
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Stuart Wagland | -- | 3272 | 2023-02-21 11:08:00 | | | |
| 2 | Rita Xu | Meta information modification | 3272 | 2023-02-22 03:30:03 | | |
Video Upload Options
Global net-zero pledges are instigating a societal shift from a fossil-fuel-based economy to renewables. This change facilitates the use of batteries, solar photovoltaic (PV), wind turbines, etc., all of which are underpinned by critical metals. Raw metal extraction is not renewable and environmental pledges made by the government will not be met if this continues. Historic industrial sites contain vast waste stocks. These sites already have an established infrastructure for resource extraction. Applying green solvents and deep eutectic solvents (DES) to such sites for resource recovery alleviates pressure on existing raw extraction processes whilst generating more immediate stores of critical metal along with relatively insignificant environmental impacts.
1. Introduction
2. Remediation of Contaminated Sites
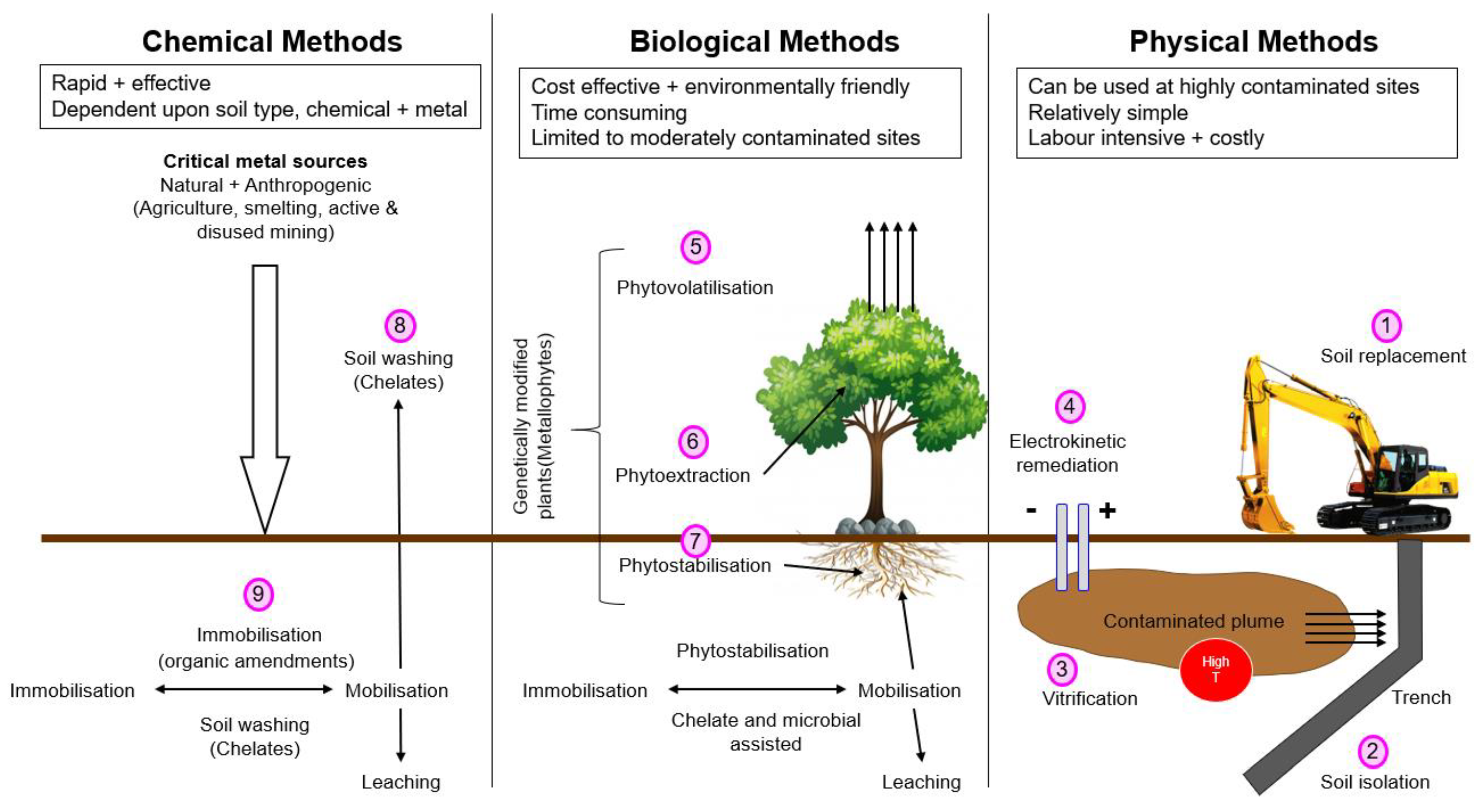
3. Management of Contaminated Soil
3.1. Physical Remediation Approaches
3.2. Phytoremediation Approaches
3.3. Chemical Remediation Approaches
4. Remediation vs. Recovery
5. Emerging Extraction Techniques
References
- Watari, T.; Nansai, K.; Nakajima, K. Major metals demand, supply, and environmental impacts to 2100: A critical review. Resour. Conserv. Recycl. 2021, 164, 105107.
- Grandell, L.; Lehtilä, A.; Kivinen, M.; Koljonen, T.; Kihlman, S.; Lauri, L.S. Role of critical metals in the future markets of clean energy technologies. Renew. Energy 2016, 95, 53–62.
- Werner, D.; Peuker, U.A.; Mütze, T. Recycling Chain for Spent Lithium-Ion Batteries. Metals 2020, 10, 316.
- Moreau, V.; Dos Reis, P.C.; Vuille, F. Enough Metals? Resource Constraints to Supply a Fully Renewable Energy System. Resources 2019, 8, 29.
- Timperley, J. Explainer: These Six Metals Are Key to a Low-Carbon Future. Carbon Brief Website. 2018. Available online: https://www.carbonbrief.org/explainer-these-six-metals-are-key-to-a-low-carbon-future/ (accessed on 13 March 2022).
- Jones, P.T.; Geysen, D.; Tielemans, Y.; Van Passel, S.; Pontikes, Y.; Blanpain, B.; Quaghebeur, M.; Hoekstra, N. Enhanced Landfill Mining in view of multiple resource recovery: A critical review. J. Clean. Prod. 2012, 55, 45–55.
- International Renewable Energy Agency. Global Energy Transformation: A Roadmap to 2050 (2019 Edition); International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2019.
- USGS National Minerals Information Center. 2013 Minerals Yearbook—Copper; USGS National Minerals Information Center: Reston, VA, USA, 2015.
- Brinded, A. Metal Madness? Mater. World 2022, 30, 6.
- Kavlak, G.; McNerney, J.; Jaffe, R.L.; Trancik, J.E. Metal production requirements for rapid photovoltaics deployment. Energy Environ. Sci. 2015, 8, 1651–1659.
- Kennedy, S.; Toledano, P.; Rietbergen, J.; Villiers-Piaget, D. Mining and the SDGS: A 2020 Status Update; Responsible Mining Foundation: Geneva, Switzerland, 2020.
- Lèbre, É.; Corder, G.; Golev, A. The Role of the Mining Industry in a Circular Economy: A Framework for Resource Management at the Mine Site Level. J. Ind. Ecol. 2017, 21, 662–672.
- Schipper, B.W.; Lin, H.-C.; Meloni, M.A.; Wansleeben, K.; Heijungs, R.; van der Voet, E. Estimating global copper demand until 2100 with regression and stock dynamics. Resour. Conserv. Recycl. 2018, 132, 28–36.
- Jadhao, P.R.; Mishra, S.; Pandey, A.; Pant, K.K.; Nigam, K.D.P. Biohydrometallurgy: A Sustainable Approach for Urban Mining of Metals and Metal Refining. In Catalysis for Clean Energy and Environmental Sustainability; Pant, K.K., Gupta, S.K., Ahmad, E., Eds.; Springer: Cham, Switzerland, 2021; pp. 865–892.
- Santana-Mayor, Á.; Rodríguez-Ramos, R.; Herrera-Herrera, A.V.; Socas-Rodríguez, B.; Rodríguez-Delgado, M. Deep eutectic solvents. The new generation of green solvents in analytical chemistry. TrAC Trends Anal. Chem. 2021, 134, 116108.
- Harris, B. Toward a Sustainable Metals Extraction Technology. In Mine Wastes and Water, Ecological Engineering and Metals Extraction; Kalin-Seidenfaden, M., Wheeler, W.N., Eds.; Springer: Cham, Switzerland, 2022; pp. 17–28.
- Jaffe, S. Balancing Li battery DEMAND with Materials Supply. 2018. Available online: https://www.vaneck.com/uploadfiles/conference/reports/cairn.pdf (accessed on 4 April 2022).
- Katwala, A. The spiralling environmental cost of our lithium battery addiction. WIRED, 5 September 2018.
- British Lithium. Battery Grade Lithium Produced from Cornish Granite 2020. Available online: https://britishlithium.co.uk/ (accessed on 21 February 2021).
- Caliman, F.A.; Robu, B.M.; Smaranda, C.; Pavel, V.L.; Gavrilescu, M. Soil and groundwater cleanup: Benefits and limits of emerging technologies. Clean Technol. Environ. Policy 2010, 13, 241–268.
- Sharma, S.; Tiwari, S.; Hasan, A.; Saxena, V.; Pandey, L.M. Recent advances in conventional and contemporary methods for remediation of heavy metal-contaminated soils. 3 Biotech 2018, 8, 216.
- Hogland, W.; Hogland, M.; Marques, M. Enhanced landfill mining: Material recovery, energy utilization and economics in the EU (Directive) perspective. In Proceedings of the International Academic Symposium on Enhanced Landfill Mining, Houthalen-Helchteren, Belgium, January 2011; pp. 209–222.
- Shahid, M.; Niazi, N.K.; Dumat, C.; Naidu, R.; Khalid, S.; Rahman, M.M.; Bibi, I. A meta-analysis of the distribution, sources and health risks of arsenic-contaminated groundwater in Pakistan. Environ. Pollut. 2018, 242, 307–319.
- Yao, Z.; Li, J.; Xie, H.; Yu, C. Review on Remediation Technologies of Soil Contaminated by Heavy Metals. Procedia Environ. Sci. 2012, 16, 722–729.
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A Review on Heavy Metals Contamination in Soil: Effects, Sources, and Remediation Techniques. Soil Sediment Contam. Int. J. 2019, 28, 380–394.
- Zheng, C.; Wang, P.P. A field demonstration of the simulation optimisation approach for remediation system design. Groundwater 2002, 40, 258–266.
- Jankaite, A.; Vasarevičius, S. Remediation technologies for soils contaminated with heavy metals. J. Environ. Eng. Landsc. Manag. 2005, 13, 109–113.
- British Geological Survey. Minerals Produced in the United Kingdom in 2017; British Geological Survey: Nottinghamshire, UK, 2017.
- Dellisanti, F.; Rossi, P.L.; Valdrè, G. In-field remediation of tons of heavy metal-rich waste by Joule heating vitrification. Int. J. Miner. Process. 2009, 93, 239–245.
- Guo, X.; Wei, Z.; Wu, Q.; Li, C.; Qian, T.; Zheng, W. Effect of soil washing with only chelators or combining with ferric chloride on soil heavy metal removal and phytoavailability: Field experiments. Chemosphere 2016, 147, 412–419.
- Li, C.-T.; Lee, W.-J.; Huang, K.-L.; Fu, S.-F.; Lai, Y.-C. Vitrification of Chromium Electroplating Sludge. Environ. Sci. Technol. 2007, 41, 2950–2956.
- Navarro, A.; Cardellach, E.; Cañadas, I.; Rodríguez, J. Solar thermal vitrification of mining contaminated soils. Int. J. Miner. Process. 2013, 119, 65–74.
- Song, Y.; Ammami, M.; Benamar, A.; Mezazigh, S.; Wang, H. Effect of EDTA, EDDS, NTA and citric acid on electrokinetic remediation of As, Cd, Cr, Cu, Ni, Pb and Zn contaminated dredged marine sediment. Environ. Sci. Pollut. Res. 2016, 23, 10577–10586.
- Wang, H.; Ma, J.; Fan, X.; Luo, Q. Research progress on enhancement of in situ remediation of heavy metal by electrokinetics. Environ. Sci. 2007, 16, 223–227.
- Ghosh, M.; Singh, S. A review on phytoremediation of heavy metals and utilization of it’s by products. Asian J. Energy Environ. 2005, 6, 214–231.
- Khalid, H.; Zia-Ur-Rehman, M.; Naeem, A.; Khalid, M.U.; Rizwan, M.; Ali, S.; Umair, M.; Sohail, M.I. Solanum nigrum L.: A Novel Hyperaccumulator for the Phyto-Management of Cadmium Contaminated Soils. In Cadmium Toxicity and Tolerance in Plants; Hasanuzzaman, M., Vara Prasad, M.N., Fujita, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 451–477.
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881.
- Sylvain, B.; Mikael, M.-H.; Florie, M.; Emmanuel, J.; Marilyne, S.; Sylvain, B.; Domenico, M. Phytostabilization of As, Sb and Pb by two willow species (S. viminalis and S. purpurea) on former mine technosols. Catena 2016, 136, 44–52.
- Bhalerao, S. Arbuscular mycorrhizal fungi: A potential biotechnology tool for phytoremediation of heavy metal contaminated soils. Int. J. Sci. Nat. 2013, 4, 1–15.
- Bolan, N.S.; Park, J.H.; Robinson, B.; Naidu, R.; Huh, K.Y. Phytostabilisation: A green approach to contaminant containment. Adv. Agron. 2011, 112, 145–204.
- Sakakibara, M.; Watanabe, A.; Inoue, M.; Sano, S.; Kaise, T. Phytoextraction And Phytovolatilization Of Arsenic From As-Contaminated Soils By Pteris vittata. Proc. Annu. Int. Conf. Soils Sediments Water Energy 2010, 12, 26.
- Nikolić, M.; Stevović, S. Family Asteraceae as a sustainable planning tool in phytoremediation and its relevance in urban areas. Urban For. Urban Green. 2015, 14, 782–789.
- Udovic, M.; Lestan, D. Fractionation and bioavailability of Cu in soil remediated by EDTA leaching and processed by earthworms (Lumbricus terrestris L.). Environ. Sci. Pollut. Res. 2009, 17, 561–570.
- Makino, T.; Takano, H.; Kamiya, T.; Itou, T.; Sekiya, N.; Inahara, M.; Sakurai, Y. Restoration of cadmium-contaminated paddy soils by washing with ferric chloride: Cd extraction mechanism and bench-scale verification. Chemosphere 2008, 70, 1035–1043.
- Wei, M.; Chen, J.; Wang, X. Removal of arsenic and cadmium with sequential soil washing techniques using Na 2 EDTA, oxalic and phosphoric acid: Optimization conditions, removal effectiveness and ecological risks. Chemosphere 2016, 156, 252–261.
- Wu, J.; Zhou, Q.; Huang, R.; Wu, K.; Li, Z. Contrasting impacts of mobilisation and immobilisation amendments on soil health and heavy metal transfer to food chain. Ecotoxicol. Environ. Saf. 2020, 209, 111836.
- Blengini, G.; Mathieux, F.; Mancini, L.; Nyberg, M.; Viegas, H. Recovery of Critical and Other Raw Materials from Mining Waste and Landfills: State of Play on Existing Practices; European Commission: Kirchberg, Luxembourg, 2019; Available online: https://core.ac.uk/download/pdf/199318323.pdf (accessed on 20 May 2022).
- Vollprecht, D.; Machiels, L.; Jones, P. The EU Training Network for Resource Recovery through Enhanced Landfill Mining—A Review. Processes 2021, 9, 394.
- Winterstetter, A.; Laner, D.; Rechberger, H.; Fellner, J. Framework for the evaluation of anthropogenic resources: A landfill mining case study—Resource or reserve? Resour. Conserv. Recycl. 2015, 96, 19–30.
- Mönkäre, T.J.; Palmroth, M.R.; Rintala, J.A. Characterization of fine fraction mined from two Finnish landfills. Waste Manag. 2016, 47, 34–39.
- Silva, R.; de Brito, J.; Dhir, R. Comparative analysis of existing prediction models on the creep behaviour of recycled aggregate concrete. Eng. Struct. 2015, 100, 31–42.
- Makarichi, L.; Jutidamrongphan, W.; Techato, K.-A. The evolution of waste-to-energy incineration: A review. Renew. Sustain. Energy Rev. 2018, 91, 812–821.
- Graedel, T.E.; Allwood, J.; Birat, J.-P.; Buchert, M.; Hagelüken, C.; Reck, B.; Sibley, S.F.; Sonnemann, G. What Do We Know About Metal Recycling Rates? J. Ind. Ecol. 2011, 15, 355–366.
- Verma, A.; Kore, R.; Corbin, D.R.; Shiflett, M.B. Metal Recovery Using Oxalate Chemistry: A Technical Review. Ind. Eng. Chem. Res. 2019, 58, 15381–15393.
- Obaid, S.S.; Gaikwad, D.; Sayyed, M.; Al-Rashdi, K.; Pawar, P. Heavy metal ions removal from waste water by the natural zeolites. Mater. Today Proc. 2018, 5, 17930–17934.
- Bosso, S.; Enzweiler, J. Evaluation of heavy metal removal from aqueous solution onto scolecite. Water Res. 2002, 36, 4795–4800.
- Inglezakis, V.J. The concept of “capacity” in zeolite ion-exchange systems. J. Colloid Interface Sci. 2005, 281, 68–79.
- Tavares, M.T.; Quintelas, C.; Figueiredo, H.; Neves, I.C. Comparative Study between Natural and Artificial Zeolites as Supports for Biosorption Systems. Mater. Sci. Forum 2006, 514–516, 1294–1298.
- Silva, B.; Figueiredo, H.; Quintelas, C.; Neves, I.C.; Tavares, T. Zeolites as supports for the biorecovery of hexavalent and trivalent chromium. Microporous Mesoporous Mater. 2008, 116, 555–560.
- Vaca Mier, M.; López Callejas, R.; Gehr, R.; Jiménez Cisneros, B.E.; Alvarez, P.J.J. Heavy metal removal with Mexican clinoptilolite: Multi-component ionic exchange. Water Res. 2001, 35, 373–378.
- Zou, W.; Feng, X.; Wei, W.; Zhou, Y.; Wang, R.; Zheng, R. Converting Spent LiFePO4 Battery into Zeolitic Phosphate for Highly Efficient Heavy Metal Adsorption. Inorg. Chem. 2021, 60, 9496–9503.
- Shuya, L.; Yang, C.; Xuefeng, C.; Wei, S.; Yaqing, W.; Yue, Y. Separation of lithium and transition metals from leachate of spent lithium-ion batteries by solvent extraction method with Versatic 10. Sep. Purif. Technol. 2020, 250, 117258.
- Prior, T.; Wäger, P.A.; Stamp, A.; Widmer, R.; Giurco, D. Sustainable governance of scarce metals: The case of lithium. Sci. Total Environ. 2013, 461–462, 785–791.
- Mathuriya, A.S.; Yakhmi, J.V. Microbial fuel cells to recover heavy metals. Environ. Chem. Lett. 2014, 12, 483–494.
- Chouler, J.; Padgett, G.A.; Cameron, P.J.; Preuss, K.; Titirici, M.-M.; Ieropoulos, I.; Di Lorenzo, M. Towards effective small scale microbial fuel cells for energy generation from urine. Electrochim. Acta 2016, 192, 89–98.
- Mathuriya, A.S.; Sharma, V.N. Bioelectricity production from various wastewaters through microbial fuel cell technology. J. Biochem. Technol. 2010, 2, 133–137.
- Wu, H.; Zuo, J.; Zillante, G.; Wang, J.; Yuan, H. Status quo and future directions of construction and demolition waste research: A critical review. J. Clean. Prod. 2019, 240, 118163.
- Ezziat, L.; Elabed, A.; Ibnsouda, S.; El Abed, S. Challenges of Microbial Fuel Cell Architecture on Heavy Metal Recovery and Removal From Wastewater. Front. Energy Res. 2019, 7, 1.
- Nancharaiah, Y.; Venkata Mohan, S.; Lens, P.N.L. Recent advances in nutrient removal and recovery in biological and bioelectrochemical systems. Bioresour. Technol. 2016, 215, 173–185.
- Choi, C.; Hu, N.; Lim, B. Cadmium recovery by coupling double microbial fuel cells. Bioresour. Technol. 2014, 170, 361–369.
- Zhang, Y.; Yu, L.; Wu, D.; Huang, L.; Zhou, P.; Quan, X.; Chen, G. Dependency of simultaneous Cr(VI), Cu(II) and Cd(II) reduction on the cathodes of microbial electrolysis cells self-driven by microbial fuel cells. J. Power Sources 2015, 273, 1103–1113.
- Xu, Y.-S.; Zheng, T.; Yong, X.-Y.; Zhai, D.-D.; Si, R.-W.; Li, B.; Yu, Y.-Y.; Yong, Y.-C. Trace heavy metal ions promoted extracellular electron transfer and power generation by Shewanella in microbial fuel cells. Bioresour. Technol. 2016, 211, 542–547.
- Abourached, C.; Catal, T.; Liu, H. Efficacy of single-chamber microbial fuel cells for removal of cadmium and zinc with simultaneous electricity production. Water Res. 2014, 51, 228–233.
- Pan, J.; Hassas, B.V.; Rezaee, M.; Zhou, C.; Pisupati, S.V. Recovery of rare earth elements from coal fly ash through sequential chemical roasting, water leaching, and acid leaching processes. J. Clean. Prod. 2020, 284, 124725.





