As research on superhydrophobic materials inspired by the self-cleaning and water-repellent properties of plants and animals in nature continues, the superhydrophobic preparation methods and the applications of superhydrophobic surfaces are widely reported. Silicones are preferred for the preparation of superhydrophobic materials because of their inherent hydrophobicity and strong processing ability. In the preparation of superhydrophobic materials, silicones can both form micro-/nano-structures with dehydration condensation and reduce the surface energy of the material surface because of their intrinsic hydrophobicity. The superhydrophobic layers of silicone substrates are characterized by simple and fast reactions, high-temperature resistance, UV resistance, and anti-aging. Although silicone superhydrophobic materials have the disadvantages of relatively low mechanical stability, this can be improved by the rational design of the material structure.
1. Introduction
Nature is the result of billions of years of evolution and selection, and it has a perfect and diverse ecosystem with thought-provoking natural phenomena. In addition to being attracted to the variations in nature, inspiration ought to be drawn from nature to find better solutions for the progress and development. In recent years, superhydrophobic materials
[1], which are often the subject of research, have been inspired by flora and fauna in nature, such as lotus leaves
[2], morphology molesta floating leaves
[3], butterfly wings
[4], fly eyes
[5], cicada wings
[6], gecko feet
[7], shark skin
[8], legs of water striders
[9], and rose petals
[10]. After decades of research by researchers, it has been found that there are various artificial superhydrophobic surfaces, such as superhydrophobic particles (silica powder
[11], metal powder
[12], metal oxide powder
[13], polystyrene powder
[14]), superhydrophobic porous materials (membrane
[15][16][17], concrete
[18], textile
[19], sponge
[20]), and superhydrophobic surface coatings that can coat the surfaces of various materials (wood
[21], aluminum sheets
[22], copper sheets
[23], and various other substrates
[24]). Many methods of preparing superhydrophobic surfaces are achieved with the involvement of silicones. Previously, Zhang
[25] counted that about 25% of the papers on superhydrophobicity were based on silanes and silicones, proving the importance of silicones in the preparation of superhydrophobic materials. These advantages come from the properties of silicones: (1) Most silicones possess many hydrophobic groups similar to —C
nH
2n+1, —CH=CH
2, —C
6H
5, —X, so silicones are inherently hydrophobic materials. So, organosilicon monomers with multi-hydrolysis functional groups can provide low-surface-energy surfaces or modify the surface of other materials to make them hydrophobic; (2) silicones can easily polymerize through dehydration condensation to form micro-/nano-structures, or act as binders to stabilize other particles on the surface, both of which can generate sufficient surface roughness for superhydrophobic interfaces. (3) Most of the reaction conditions of organosilicon are relatively gentle, and the reaction can be completed under acidic and alkaline conditions, without imposing high temperature and pressure, which makes it simple and fast in the preparation process and convenient for mass production. (4) The main chain of organosilicon products is Si-O, and the double bond is less present, so it is not easy to be decomposed by ultraviolet light and ozone. Similarly, the Si-O bond of the molecule does not break or decompose under high temperature, so the organosilicon have both UV resistance and thermal stability. (5) Silicone has low toxicity, except for a tiny portion containing fluoro-silane, which facilitates mass production and practical applications in our daily life. Therefore, silicones have a significant advantage in the preparation of superhydrophobic materials.
Generally, when the contact angle between a material and a water droplet is greater than 90°, we consider this material to be hydrophobic, such as rubber, plastic, and leaves in nature, including the non-treated collard leaves in the figure
[26]. When the contact angle of a material is less than 90°, this material can be called a hydrophilic material. In addition, researchers consider those interfacial materials with contact angles greater than 150° and rolling angles less than 10° to be defined as superhydrophobic materials
[27]. The most typical superhydrophobic surface in nature is the surface of the lotus leaf. The droplet is in the Cassie state and there is an air layer between the droplet and the solid due to the high roughness of the superhydrophobic surface and the presence of many gaps; if a lubricant, such as dimethyl silicone oil, is injected into the porous gaps, a slippery liquid-infused porous surface is formed. In addition to the possibility of re-injecting lubricants into superhydrophobic surfaces
[28], it is also possible to inject lubrication into porous hydrophobic materials
[29], which can also form slippery liquid-infused porous surfaces (SLIPS) (The abbreviations appearing are summarized in the Abbreviations). The biological inspiration for SLIPS is derived from hogweed, a rough surface structure combined with a lubricating fluid.
2. Silicones
2.1. Silicone Monomer
Silanes can be divided into two main categories based on the atom to which the functional group is said to be attached.
The first category is silanes with the functional group directly attached to the silicon atom, which can be represented by RnSiX4−n, where R is alkyl, aryl, arylalkyl and hydrogen, etc.; X is a hydrolyzable functional group, such as halogen (main chlorine), alkoxy, acyloxy, amino, and hydrogen, and they are collectively called silicon-functional organosilanes.
The second category is the functional group attached to an organic group other than Si, which can be represented by (YR′)nSiX4−n, where Y is a functional group (such as NH2, OCOCMe=CH2, Cl, OH, etc.); R′ is a sub-alkyl group; and X is a hydrolyzable functional group such as halogen, MeO, EtO, AcO, MeOC2H4O, etc. When n is 1~3, these silanes are collectively referred to as carbon-functional organosilanes.
2.1.1. Organohalogenated Silanes
Chemical formula of organohalogen silane can be represented as RnSiX4−n (R is Me, Et, Vi, Pr, Ph, etc.; X is F, Cl, Br, and I; and n = 1~3). The most representative one is organochlorosilane, which is the most important raw material for the preparation of silicone polymers (industrial silicone oil, silicone rubber, and silicone resin) and other silicon-functional silanes.
Si-X bonds are much more active than Si-C and C-X bonds because of their ionic, covalent, and double-bonding properties, and Si-X bonds can be replaced by nucleophilic atoms or groups to produce silanes with corresponding substituents.
Organohalosilanes can undergo hydrolysis to form silanols. The rate of the hydrolysis reaction of organohalosilanes accelerates with an increase in polarity and the number of Si-X bonds, but slows down with an increase in site resistance and the number of organic groups. The Si-OH bond can be easily condensed to polysiloxane via dehydration, because silanol is not very stable, especially under the action of real acids.
2.1.2. Alkoxysilanes
Alkoxysilanes conform to HnSi(OR)4−n. The Si-O bond is much more reactive than the Si-C bond, especially when OMe or OEt is linked to the silicon atom. However, when the R in -SiOR has a large spatial site resistance, it becomes very inactive and also has good heat resistance when R is an aryl group.
HnSi(OR)4−n can undergo hydrolysis reactions under certain conditions:
However, the hydrolytic activity of the Si-OR bond is much lower than that of Si-X. In addition, the structure of alkoxysilane and the hydrolysis conditions also greatly influence the hydrolysis reaction. The hydrolysis activity of Si(OR)4 decreases with an increase in the number of carbon atoms of R in OR. The commonly used catalysts for the hydrolysis reaction of Si-OC bonds are HCl, H2SO4, AcOH, Al2O3, ZnO, HNR2, and M(OH)n (M is a metal). Chemical formula of organoalkoxysilane replaces the above —H with R (organic group), becoming RnSi(OR)4−n. Its properties are similar to alkoxysilane.
2.1.3. Organohydrosilanes
Organohydrosilanes are broadly divided into two categories, those with only hydrocarbon and hydrogen atoms on the silicon atom, and those containing Si-Cl, Si-OR, or Si-OAc bonds. The second group is most commonly used because it can produce a variety of hydrogen-containing silicone oils, silicone rubbers, and silicone resins, as well as a range of carbon-functional alkyl groups through hydrosilation.
The addition of hydrosilanes containing Si-H bonds to unsaturated hydrocarbon groups and carbonyl groups is hydrosilation:
2.1.4. Organoyloxy Silanes
Organoxysilanes can be expressed as RnSi(OCOR′)4−n. The hydrolytic activity of organoxysilanes is intermediate between that of chlorosilanes and alkoxysilanes, and hydrolysis reactions can occur at room temperature without a catalyst:
The rate of hydrolysis of organoyloxy silanes increases with the number of acyloxy groups on the silicon atom.
2.1.5. Haloalkylsilanes
In haloalkylsilanes, the reactivity of the C-X bond decreases in the following order: C-I > C-Br > C-Cl > C-F.
It can be found that the C-F bond is the least chemically active, but people synthesize fluoroalkyl silane because it can give excellent oil resistance, solvent resistance, hydrophobicity, stain resistance, etc. to polymerization.
2.1.6. Silane Coupling Agents
The traditional silane coupling agent is a class of silane containing three hydrolyzable silicon functional groups; some of the silanes mentioned earlier also belong to the silane coupling agent. The silane coupling agent has three major functions. The first point is it can improve the bonding of the interface of the materials, playing the role of the adhesive and the coating adhesive. The second point is it can modify the surface of the material, giving the surface waterproof, anti-static, and anti-mold functions, as well as other functions. The third point is it can be used as a filler to increase the bonding between different chemical properties of materials, improving the mechanical properties of the product such as insulation, anti-aging, and hydrophobicity.
2.2. Polymerization of Silicones
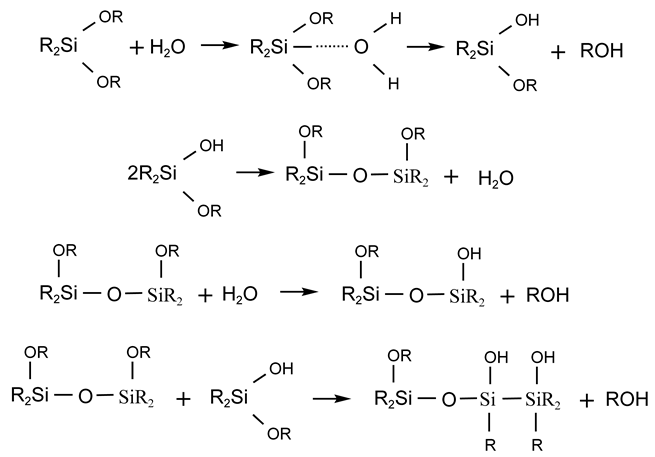
The polymerization between silicones mainly relies on the dehydration condensation of silicone monomers. As mentioned before, some functional groups are easily hydrolyzed to produce silanols, which are dehydrated and condensed to obtain siloxanes, and it is the simplest and most effective way to synthesis polysiloxanes. The hydrolysis process can be expressed as follows:
where R is alkyl, aryl, alkenyl, etc., and X is halogen, alkoxy, and acyloxy. The hydrolysis rate of silico-functional silanes decreases in the order of halogen > acyloxy > alkoxy, and the hydrolysis rate of organohalogen silanes decreases in the order of I > Br > Cl > F. When trifunctional silanes are hydrolyzed, generally less than trisilanols are obtained, but rather condensed siloxanes are obtained.
2.2.1. Hydrolytic Condensation of Difunctional Silicone Monomers
For example, the partial hydrolytic condensation of dialkyl dialkoxysilanes with small amounts of water produces high molecular weight polydialkyl siloxanes:
If the functional group organic monomer is hydrolyzed in excess water, a mixture of straight-chain and cyclic siloxanes is produced via dehydration polymerization. The proportion of the mixture is related to many factors: if an oxygen-containing reactive solvent is used, the proportion of cyclic siloxanes in the product increases; if straight-chain polysiloxanes are more easily produced under alkaline conditions, cyclic siloxanes are more easily produced under acidic conditions; and if the volume of substituents on the silicon atom is larger, cyclic siloxanes are more easily produced.
2.2.2. Hydrolytic Condensation of Trifunctional Organosilicon Monomers
When trifunctional silicone monomers interact with small amounts of water, straight-chain siloxanes can be obtained, and the polymerization process is similar to that of dialkyl dialkoxysilanes.
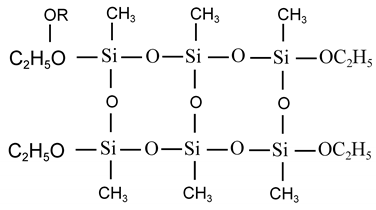
Trifunctional organosilicon monomers undergo hydrolysis and condensation reaction in excess water, which can generate polymers with complex components, and cyclic compounds can be obtained in addition to cross-linked structures. For example, when methyltriethoxysilane is hydrolyzed and a condensation reaction occurs in excess water, the condensed cyclic compound can be separated:
The higher degree of polymerization of the network structure requires further polymerization to complete the cross-linking, such as chain polysiloxane branched-chain condensation to complete the cross-linking, or cyclic polysiloxane ring-opening polymerization, or carbon tubular energy groups containing double bonds can be completed by hydrosilation to complete the cross-linking.
2.3. Surface Modification with Silicones
—CnH2n+1, —CH=CH2, —C6H5, —X, etc., are some representative hydrophobic groups, so it is possible to use the silane coupling agent, and the hydroxyl group on the surface to be treated is dehydrated and condensed; the hydrophilic group on the surface of the substrate hydroxyl disappears and is replaced by the hydrophobic group on top of the silane coupling agent, completing the hydrophobic treatment of the surface. If the surface substrate is a rough surface with a micro–nano structure, the grafting method can be used to complete the preparation of superhydrophobicity. This grafting method is used in many superhydrophobic preparation processes.
2.4. Silicones Build Micro- and Nano-Structures
2.4.1. Stöber Method
Hydrophilic silica was generated using the Stöber method and then the silica was modified using other silane coupling agents while firmly bonding the silica powder to the substrate surface.
Zhao et al. reported a silica nanoparticle precursor dispersion prepared by the Stober process followed by surface modification to prepare a superhydrophobic surface
[30]. The modification process utilizes the hydrolysis–condensation reaction of tetraethoxysilane (TEOS) and 1H,1H,2H,2H-perfluorodecyltriethoxysilane (HDFTES) to branch on fluorinated groups on top of the silica nanoparticles.
2.4.2. PDMS
Polydimethylsiloxane (PDMS) possesses good elasticity and demolding properties, so PDMS is also often used to build micro–nano mechanisms.
Oh et al. reported a template method using PDMS to prepare superhydrophobic surfaces
[31]. The scholars formed regular micron-sized column arrays on silicon wafers using conventional lithography and inverted the cylindrical hole arrays of the silicon master into PDMS molds. The resulting films were separated from the molds without damage or deformation due to the elasticity, high chemical stability, and low interfacial energy of PDMS.
2.4.3. Polymerization of Silicones
According to the hydrolytic condensation of silicones, silicon–hydrogen reactions can then be cross-linked with each other to form rough structures.
Qu et al. reported a method for constructing multivacancy rough structures through organosilicon hinges
[32]. A lightweight, mechanically flexible, and surface superhydrophobic organosilica aerogel was used to tune the cross-linked network via a simple environmental drying process. Polymethylhydrosiloxane with different hydrogen content was utilized to react with vinylmethyldimethoxysilane under the Pt catalyst, thus effectively tuning the cross-linking network in the organosilicon aerogel.
3. Silicone-Based Water-Repellent Surface Preparation
3.1. Electrostatic Spinning
Electrostatic spinning is a simple but versatile method for producing continuous fibers with diameters ranging from nanometer to submicron scales. The principle is to use a high DC electric field or high electric force to overcome the surface tension on the surface of the polymer solution and produce very thin jets. Therefore, electrostatic spinning is very suitable for preparing superhydrophobic materials, and silicone materials also play a key role in the preparation of many electrostatic spinning processes.
Organic silicon materials can be blended with their organic polymers as electrostatic spinning feedstock to prepare superhydrophobic materials. Song et al. reported an electrostatic spinning method to prepare superhydrophobic materials with polyvinylidene fluoride (PVDF)
[33]. The scholars added TEOS to the electrostatic spinning solution of PVDF, and then applied a voltage to complete the electrostatic spinning precursor solution. When the electrospun nanofibers were annealed at 100 °C under room temperature, the condensation reaction was accelerated to form a cross-linked silica network and PVDF was embedded in the TEOS network through chemical bonding chains. The contact angle of the final prepared superhydrophobic material reached 155.5°.
Organosilicon can also modify some of the raw materials hydrophobically and then perform electrostatic spinning experiments in configuring the reaction solution. Mookgo et al. reported a method to prepare superhydrophobic-superoleophilic nanofiber membranes via electrostatic spinning
[34]. Perfluorooctyltriethoxysilane was deposited on carbon nanotubes (CNTs). Then, the treated CNTs were added to a PVDF polymer solution for electrostatic spinning to finally prepare superhydrophobic fiber membranes. This nanofiber membrane has good oil–water separation capability.
Silicones can be silanized on their rough surfaces after the completion of electrostatic spinning to complete the preparation of superhydrophobic fiber membranes. Faride et al. reported a combination of electrostatic spinning techniques and the dichlorodimethylsilane deposition method to prepare superhydrophobic nanofiber membranes
[35]. The electrostatic spun nanofiber membranes formed from PVDF were modified with dichlorodimethylsilane (DMDCS) reagent to successfully prepare superhydrophobic and super lipophilic functionalized electrostatic spun PVDF membranes.
3.2. Spray Method
The spray method is a working method in which the coating is pressed or sucked out of the container by an external force and forms a mist to adhere to the object’s surface. In the superhydrophobic preparation process, silicones can either bond the micro–nano particles in the spray solution together or hydrophobically modify the surface.
3.3. Template Method
The stencil method is a commonly used method for preparing superhydrophobic coated films, which is a monolithic coverage surface technology. The template method uses a solid with a rough structure as a template, and the hydrophobic material is formed and demolded on the rough solid surface via extrusion or coating followed by light curing on a specific template to produce a superhydrophobic film. The preparation of superhydrophobic coatings using the template method has the advantages of simple operation, good reproducibility, and controllable nanowire diameter ratio. In the preparation of superhydrophobic water using the template method, PDMS demonstrates its excellent characteristics because PDMS is flexible and can be easily peeled off during the demolding process while the processed PDMS keeps the mold intact and undamaged, and PDMS is flexible and contacts the relatively rough surface very closely after processing. So, almost superhydrophobic materials on silicone substrates use PDMS when using the template method.
3.4. Particle Filled Method
The particle filled method is a direct combination of silicone and various nano-powders to form a superhydrophobic surface with a particle-based surface. The superhydrophobic surface of the silicone substrate is mainly formed by silica prepared using the Stöber method, or silicone acts as a binder and modifier in other nano-powders to form a superhydrophobic surface. This method has the advantage of simple and convenient operation, but because the powder is the main body, it tends to lead to poor surface durability and wear resistance.
3.5. Chemical Deposition Method
Chemical deposition methods are widely used to prepare superhydrophobic surfaces because they can directly and effectively construct suitable surface roughness and reduce surface energy. The preparation of superhydrophobic surfaces on silicone substrates via chemical deposition is usually accompanied by chemical reactions, such as the cross-linking of silicone polymerization and grafting rough surfaces. They can be divided into vapor deposition, solution deposition, and electrochemical deposition.
4. Silicone-Based Superhydrophobic Applications
4.1. Self-Cleaning
Self-cleaning is the most basic application of superhydrophobic surfaces. After being contaminated, the superhydrophobic surfaces can take the contaminants away by themselves when rainwater rolls over the surface to achieve self-cleaning. The principle is that on a rough, low-energy surface, if the surface is contaminated with dust particles, the interface area between the contaminated particles and the surface is relatively small, resulting in reduced adhesion. Water droplets from rainfall can absorb contaminated particles and carry them away as the droplets roll off the surface, achieving a self-cleaning effect. Simulated pollution experiments on various natural plant leaves (naturally hydrophobic surfaces) show that pollutant particles on hydrophobic superhydrophobic leaves are almost completely removed by water droplets, while a considerable number of particles remain on non-superhydrophobic leaves.
4.2. Corrosion Resistant
Superhydrophobicity has good corrosion resistance; on the one hand, the air film in the superhydrophobic micro–nano structure will directly isolate the metal from the external corrosive solution. On the other hand, when exposed to air, metals tend to form electrolyte films easily on the surface, and the formation of such electrolyte films can lead to a sudden increase in the corrosion rate as they are carriers of electrons, oxygen, and carbon dioxide
[36]. On superhydrophobic surfaces, the droplets produced after condensation are separated from each other by gas films in the surface roughness and electrons cannot move freely. As a result, electrochemical reactions are hindered, leading to superhydrophobic surfaces with good corrosion resistance
[37].
4.3. Oil-Water Separation
Oil-water separation is generally gravity-driven, based on the difference in surface wettability for water and oily liquids. Most superhydrophobic surfaces are hydrophobic and lipophilic, allowing oily liquids to pass and blocking the passage of water.
4.4. Anti-Icing
An ideal superhydrophobic surface should have a long delayed icing time
[38] and a small ice adhesion
[39] so that the ice formed on the surface can be removed by its own gravity or natural wind. Therefore, superhydrophobic surfaces possess good anti-icing capability.