
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hooi-Leng Ser | -- | 1939 | 2023-02-22 21:15:20 | | | |
| 2 | Catherine Yang | Meta information modification | 1939 | 2023-02-23 01:57:44 | | |
Video Upload Options
Endometriosis affects approximately 6 to 10% of reproductive-age women globally. Despite much effort invested, the pathogenesis that promotes the development, as well as the progression of this chronic inflammatory disease, is poorly understood. The imbalance in the microbiome or dysbiosis has been implicated in a variety of human diseases, especially the gut microbiome. In the case of endometriosis, emerging evidence suggests that there may be urogenital-gastrointestinal crosstalk that leads to the development of endometriosis. Along with these findings, several studies have reported the potential of probiotics in managing endometriosis, however subsequent investigations on microbial dynamics post administration of probiotics as well as route of administration and formulation of probiotics would be needed to strengthen the rationale of using such microbiome-based intervention in the management of endometriosis.
1. The Intricate Relationship between the Female Reproductive Tract Microbiome and Gut Microbiome in the Development and Progression of Endometriosis
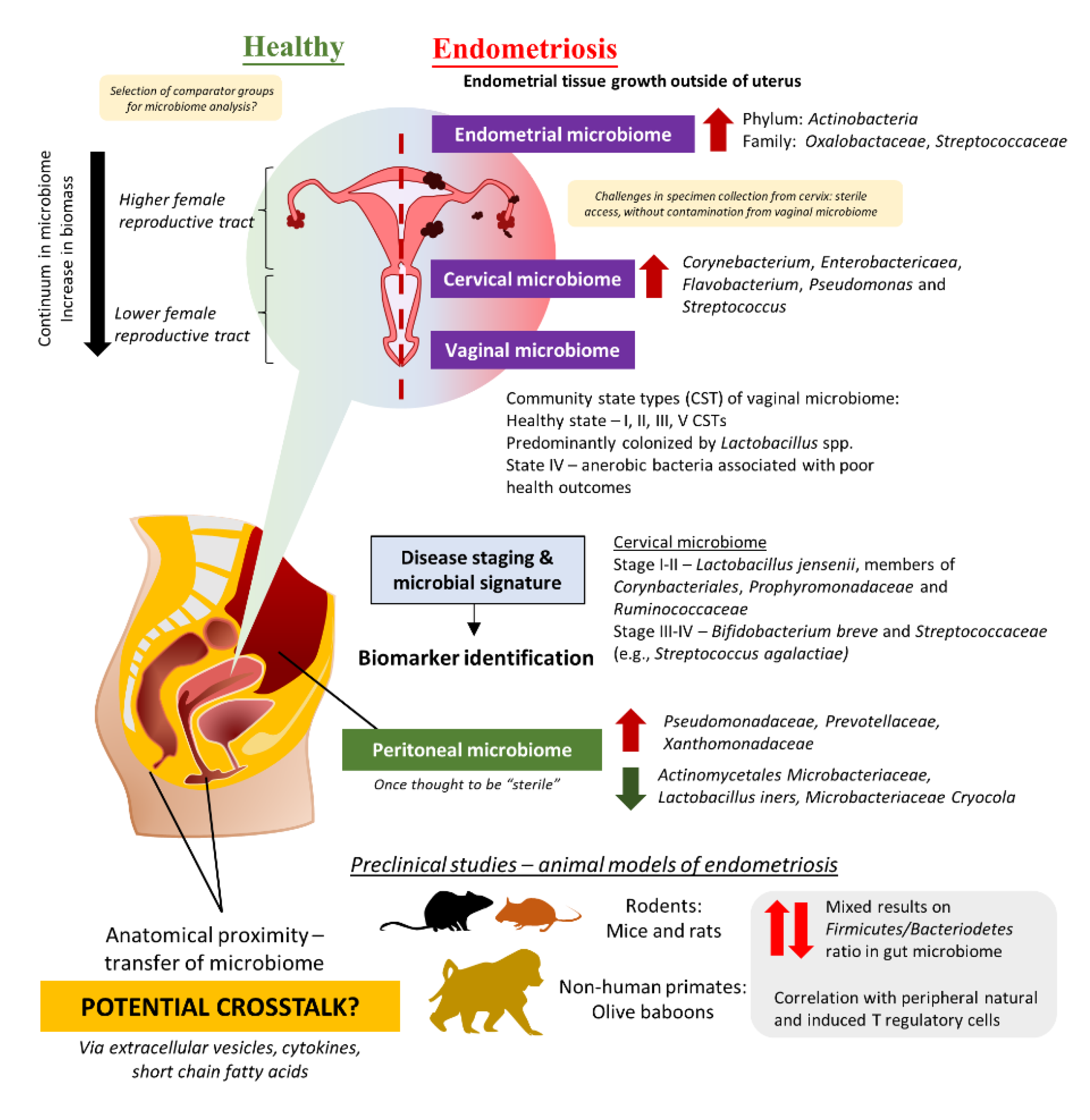
2. Evidence from Clinical Studies: Are There Any Distinct Microbiome Changes in the Vaginal Microbiome?
Given the challenges in obtaining cervical specimens without cervicovaginal contamination and the nature of biomass in the upper FRT, several teams have attempted to study the differences in the microbiome of the lower FRT. For instance, three studies in Brazil and China studied the vaginal swab or fluid obtained from patients and observed a lower abundance of Lactobacillus in the endometriosis group as compared to the control [24][28][29]. Besides that, the study by Ata et al. discussed the differences in vaginal samples obtained from Stage III or IV endometriosis patients as compared to healthy women [18]. At the genus level, Gemella and Atopobium spp. was absent in the vaginal samples obtained from the endometriosis group. A similar approach was taken by Perrotta et al., but the team took a broader approach to look at the vaginal CST rather than looking at just a specific group of microbes [30]. These data then allowed the team to build a random forest-based classification model with machine-learning methods on microbiota composition to predict r-ASRM stages of endometriosis. Analyzing the changes during follicular and menstrual phases yielded highly predictive taxa which can be used to predict either stage I-II or stage III-IV endometriosis—the genus Anaerococcus (phylum Firmicutes).
3. Connections between Gut Microbiome, Peritoneal Microbiome, and Endometriosis
4. Potential Benefits of Probiotics in the Management of Endometriosis
References
- Blum, H.E. The human microbiome. Adv. Med. Sci. 2017, 62, 414–420.
- Martinez-Guryn, K.; Leone, V.; Chang, E.B. Regional Diversity of the Gastrointestinal Microbiome. Cell Host Microbe 2019, 26, 314–324.
- Lopetuso, L.R.; Scaldaferri, F.; Franceschi, F.; Gasbarrini, A. The gastrointestinal microbiome—Functional interference between stomach and intestine. Best Pr. Res. Clin. Gastroenterol. 2014, 28, 995–1002.
- Noto, J.M.; Peek, R.M., Jr. The gastric microbiome, its interaction with Helicobacter pylori, and its potential role in the progression to stomach cancer. PLoS Pathog. 2017, 13, e1006573.
- Ruan, W.; Engevik, M.A.; Spinler, J.K.; Versalovic, J. Healthy Human Gastrointestinal Microbiome: Composition and Function After a Decade of Exploration. Dig. Dis. Sci. 2020, 65, 695–705.
- Mason, K.L.; Huffnagle, G.B.; Noverr, M.C.; Kao, J.Y. Overview of gut immunology. Adv. Exp. Med. Biol. 2008, 635, 1–14.
- Morbe, U.M.; Jorgensen, P.B.; Fenton, T.M.; von Burg, N.; Riis, L.B.; Spencer, J.; Agace, W.W. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. 2021, 14, 793–802.
- Li, S.; Chen, S.; Nie, M.; Wen, L.; Zou, B.; Zhang, L.; Xie, J.; Ser, H.L.; Lee, L.H.; Wang, S.; et al. Salt-Sensitive Ileal Microbiota Plays a Role in Atrial Natriuretic Peptide Deficiency-Induced Cardiac Injury. Nutrients 2022, 14, 3129.
- Ser, H.-L.; Wong, J.Y.-J.; Goh, B.-H.; Reginald, K. IDDF2022-ABS-0236 Healing the GUT with probiotics: Can probiotics help relieve allergic rhinitis? Gut 2022, 71, A63–A64.
- Joseph, R.J.; Ser, H.L.; Kuai, Y.H.; Tan, L.T.; Arasoo, V.J.T.; Letchumanan, V.; Wang, L.; Pusparajah, P.; Goh, B.H.; Ab Mutalib, N.S.; et al. Finding a Balance in the Vaginal Microbiome: How Do We Treat and Prevent the Occurrence of Bacterial Vaginosis? Antibiotics 2021, 10, 719.
- Mestrovic, T.; Matijasic, M.; Peric, M.; Cipcic Paljetak, H.; Baresic, A.; Verbanac, D. The Role of Gut, Vaginal, and Urinary Microbiome in Urinary Tract Infections: From Bench to Bedside. Diagnostics 2020, 11, 7.
- Ser, H.-L.; Wong, J.Y.J.; Letchumanan, V.; Law, J.W.-F.; Tan, L.T.-H.; Lee, L.-H. IDDF2021-ABS-0132 Moving beyond the gastrointestinal tract: The involvement of gut microbiome in endometriosis. Gut 2021, 70, A46–A47.
- Plesniarski, A.; Siddik, A.B.; Su, R.C. The Microbiome as a Key Regulator of Female Genital Tract Barrier Function. Front. Cell. Infect. Microbiol. 2021, 11, 790627.
- Punzon-Jimenez, P.; Labarta, E. The impact of the female genital tract microbiome in women health and reproduction: A review. J. Assist. Reprod. Genet. 2021, 38, 2519–2541.
- Gajer, P.; Brotman, R.M.; Bai, G.; Sakamoto, J.; Schutte, U.M.; Zhong, X.; Koenig, S.S.; Fu, L.; Ma, Z.S.; Zhou, X.; et al. Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 2012, 4, 132ra152.
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687.
- Akiyama, K.; Nishioka, K.; Khan, K.N.; Tanaka, Y.; Mori, T.; Nakaya, T.; Kitawaki, J. Molecular detection of microbial colonization in cervical mucus of women with and without endometriosis. Am. J. Reprod. Immunol. 2019, 82, e13147.
- Ata, B.; Yildiz, S.; Turkgeldi, E.; Brocal, V.P.; Dinleyici, E.C.; Moya, A.; Urman, B. The endobiota study: Comparison of vaginal, cervical and gut microbiota between women with stage 3/4 endometriosis and healthy controls. Sci. Rep. 2019, 9, 1–9.
- Chen, S.; Gu, Z.; Zhang, W.; Jia, S.; Wu, Y.; Zheng, P.; Dai, Y.; Leng, J. Microbiome of the lower genital tract in Chinese women with endometriosis by 16s-rRNA sequencing technique: A pilot study. Ann. Transl. Med. 2020, 8, 1440.
- Wei, W.; Zhang, X.; Tang, H.; Zeng, L.; Wu, R. Microbiota composition and distribution along the female reproductive tract of women with endometriosis. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 15.
- Lee, S.R.; Lee, J.C.; Kim, S.H.; Oh, Y.S.; Chae, H.D.; Seo, H.; Kang, C.S.; Shin, T.S. Altered Composition of Microbiota in Women with Ovarian Endometrioma: Microbiome Analyses of Extracellular Vesicles in the Peritoneal Fluid. Int. J. Mol. Sci. 2021, 22, 4608.
- Huang, L.; Liu, B.; Liu, Z.; Feng, W.; Liu, M.; Wang, Y.; Peng, D.; Fu, X.; Zhu, H.; Cui, Z.; et al. Gut Microbiota Exceeds Cervical Microbiota for Early Diagnosis of Endometriosis. Front. Cell. Infect. Microbiol. 2021, 11, 788836.
- Shan, J.; Ni, Z.; Cheng, W.; Zhou, L.; Zhai, D.; Sun, S.; Yu, C. Gut microbiota imbalance and its correlations with hormone and inflammatory factors in patients with stage 3/4 endometriosis. Arch. Gynecol. Obs. 2021, 304, 1363–1373.
- Chao, X.; Liu, Y.; Fan, Q.; Shi, H.; Wang, S.; Lang, J. The role of the vaginal microbiome in distinguishing female chronic pelvic pain caused by endometriosis/adenomyosis. Ann. Transl. Med. 2021, 9, 771.
- Chang, C.Y.; Chiang, A.J.; Lai, M.T.; Yan, M.J.; Tseng, C.C.; Lo, L.C.; Wan, L.; Li, C.J.; Tsui, K.H.; Chen, C.M.; et al. A more diverse cervical microbiome associates with better clinical outcomes in patients with endometriosis: A pilot study. Biomedicines 2022, 10, 174.
- Oishi, S.; Mekaru, K.; Tanaka, S.E.; Arai, W.; Ashikawa, K.; Sakuraba, Y.; Nishioka, M.; Nakamura, R.; Miyagi, M.; Akamine, K. Microbiome analysis in women with endometriosis: Does a microbiome exist in peritoneal fluid and ovarian cystic fluid? Reprod. Med. Biol. 2022, 21, e12441.
- Yuan, W.; Wu, Y.; Chai, X.; Wu, X. The colonized microbiota composition in the peritoneal fluid in women with endometriosis. Arch. Gynecol. Obs. 2022, 305, 1573–1580.
- Lu, F.; Wei, J.; Zhong, Y.; Feng, Y.; Ma, B.; Xiong, Y.; Wei, K.; Tan, B.; Chen, T. Antibiotic Therapy and Vaginal Microbiota Transplantation Reduce Endometriosis Disease Progression in Female Mice via NF-kappaB Signaling Pathway. Front. Med. 2022, 9, 831115.
- Hernandes, C.; Silveira, P.; Rodrigues Sereia, A.F.; Christoff, A.P.; Mendes, H.; Valter de Oliveira, L.F.; Podgaec, S. Microbiome Profile of Deep Endometriosis Patients: Comparison of Vaginal Fluid, Endometrium and Lesion. Diagnostics 2020, 10, 163.
- Perrotta, A.R.; Borrelli, G.M.; Martins, C.O.; Kallas, E.G.; Sanabani, S.S.; Griffith, L.G.; Alm, E.J.; Abrao, M.S. The vaginal microbiome as a tool to predict rASRM stage of disease in endometriosis: A pilot study. Reprod. Sci. 2020, 27, 1064–1073.
- Le, N.; Cregger, M.; Fazleabas, A.; Braundmeier-Fleming, A. Effects of endometriosis on immunity and mucosal microbial community dynamics in female olive baboons. Sci. Rep. 2022, 12, 1590.
- Svensson, A.; Brunkwall, L.; Roth, B.; Orho-Melander, M.; Ohlsson, B. Associations between endometriosis and gut microbiota. Reprod. Sci. 2021, 28, 2367–2377.
- Wessels, J.M.; Domínguez, M.A.; Leyland, N.A.; Agarwal, S.K.; Foster, W.G. Endometrial microbiota is more diverse in people with endometriosis than symptomatic controls. Sci. Rep. 2021, 11, 1–12.
- Simoes-Silva, L.; Araujo, R.; Pestana, M.; Soares-Silva, I.; Sampaio-Maia, B. Peritoneal Microbiome in End-Stage Renal Disease Patients and the Impact of Peritoneal Dialysis Therapy. Microorganisms 2020, 8, 173.
- Gilbreath, J.J.; Semino-Mora, C.; Friedline, C.J.; Liu, H.; Bodi, K.L.; McAvoy, T.J.; Francis, J.; Nieroda, C.; Sardi, A.; Dubois, A.; et al. A core microbiome associated with the peritoneal tumors of pseudomyxoma peritonei. Orphanet J. Rare Dis. 2013, 8, 105.
- Semino-Mora, C.; Liu, H.; McAvoy, T.; Nieroda, C.; Studeman, K.; Sardi, A.; Dubois, A. Pseudomyxoma peritonei: Is disease progression related to microbial agents? A study of bacteria, MUC2 AND MUC5AC expression in disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. Ann. Surg. Oncol. 2008, 15, 1414–1423.
- Ferrero, S.; Vellone, V.G.; Barra, F. Pathophysiology of pain in patients with peritoneal endometriosis. Ann. Transl. Med. 2019, 7, S8.
- Khodaverdi, S.; Mohammadbeigi, R.; Khaledi, M.; Mesdaghinia, L.; Sharifzadeh, F.; Nasiripour, S.; Gorginzadeh, M. Beneficial Effects of Oral Lactobacillus on Pain Severity in Women Suffering from Endometriosis: A Pilot Placebo-Controlled Randomized Clinical Trial. Int. J. Fertil. Steril. 2019, 13, 178–183.
- Itoh, H.; Sashihara, T.; Hosono, A.; Kaminogawa, S.; Uchida, M. Lactobacillus gasseri OLL2809 inhibits development of ectopic endometrial cell in peritoneal cavity via activation of NK cells in a murine endometriosis model. Cytotechnology 2011, 63, 205–210.
- Ibrahim, A.; Ali, R.A.R.; Manaf, M.R.A.; Ahmad, N.; Tajurruddin, F.W.; Qin, W.Z.; Desa, S.H.M.; Ibrahim, N.M. Multi-strain probiotics (Hexbio) containing MCP BCMC strains improved constipation and gut motility in Parkinson’s disease: A randomised controlled trial. PLoS ONE 2020, 15, e0244680.
- Itoh, H.; Uchida, M.; Sashihara, T.; Ji, Z.S.; Li, J.; Tang, Q.; Ni, S.; Song, L.; Kaminogawa, S. Lactobacillus gasseri OLL2809 is effective especially on the menstrual pain and dysmenorrhea in endometriosis patients: Randomized, double-blind, placebo-controlled study. Cytotechnology 2011, 63, 153–161.
- Uchida, M.; Kobayashi, O. Effects of Lactobacillus gasseri OLL2809 on the induced endometriosis in rats. Biosci. Biotechnol. Biochem. 2013, 77, 1879–1881.
- Del Piano, M.; Carmagnola, S.; Ballare, M.; Sartori, M.; Orsello, M.; Balzarini, M.; Pagliarulo, M.; Tari, R.; Anderloni, A.; Strozzi, G.P.; et al. Is microencapsulation the future of probiotic preparations? The increased efficacy of gastro-protected probiotics. Gut Microbes 2011, 2, 120–123.
- Sanders, M.E.; Klaenhammer, T.R.; Ouwehand, A.C.; Pot, B.; Johansen, E.; Heimbach, J.T.; Marco, M.L.; Tennila, J.; Ross, R.P.; Franz, C.; et al. Effects of genetic, processing, or product formulation changes on efficacy and safety of probiotics. Ann. New York Acad. Sci. 2014, 1309, 1–18.




