
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Joyce W.H. Chan | -- | 2240 | 2023-02-22 16:48:18 | | | |
| 2 | Rita Xu | -1 word(s) | 2239 | 2023-02-23 02:30:39 | | |
Video Upload Options
The demand for parenchyma-sparing local therapies for lung cancer is rising owing to an increasing incidence of multifocal lung cancers and patients who are unfit for surgery. With the latest evidence of the efficacy of lung cancer screening, more premalignant or early-stage lung cancers are being discovered and the paradigm has shifted from treatment to prevention. Transbronchial therapy is an important armamentarium in the local treatment of lung cancers, with microwave ablation being the most promising based on early to midterm results. Adjuncts to improve transbronchial ablation efficiency and accuracy include mobile C-arm platforms, software to correct for the CT-to-body divergence, metal-containing nanoparticles, and robotic bronchoscopy.
1. Introduction
2. Transbronchial Microwave Ablation
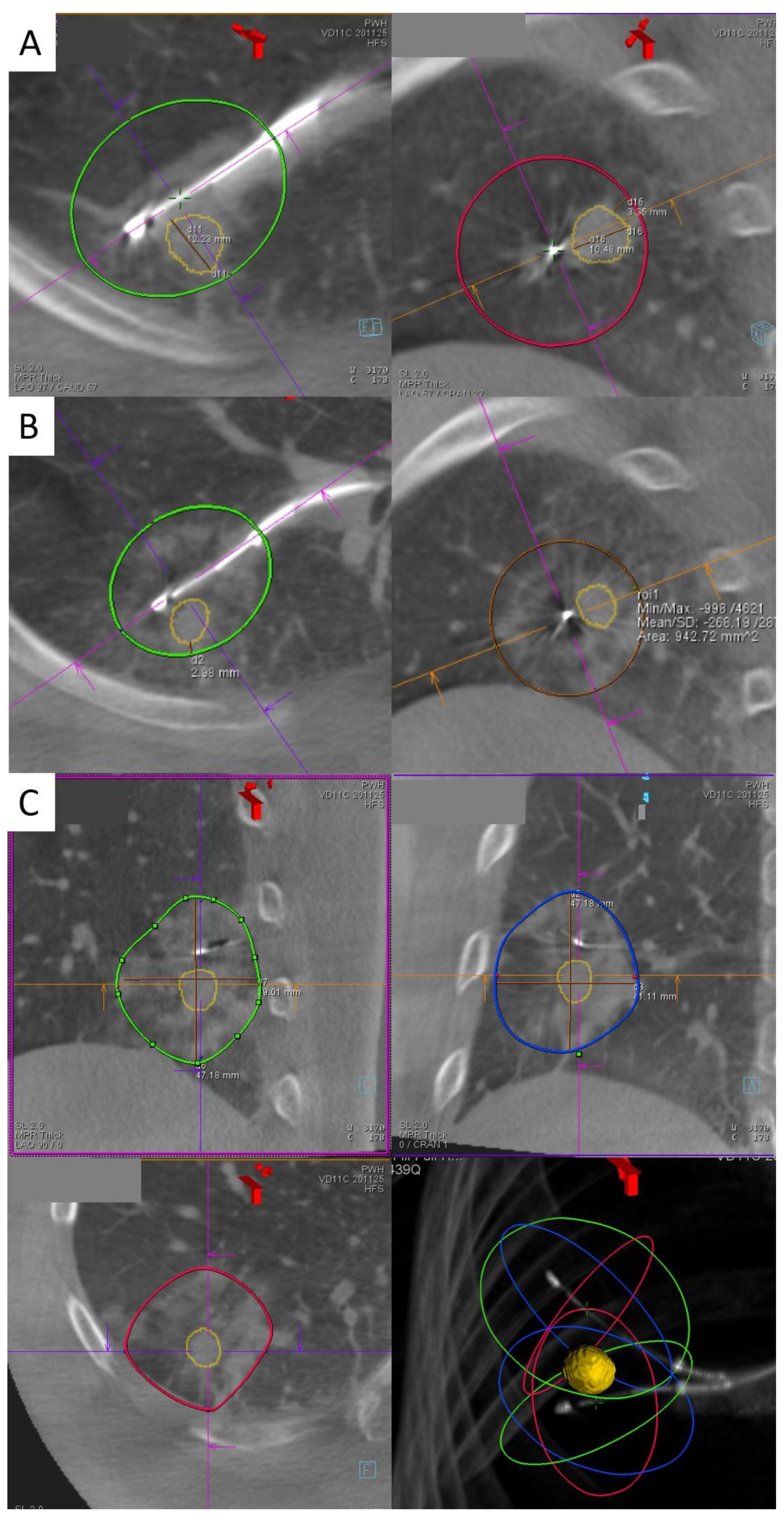
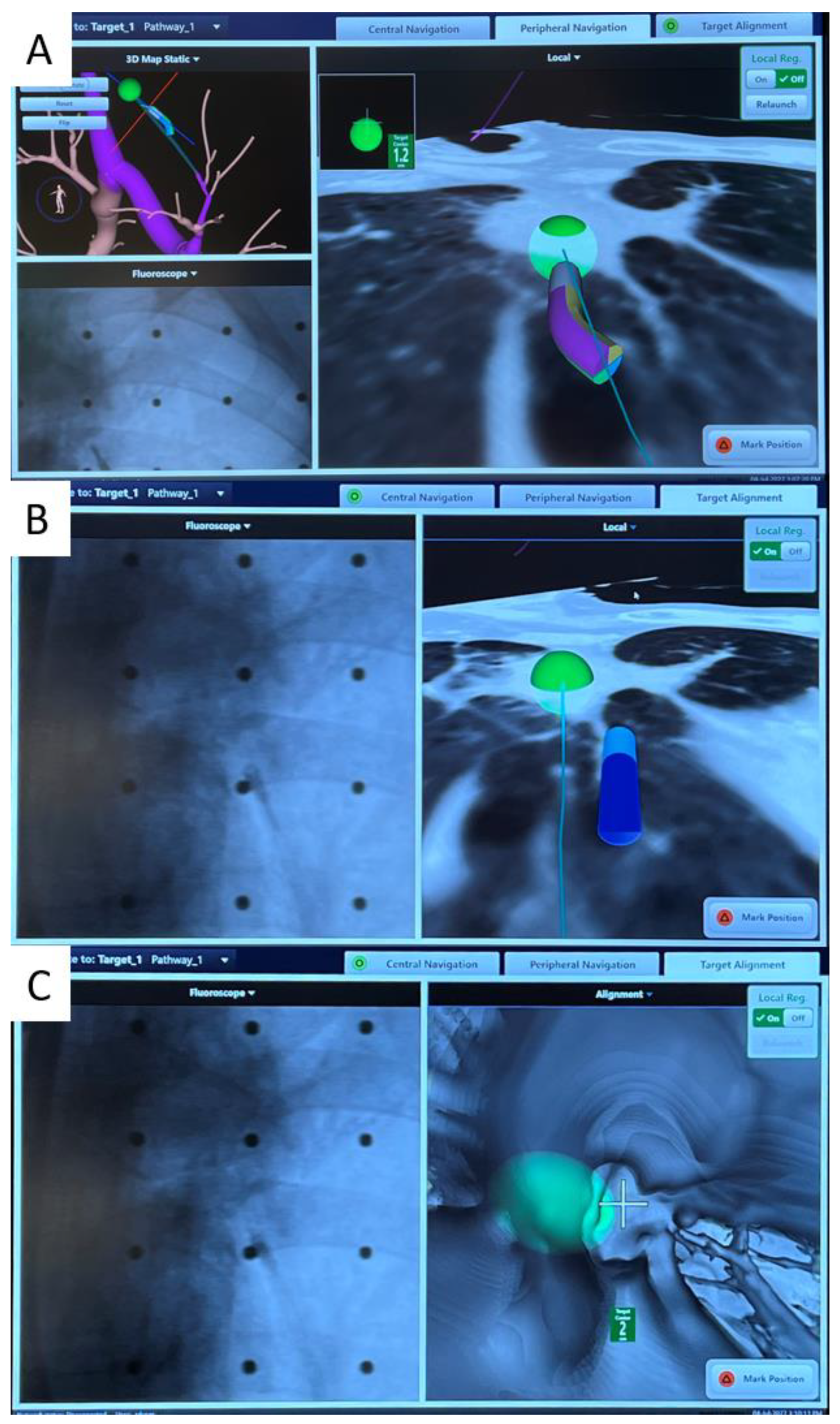
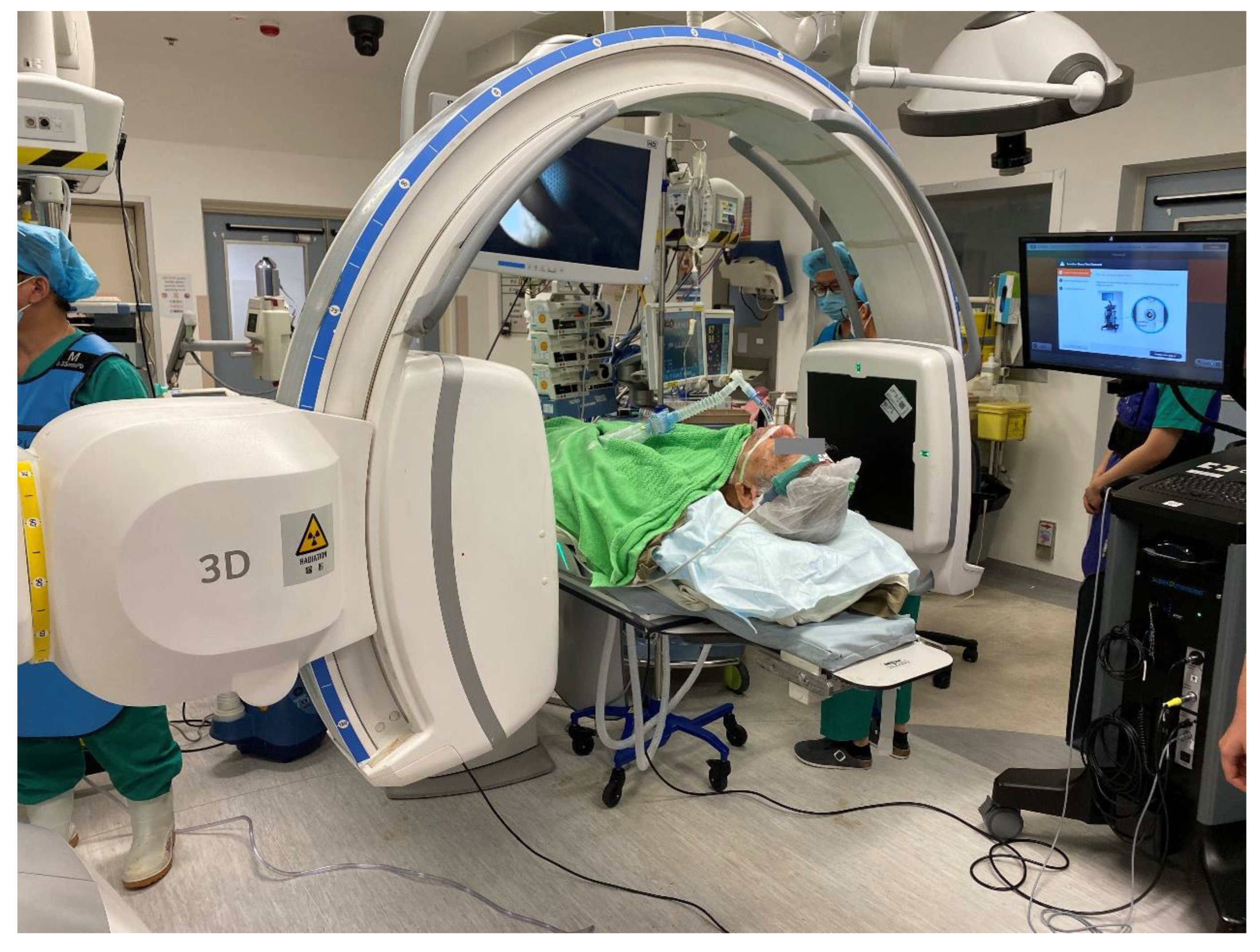
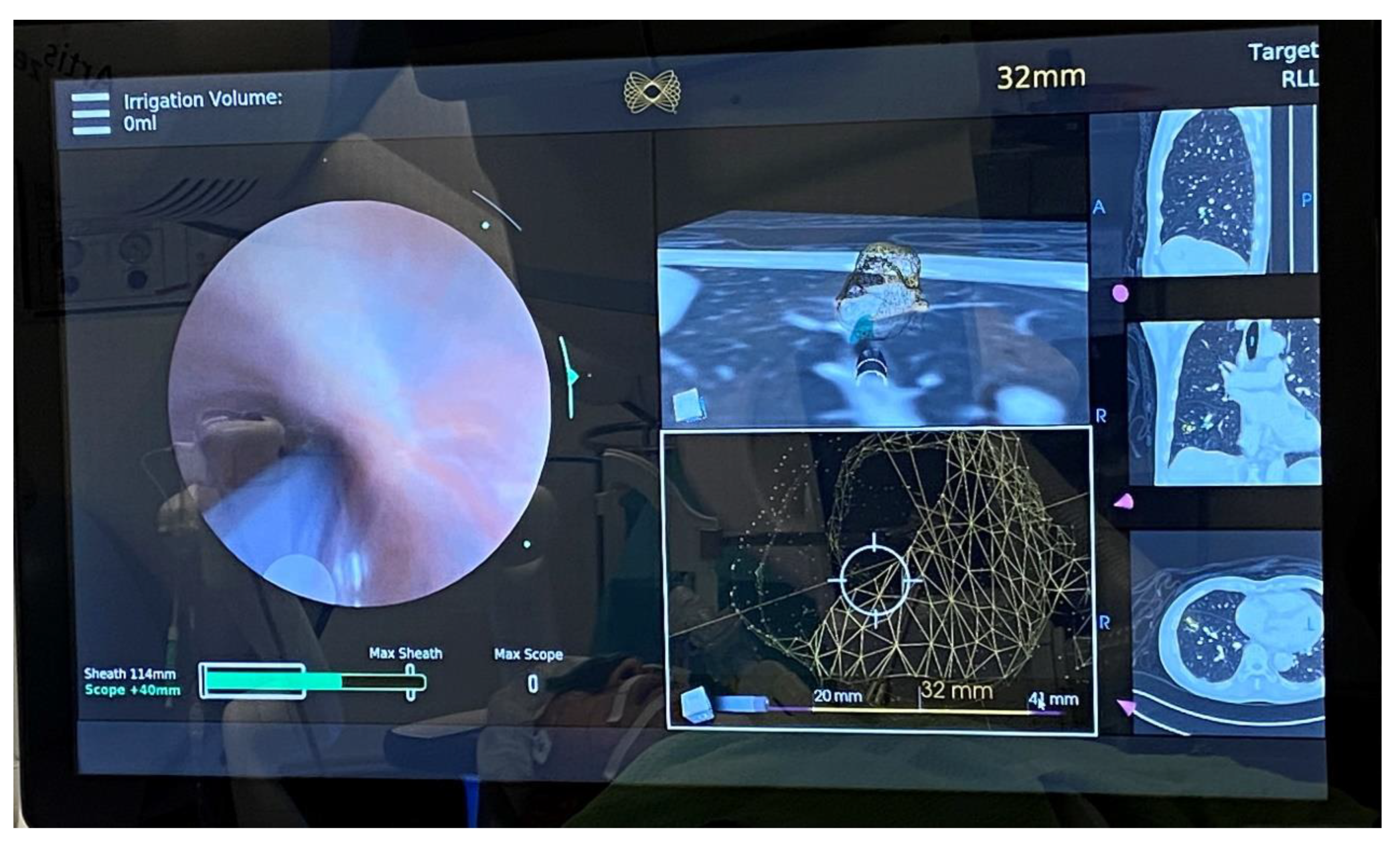
4. Robotic Bronchoscopy to Potentially Improve Transbronchial Ablation Accuracy and Efficacy
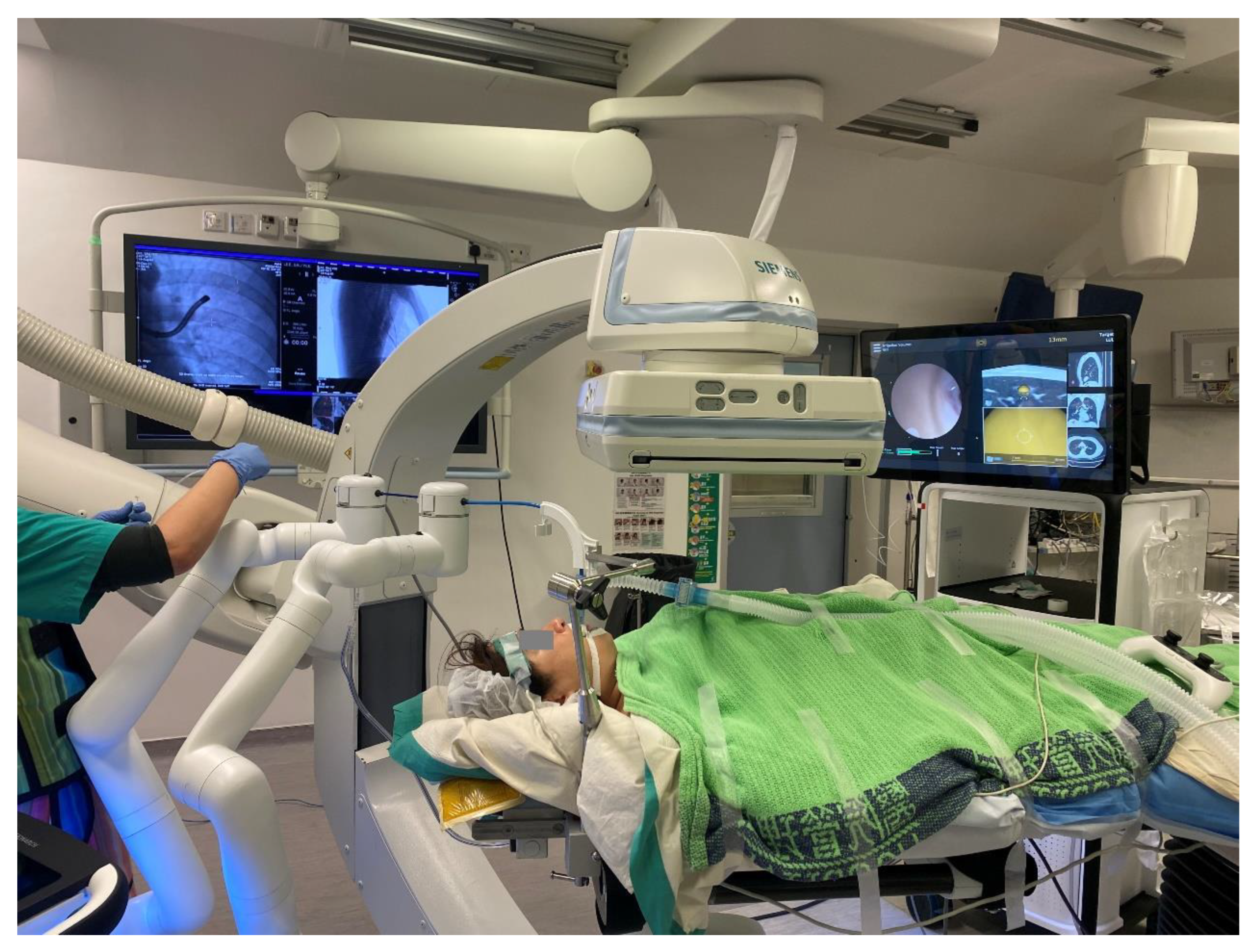
References
- Ginsberg, R.J.; Rubinstein, L.V. Randomized Trial of Lobectomy Versus Limited Resection for T1 N0 Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 1995, 60, 615–623. Available online: https://pubmed.ncbi.nlm.nih.gov/7677489/ (accessed on 8 November 2022).
- Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; The National Lung Screening Trial Research Team. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N. Engl. J. Med. 2011, 365, 395–409. Available online: http://www.nejm.org/doi/10.1056/NEJMoa1102873 (accessed on 10 April 2020).
- De Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. Available online: https://pubmed.ncbi.nlm.nih.gov/31995683/ (accessed on 10 May 2022).
- Leventakos, K.; Peikert, T.; Midthun, D.E.; Molina, J.R.; Blackmon, S.; Nichols, F.C.; Garces, Y.I.; Hallemeier, C.L.; Murphy, S.J.; Vasmatzis, G.; et al. Management of Multifocal Lung Cancer: Results of a Survey. J. Thorac. Oncol. 2017, 12, 1398–1402. Available online: https://pubmed.ncbi.nlm.nih.gov/28583587/ (accessed on 8 November 2022).
- Warth, A.; Macher-Goeppinger, S.; Muley, T.; Thomas, M.; Hoffmann, H.; Schnabel, P.A.; Penzel, R.; Schirmacher, P.; Aulmann, S. Clonality of Multifocal Nonsmall Cell Lung Cancer: Implications for Staging and Therapy. Eur. Respir. J. 2012, 39, 1437–1442. Available online: https://pubmed.ncbi.nlm.nih.gov/22075483/ (accessed on 8 November 2022).
- Rami-Porta, R.; Tsuboi, M. Sublobar Resection for Lung Cancer. Eur. Respir. J. 2009, 33, 426–435. Available online: https://pubmed.ncbi.nlm.nih.gov/19181916/ (accessed on 20 September 2020).
- El-Sherif, A.; Gooding, W.E.; Santos, R.; Pettiford, B.; Ferson, P.F.; Fernando, H.C.; Urda, S.J.; Luketich, J.D.; Landreneau, R.J. Outcomes of Sublobar Resection Versus Lobectomy for Stage I Non-Small Cell Lung Cancer: A 13-Year Analysis. Ann. Thorac. Surg. 2006, 82, 408–416. Available online: https://pubmed.ncbi.nlm.nih.gov/16863738/ (accessed on 8 August 2020).
- Berfield, K.S.; Wood, D.E. Sublobar resection for stage IA non-small cell lung cancer. J. Thorac. Dis. AME Publ. Co. 2017, 9, 208–210.
- Abreu, C.E.C.V.; Ferreira, P.P.R.; Moraes, F.Y.D.; Neves, W.F.P.; Gadia, R.; Carvalho, H.D.A. Radioterapia Estereotáxica Extracraniana em Câncer de Pulmão: Atualização. J. Bras. Pneumol. Soc. Bras. Pneumol. E Tisiol. 2015, 41, 376–387. Available online: https://www.jornaldepneumologia.com.br/details/2431/pt-BR/radioterapia-estereotaxica-extracraniana-em-cancer-de-pulmao--atualizacao (accessed on 20 September 2020).
- Long-Term Results of Stereotactic Body Radiation Therapy in Medically Inoperable Stage I Non-Small Cell Lung Cancer—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/29852036/ (accessed on 20 September 2020).
- Dupuy, D.E.; Zagoria, R.J.; Akerley, W.; Mayo-Smith, W.W.; Kavanagh, P.V.; Safran, H. Technical Innovation: Percutaneous Radiofrequency Ablation of Malignancies in the Lung. Am. J. Roentgenol. 2000, 174, 57–59. Available online: https://pubmed.ncbi.nlm.nih.gov/10628454/ (accessed on 20 September 2020).
- Tanabe, T.; Koizumi, T.; Tsushima, K.; Ito, M.; Kanda, S.; Kobayashi, T.; Yasuo, M.; Yamazaki, Y.; Kubo, K.; Honda, T.; et al. Comparative Study of Three Different Catheters for ct Imaging-Bronchoscopy- Guided Radiofrequency Ablation as a Potential and Novel Interventional Therapy for Lung Cancer. Chest 2010, 137, 890–897. Available online: https://pubmed.ncbi.nlm.nih.gov/19858232/ (accessed on 8 August 2020).
- Koizumi, T.; Tsushima, K.; Tanabe, T.; Agatsuma, T.; Yokoyama, T.; Ito, M.; Kanda, S.; Kobayashi, T.; Yasuo, M. Bronchoscopy-Guided Cooled Radiofrequency Ablation as a Novel Intervention Therapy for Peripheral Lung Cancer. Respiration 2015, 90, 47–55. Available online: https://pubmed.ncbi.nlm.nih.gov/26044954/ (accessed on 8 August 2020).
- Xie, F.; Zheng, X.; Xiao, B.; Han, B.; Herth, F.J.F.; Sun, J. Navigation Bronchoscopy-Guided Radiofrequency Ablation for Nonsurgical Peripheral Pulmonary Tumors. Respiration 2017, 94, 293–298. Available online: https://pubmed.ncbi.nlm.nih.gov/28683443/ (accessed on 8 August 2020).
- Yuan, H.B.; Wang, X.Y.; Sun, J.Y.; Xie, F.F.; Zheng, X.X.; Tao, G.Y.; Pan, L.; Hogarth, D.K. Flexible Bronchoscopy-Guided Microwave Ablation in Peripheral Porcine Lung: A New Minimally-Invasive Ablation. Transl. Lung Cancer Res. 2019, 8, 787–796. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6976383/ (accessed on 8 August 2020).
- Medtronic Announces NAVABLATE Study Results Released in Late-Breaking Podium Presentation at European Respiratory Society International Congress 2021—7 September 2021. Available online: https://news.medtronic.com/2021-09-07-Medtronic-Announces-NAVABLATE-Study-Results-Released-in-Late-Breaking-Podium-Presentation-at-European-Respiratory-Society-International-Congress-2021 (accessed on 12 November 2022).
- Chan, J.; Yu, P.; Lau, R.; Ng, C. P02.02 Transbronchial Microwave Ablation of Lung Nodules in the Hybrid Operating Room—Mid-Term Follow Up of a Novel Technique. J. Thorac. Oncol. 2021, 16, S977.
- Mak, K.L.; Chan, J.W.Y.; Lau, R.W.H.; Ng, C.S.H. Management of Bronchopleural Fistula with Endobronchial Valve in Hybrid Operating Room Following Transbronchial Microwave Ablation. Interact. Cardiovasc. Thorac. Surg. 2021, 33, 992–994. Available online: https://academic.oup.com/icvts/article/33/6/992/6318715 (accessed on 8 November 2022).
- Chan, J.W.; Lau, R.W.; Ngai, J.C.; Tsoi, C.; Chu, C.M.; Mok, T.S.; Ng, C.S. Transbronchial Microwave Ablation of Lung Nodules with Electromagnetic Navigation Bronchoscopy Guidance-A Novel Technique and Initial Experience with 30 Cases. Transl. Lung Cancer Res. 2021, 10, 1608–1622. Available online: https://tlcr.amegroups.com/article/view/51418/html (accessed on 8 May 2021).
- Xie, F.; Chen, J.; Jiang, Y.; Sun, J.; Hogarth, D.K.; Herth, F.J.F. Microwave Ablation Via a Flexible Catheter for the Treatment of Nonsurgical Peripheral Lung Cancer: A Pilot Study. Thorac. Cancer 2022, 13, 1014–1020. Available online: https://pubmed.ncbi.nlm.nih.gov/35166043/ (accessed on 8 November 2022).
- Bao, F.; Yu, F.; Wang, R.; Chen, C.; Zhang, Y.; Lin, B.; Wang, Y.; Hao, X.; Gu, Z.; Fang, W. Electromagnetic Bronchoscopy Guided Microwave Ablation for Early Stage Lung Cancer Presenting as Ground Glass Nodule. Transl. Lung Cancer Res. 2021, 10, 3759–3770. Available online: https://pubmed.ncbi.nlm.nih.gov/34733626/ (accessed on 8 November 2022).
- Cios Spin® by Siemens Healthineers. Available online: https://www.siemens-healthineers.com/surgical-c-arms-and-navigation/mobile-c-arms/cios-spin (accessed on 19 November 2022).
- Chen, J.; Xie, F.; Zheng, X.; Li, Y.; Liu, S.; Ma, K.C.; Goto, T.; Müller, T.; Chan, E.D.; Sun, J. Mobile 3-Dimensional (3D) C-Arm System-Assisted Transbronchial Biopsy and Ablation for Ground-Glass Opacity Pulmonary Nodules: A case Report. Transl Lung Cancer Res. 2021, 10, 3312–3319. Available online: https://tlcr.amegroups.com/article/view/54398/html (accessed on 7 August 2021).
- Cho, R.J.; Senitko, M.; Wong, J.; Dincer, E.H.; Khosravi, H.; Abraham, G.E., III. Feasibility of Using the O-Arm Imaging System During ENB-rEBUS-Guided Peripheral Lung Biopsy: A Dual-Center Experience. J. Bronchol. Interv. Pulmonol. 2020. Available online: https://pubmed.ncbi.nlm.nih.gov/34085805/ (accessed on 11 July 2021).
- Chan, J.W.Y.; Lau, R.W.H.; Chu, C.M.; Ng, C.S.H. Expanding the Scope of Electromagnetic Navigation Bronchoscopy-Guided Transbronchial Biopsy and Ablation with Mobile 3D C-Arm Machine Cios Spin ®-Feasibility and Challenges. Transl. lung Cancer Res. 2021, 10, 4043–4046. Available online: https://pubmed.ncbi.nlm.nih.gov/34858793/ (accessed on 10 May 2022).
- “Ion Cios Spin”|Search|LinkedIn. Available online: https://www.linkedin.com/search/results/all/?keywords=ionciosspin&origin=GLOBAL_SEARCH_HEADER&sid=9cG (accessed on 19 November 2022).
- Chheang, S.; Abtin, F.; Guteirrez, A.; Genshaft, S.; Suh, R. Imaging Features Following Thermal Ablation of Lung Malignancies. Semin. Intervent. Radiol. 2013, 30, 157–168. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3709948/ (accessed on 8 August 2020).
- Yamamoto, A.; Nakamura, K.; Matsuoka, T.; Toyoshima, M.; Okuma, T.; Oyama, Y.; Ikura, Y.; Ueda, M.; Inoue, Y. Radiofrequency Ablation in a Porcine Lung Model: Correlation Between CT and Histopathologic Findings. Am. J. Roentgenol. 2005, 185, 1299–1306. Available online: http://www.ajronline.org/doi/10.2214/AJR.04.0968 (accessed on 8 August 2020).
- Anderson, E.M.; Lees, W.R.; Gillams, A.R. Early Indicators of Treatment Success after Percutaneous Radiofrequency of Pulmonary Tumors. Cardiovasc. Intervent. Radiol. 2009, 32, 478–483. Available online: https://pubmed.ncbi.nlm.nih.gov/19127381/ (accessed on 8 August 2020).
- Wolf, F.J.; Grand, D.J.; Machan, J.T.; DiPetrillo, T.A.; Mayo-Smith, W.W.; Dupuy, D.E. Microwave Ablation of Lung Malignancies: Effectiveness, CT Findings, and Safety in 50 Patients. Radiology 2008, 247, 871–879. Available online: https://pubmed.ncbi.nlm.nih.gov/18372457/ (accessed on 8 August 2020).
- Chen, A.C.; Gillespie, C.T. Robotic Endoscopic Airway Challenge: REACH Assessment. Ann. Thorac. Surg. 2018, 106, 293–297. Available online: https://pubmed.ncbi.nlm.nih.gov/29486178/ (accessed on 10 May 2022).
- Monarch Platform by Auris Health. Available online: https://www.aurishealth.com/monarch-platform (accessed on 19 November 2022).
- Intuitive|Robotic-Assisted Bronchoscopy|Ion Platform. Available online: https://www.intuitive.com/en-us/products-and-services/ion (accessed on 12 November 2022).
- Rojas-Solano, J.R.; Ugalde-Gamboa, L.; MacHuzak, M. Robotic Bronchoscopy for Diagnosis of Suspected Lung Cancer: A Feasibility Study. J. Bronchol. Interv. Pulmonol. 2018, 25, 168–175. Available online: https://pubmed.ncbi.nlm.nih.gov/29762461/ (accessed on 10 May 2022).
- Chen, A.C.; Pastis, N.J.; Machuzak, M.S.; Gildea, T.R.; Simoff, M.J.; Gillespie, C.T.; Mahajan, A.K.; Oh, S.S.; Silvestri, G.A. Accuracy of a Robotic Endoscopic System in Cadaver Models with Simulated Tumor Targets: ACCESS Study. Respiration 2020, 99, 56–61. Available online: https://pubmed.ncbi.nlm.nih.gov/31805570/ (accessed on 10 May 2022).
- Chaddha, U.; Kovacs, S.P.; Manley, C.; Hogarth, D.K.; Cumbo-Nacheli, G.; Bhavani, S.V.; Kumar, R.; Shende, M.; Egan, J.P.; Murgu, S. Robot-Assisted Bronchoscopy for Pulmonary Lesion Diagnosis: Results from the Initial Multicenter Experience. BMC Pulm. Med. 2019, 19, 1–7. Available online: https://pubmed.ncbi.nlm.nih.gov/31829148/ (accessed on 10 May 2022).
- Agrawal, A.; Hogarth, D.K.; Murgu, S. Robotic Bronchoscopy for Pulmonary Lesions: A Review of Existing Technologies and Clinical Data. J. Thorac. Dis. 2020, 12, 3279–3286. Available online: https://pubmed.ncbi.nlm.nih.gov/32642251/ (accessed on 10 May 2022).
- Kalchiem-Dekel, O.; Connolly, J.G.; Lin, I.H.; Husta, B.C.; Adusumilli, P.S.; Beattie, J.A.; Buonocore, D.J.; Dycoco, J.; Fuentes, P.; Jones, D.R.; et al. Shape-Sensing Robotic-Assisted Bronchoscopy in the Diagnosis of Pulmonary Parenchymal Lesions. Chest 2022, 161, 572–582. Available online: https://pubmed.ncbi.nlm.nih.gov/34384789/ (accessed on 8 November 2022).
- Benn, B.S.; Romero, A.O.; Lum, M.; Krishna, G. Robotic-Assisted Navigation Bronchoscopy as a Paradigm Shift in Peripheral Lung Access. Lung 2021, 199, 177–186. Available online: https://pubmed.ncbi.nlm.nih.gov/33547938/ (accessed on 8 November 2022).
- Fielding, D.I.; Bashirzadeh, F.; Son, J.H.; Todman, M.; Chin, A.; Tan, L.; Steinke, K.; Windsor, M.N.; Sung, A.W. First Human Use of a New Robotic-Assisted Fiber Optic Sensing Navigation System for Small Peripheral Pulmonary Nodules. Respiration 2019, 98, 142–150. Available online: https://pubmed.ncbi.nlm.nih.gov/31352444/ (accessed on 8 November 2022).
- Seijo, L.M.; de Torres, J.P.; Lozano, M.D.; Bastarrika, G.; Alcaide, A.B.; Lacunza, M.M.; Zulueta, J.J. Diagnostic Yield of Electromagnetic Navigation Bronchoscopy Is Highly Dependent on the Presence of a Bronchus Sign on CT Imaging: Results from A Prospective Study. Chest 2010, 138, 1316–1321. Available online: https://pubmed.ncbi.nlm.nih.gov/20435658/ (accessed on 11 July 2021).
- Transbronchial Biopsy Assisted by Robot Guidance in the Evaluation of Tumors of the Lung—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04182815 (accessed on 12 November 2022).




