; Akchurin, G.G.; Maksimova, I.L.; Tuchin, V.V. Laser-induced tissue hyperthermia mediated by gold nanoparticles: Toward cancer phototherapy. J. Biomed. Opt. 2009, 14, 021016.
- Rinoldi, C.; Zargarian, S.S.; Nakielski, P.; Li, X.; Liguori, A.; Petronella, F.; Presutti, D.; Wang, Q.; Costantini, M.; De Sio, L.; et al. Nanotechnology-Assisted RNA Delivery: From Nucleic Acid Therapeutics to COVID-19 Vaccines. Small Methods 2021, 5, e2100402.
- Norouzi, H.; Khoshgard, K.; Akbarzadeh, F. In vitro outlook of gold nanoparticles in photo-thermal therapy: A literature review. Lasers Med. Sci. 2018, 33, 917–926.
- Hwang, S.; Nam, J.; Jung, S.; Song, J.; Doh, H.; Kim, S. Gold nanoparticle-mediated photothermal therapy: Current status and future perspective. Nanomedicine 2014, 9, 2003–2022.
- Yamada, M.; Foote, M.; Prow, T.W. Therapeutic gold, silver, and platinum nanoparticles. Wiley Interdiscip Rev. Nanomed. Nanobiotechnol. 2015, 7, 428–445.
- Ban, Q.; Bai, T.; Duan, X.; Kong, J. Noninvasive photothermal cancer therapy nanoplatforms via integrating nanomaterials and functional polymers. Biomater. Sci. 2017, 5, 190–210.
- Pitsillides, C.M.; Joe, E.K.; Wei, X.; Anderson, R.R.; Lin, C.P. Selective cell targeting with light-absorbing microparticles and nanoparticles. Biophys. J. 2003, 84, 4023–4032.
- El-Sayed, I.H.; Huang, X.; El-Sayed, M.A. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006, 239, 129–135.
- Onaciu, A.; Braicu, C.; Zimta, A.A.; Moldovan, A.; Stiufiuc, R.; Buse, M.; Ciocan, C.; Buduru, S.; Berindan-Neagoe, I. Gold nanorods: From anisotropy to opportunity. An evolution update. Nanomedicine 2019, 14, 1203–1226.
- Campu, A.; Susu, L.; Orzan, F.; Maniu, D.; Craciun, A.M.; Vulpoi, A.; Roiban, L.; Focsan, M.; Astilean, S. Multimodal Biosensing on Paper-Based Platform Fabricated by Plasmonic Calligraphy Using Gold Nanobypiramids Ink. Front Chem. 2019, 7, 55.
- Zhang, M.; Kim, H.S.; Jin, T.; Woo, J.; Piao, Y.J.; Moon, W.K. Near-infrared photothermal therapy using anti-EGFR-gold nanorod conjugates for triple negative breast cancer. Oncotarget 2017, 8, 86566–86575.
- Lu, W.; Singh, A.K.; Khan, S.A.; Senapati, D.; Yu, H.; Ray, P.C. Gold Nano-Popcorn-Based Targeted Diagnosis, Nanotherapy Treatment, and In Situ Monitoring of Photothermal Therapy Response of Prostate Cancer Cells Using Surface-Enhanced Raman Spectroscopy. J. Am. Chem. Soc. 2010, 132, 18103–18114.
- Black, K.C.; Yi, J.; Rivera, J.G.; Zelasko-Leon, D.C.; Messersmith, P.B. Polydopamine-enabled surface functionalization of gold nanorods for cancer cell-targeted imaging and photothermal therapy. Nanomedicine 2013, 8, 17–28.
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.E.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554.
- Au, L.; Zheng, D.; Zhou, F.; Li, Z.Y.; Li, X.; Xia, Y. A quantitative study on the photothermal effect of immuno gold nanocages targeted to breast cancer cells. ACS Nano 2008, 2, 1645–1652.
- Yuan, H.; Khoury, C.G.; Wilson, C.M.; Grant, G.A.; Bennett, A.J.; Vo-Dinh, T. In vivo particle tracking and photothermal ablation using plasmon-resonant gold nanostars. Nanomedicine 2012, 8, 1355–1363.
- Chen, H.; Zhang, X.; Dai, S.; Ma, Y.; Cui, S.; Achilefu, S.; Gu, Y. Multifunctional gold nanostar conjugates for tumor imaging and combined photothermal and chemo-therapy. Theranostics 2013, 3, 633–649.
- Simón, M.; Norregaard, K.; Jørgensen, J.T.; Oddershede, L.B.; Kjaer, A. Fractionated photothermal therapy in a murine tumor model: Comparison with single dose. Int. J. Nanomed. 2019, 14, 5369–5379.
- Jørgensen, J.T.; Norregaard, K.; Simón Martín, M.; Oddershede, L.B.; Kjaer, A. Non-invasive Early Response Monitoring of Nanoparticle-assisted Photothermal Cancer Therapy Using (18)F-FDG, (18)F-FLT, and (18)F-FET PET/CT Imaging. Nanotheranostics 2018, 2, 201–210.
- Norregaard, K.; Jørgensen, J.T.; Simón, M.; Melander, F.; Kristensen, L.K.; Bendix, P.M.; Andresen, T.L.; Oddershede, L.B.; Kjaer, A. 18F-FDG PET/CT-based early treatment response evaluation of nanoparticle-assisted photothermal cancer therapy. PLoS ONE 2017, 12, e0177997.
- Ying, H.; Wang, H.; Jiang, G.; Tang, H.; Li, L.; Zhang, J. Injectable agarose hydrogels and doxorubicin-encapsulated iron-gallic acid nanoparticles for chemodynamic-photothermal synergistic therapy against osteosarcoma. Front. Chem. 2022, 10, 1045612.
- Zang, S.; Deng, X.; Wang, J.; Zhao, Y.; Wu, S. Smart DNA nanogel coated polydopamine nanoparticle with high drug loading for chemo-photothermal therapy of cancer. Biointerphases 2022, 17, 061006.
- Fan, X.; Yue, T.; Liu, A.; Xie, X.; Fang, W.; Wei, Y.; Zheng, H.; Zheng, H.; Zhou, M.; Piao, J.; et al. Lignin-assisted construction of sub-10 nm supramolecular self-assembly for photothermal immunotherapy and potentiating anti-PD-1 therapy against primary and distant breast tumors. Asian J. Pharm. Sci. 2022, 17, 713–727.
- Jin, F.; Qi, J.; Zhu, M.; Liu, D.; You, Y.; Shu, G.; Du, Y.; Wang, J.; Yu, H.; Sun, M.; et al. NIR-Triggered Sequentially Responsive Nanocarriers Amplified Cascade Synergistic Effect of Chemo-Photodynamic Therapy with Inspired Antitumor Immunity. ACS Appl. Mater. Interfaces 2020, 12, 32372–32387.
- Li, J.; Yu, X.; Jiang, Y.; He, S.; Zhang, Y.; Luo, Y.; Pu, K. Second Near-Infrared Photothermal Semiconducting Polymer Nanoadjuvant for Enhanced Cancer Immunotherapy. Adv. Mater. 2021, 33, e2003458.
- Zhu, Y.; Xue, J.; Chen, W.; Bai, S.; Zheng, T.; He, C.; Guo, Z.; Jiang, M.; Du, G.; Sun, X. Albumin-biomineralized nanoparticles to synergize phototherapy and immunotherapy against melanoma. J. Control. Release 2020, 322, 300–311.
- Faid, A.H.; Shouman, S.A.; Badr, Y.A.; Sharaky, M.; Mostafa, E.M.; Sliem, M.A. Gold nanoparticles loaded chitosan encapsulate 6-mercaptopurine as a novel nanocomposite for chemo-photothermal therapy on breast cancer. BMC Chem. 2022, 16, 94.
- Wang, R.; Du, N.; Jin, L.; Chen, W.; Ma, Z.; Zhang, T.; Xu, J.; Zhang, W.; Wang, X.; Li, M. Hyaluronic Acid Modified (2)@Au Nanoparticles for Photothermal Therapy of Genitourinary Tumors. Polymers 2022, 14, 4772.
- Arellano-Galindo, L.; Villar-Alvarez, E.; Varela, A.; Figueroa, V.; Fernandez-Vega, J.; Cambón, A.; Prieto, G.; Barbosa, S.; Taboada, P. Hybrid Gold Nanorod-Based Nanoplatform with Chemo and Photothermal Activities for Bimodal Cancer Therapy. Int. J Mol. Sci. 2022, 23, 13109.
- Sun, J.; Zhu, S.; Xu, W.; Jiang, G. Redox-responsive hyaluronan-conjugated polypyrrole nanoparticles targeting chemo-photothermal therapy for breast cancer. Front Bioeng. Biotechnol. 2022, 10, 1049437.
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Review of Some Interesting Surface Plasmon Resonance-enhanced Properties of Noble Metal Nanoparticles and Their Applications to Biosystems. Plasmonics 2007, 2, 107–118.
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217–228.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
729
Revisions:
2 times
(View History)
Update Date:
28 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




