
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Elisiário Tavares da Silva | -- | 2085 | 2023-02-17 17:53:01 | | | |
| 2 | Rita Xu | Meta information modification | 2085 | 2023-02-20 06:11:44 | | |
Video Upload Options
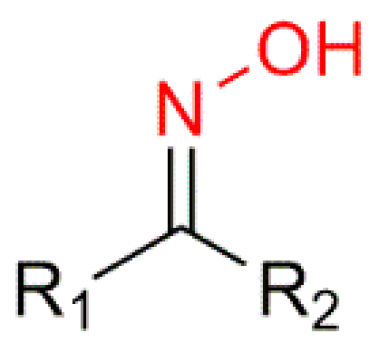
Steroids and their derivatives have been the subject of extensive research among investigators due to their wide range of pharmacological properties, in which steroidal oximes are included. Oximes are a chemical group with the general formula R1R2C=N−OH and they exist as colorless crystals and are poorly soluble in water. Oximes can be easily obtained through the condensation of aldehydes or ketones with various amine derivatives, making them a very interesting chemical group in medicinal chemistry for the design of drugs as potential treatments for several diseases. A large number of steroid oximes exhibit important biological activities, such as anticancer, anti-inflammatory, antibacterial, antifungal and antiviral, among others, through different mechanisms of action. Several steroid oximes are used clinically as drugs and many others are in clinical trials.
1. Introduction
2. Chemistry of Steroidal Oximes
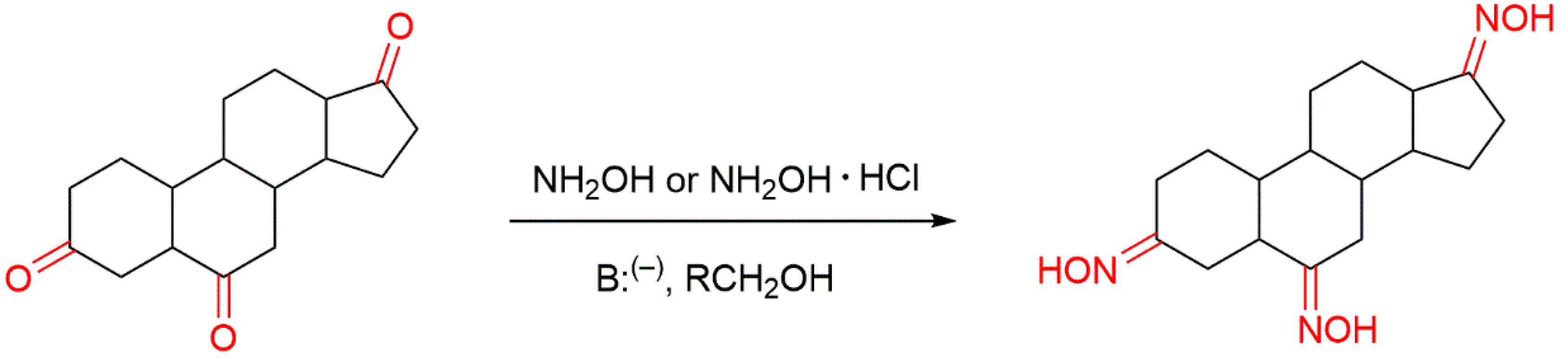
3. Mechanisms of Action of Steroidal Oximes
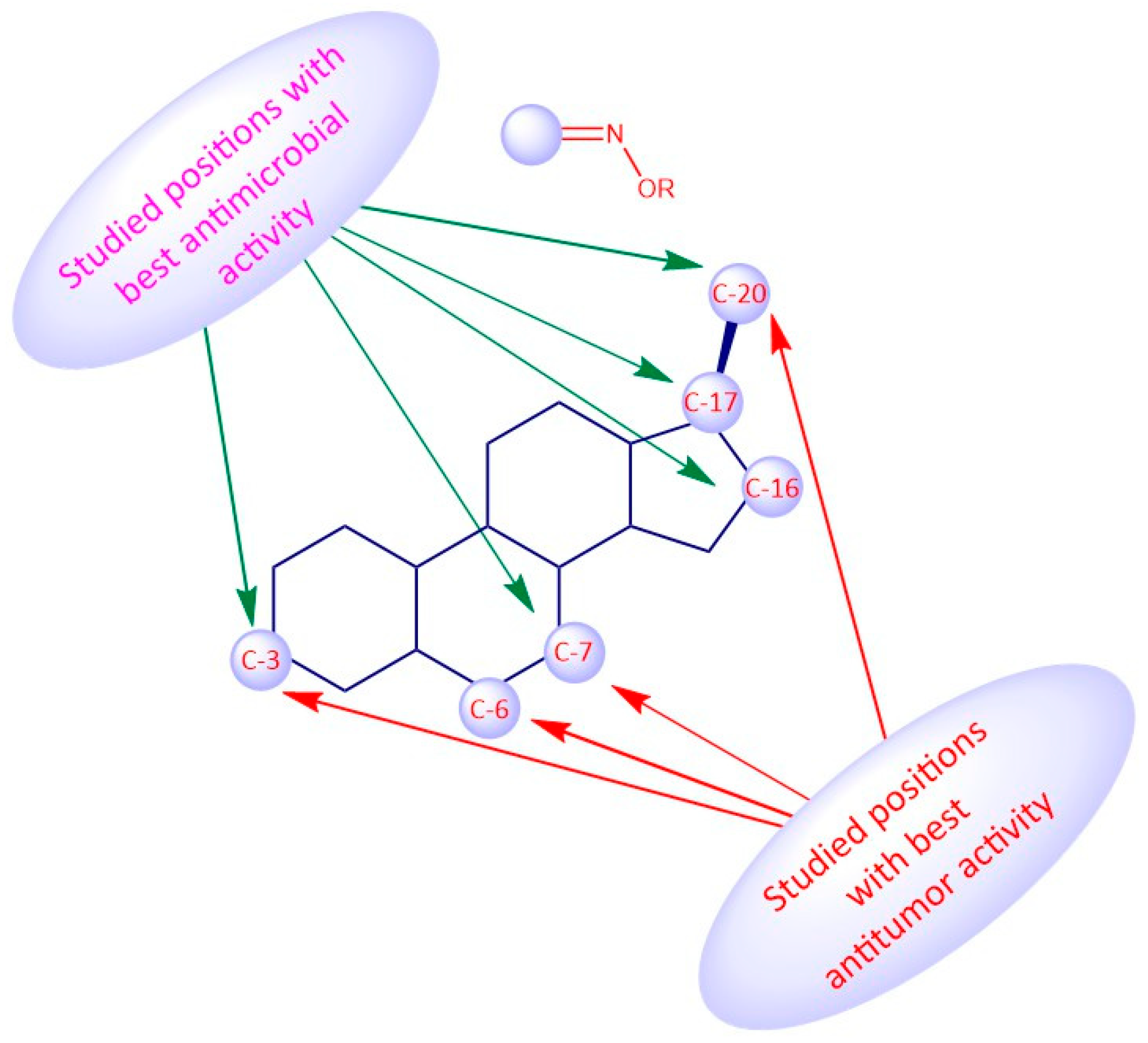
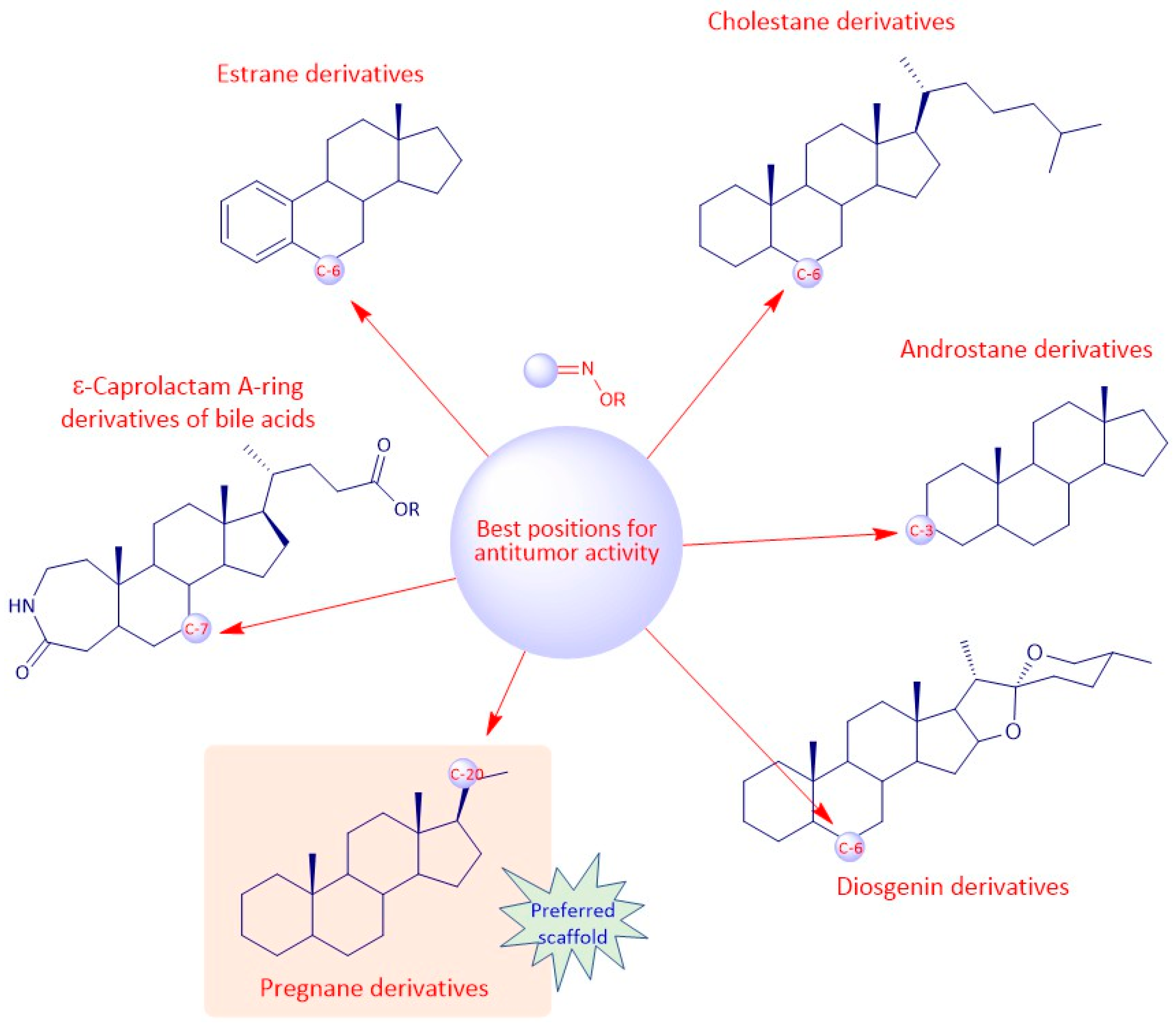
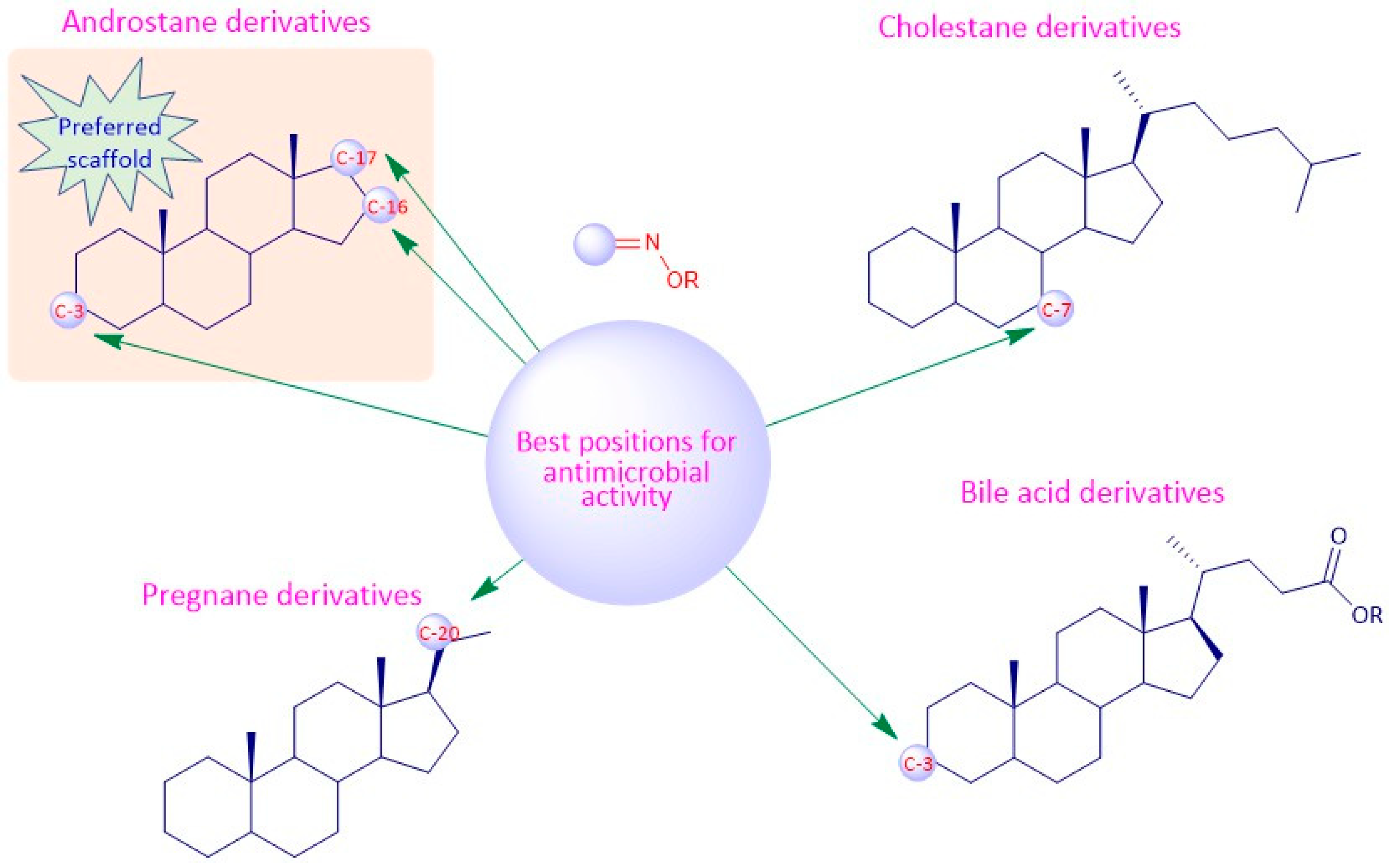
4. Structure-Activity Relationships of Steroidal Oximes
References
- Bhatti, H.N.; Khera, R.A. Biological transformations of steroidal compounds: A review. Steroids 2012, 77, 1267–1290.
- Gupta, A.; Kumar, B.S.; Negi, A.S. Current status on development of steroids as anticancer agents. J. Steroid Biochem. Mol. Biol. 2013, 137, 242–270.
- Ericson-Neilsen, W.; Kaye, A.D. Steroids: Pharmacology, complications, and practice delivery issues. Ochsner J. 2014, 14, 203–207.
- Rasheed, A.; Qasim, M. A Review of natural steroids and their applications. Int. J. Pharm. Sci. Res. 2013, 4, 520–531.
- Bansal, R.; Suryan, A. A Comprehensive Review on Steroidal Bioconjugates as Promising Leads in Drug Discovery. ACS Bio Med Chem Au 2022, 2, 340–369.
- Bai, C.; Schmidt, A.; Freedman, L.P. Steroid Hormone Receptors and Drug Discovery: Therapeutic Opportunities and Assay Designs. ASSAY Drug Dev. Technol. 2003, 1, 843–852.
- Schepetkin, I.; Plotnikov, M.; Khlebnikov, A.; Plotnikova, T.; Quinn, M. Oximes: Novel Therapeutics with Anticancer and Anti-Inflammatory Potential. Biomolecules 2021, 11, 777.
- Grob, D.; Johns, R.J. Use of oximes in the treatment of intoxication by anticholinesterase compounds in normal subjects. Am. J. Med. 1958, 24, 497–511.
- Dhuguru, J.; Zviagin, E.; Skouta, R. FDA-Approved Oximes and Their Significance in Medicinal Chemistry. Pharmaceuticals 2022, 15, 66.
- Chen, S.-R.; Shen, F.-J.; Feng, G.-L.; Yuan, R.-X. Synthesis and Anticancer Activity of 4-azasteroidal-20-oxime Derivatives. J. Chem. Res. 2015, 39, 527–530.
- Sørensen, M.; Neilson, E.H.; Møller, B.L. Oximes: Unrecognized Chameleons in General and Specialized Plant Metabolism. Mol. Plant 2017, 11, 95–117.
- Surowiak, A.K.; Lochyński, S.; Strub, D.J. Unsubstituted oximes as potential therapeutic agents. Symmetry 2020, 12, 575.
- Aakeröy, C.B.; Sinha, A.S.; Epa, K.N.; Chopade, P.D.; Smith, M.M.; Desper, J. Structural Chemistry of Oximes. Cryst. Growth Des. 2013, 13, 2687–2695.
- Canario, C.; Silvestre, S.; Falcão, A.; Alves, G. Steroidal Oximes: Useful Compounds with Antitumor Activities. Curr. Med. Chem. 2018, 25, 660–686.
- Donaruma, L.G.; Heldt, W.Z. The Beckmann rearrangement. Org. React. 2011, 15, 1–156.
- Ãbele, E.; Lukevics, E. Recent advances in the chemistry of oximes. Org. Prep. Proced. Int. 2000, 32, 235–264.
- Bolotin, D.S.; Bokach, N.A.; Demakova, M.Y.; Kukushkin, V.Y. Metal-Involving Synthesis and Reactions of Oximes. Chem. Rev. 2017, 117, 13039–13122.
- Elliott, M.C. Imines and Their N-Substituted Derivatives: Hydrazones and Other N,N-Derivatives Including Diazo Compounds. Cheminform 2005, 36, 469–523.
- Gomes, A.R.; Pires, A.S.; Abrantes, A.M.; Gonçalves, A.C.; Costa, S.C.; Varela, C.L.; Silva, E.T.; Botelho, M.F.; Roleira, F.M. Design, synthesis, and antitumor activity evaluation of steroidal oximes. Bioorg. Med. Chem. 2021, 46, 116360.
- Ajduković, J.J.; Jakimov, D.S.; Rárová, L.; Strnad, M.; Dzichenka, Y.U.; Usanov, S.; Škorić, D.; Jovanović-Šanta, S.S.; Sakač, M.N. Novel alkylaminoethyl derivatives of androstane 3-oximes as anticancer candidates: Synthesis and evaluation of cytotoxic effects. RSC Adv. 2021, 11, 37449–37461.
- Ajduković, J.J.; Penov Gaši, K.M.; Jakimov, D.S.; Klisurić, O.R.; Jovanović-Šanta, S.S.; Sakač, M.N.; Aleksić, L.D.; Djurendić, E.A. Synthesis, structural analysis and antitumor activity of novel 17α-picolyl and 17(E)-picolinylidene A-modified androstane derivatives. Bioorg. Med. Chem. 2015, 23, 1557–1568.
- Berényi, Á.; Minorics, R.; Iványi, Z.; Ocsovszki, I.; Ducza, E.; Thole, H.; Messinger, J.; Wölfling, J.; Mótyán, G.; Mernyák, E.; et al. Synthesis and investigation of the anticancer effects of estrone-16-oxime ethers in vitro. Steroids 2013, 78, 69–78.
- Mernyák, E.; Fiser, G.; Szabó, J.; Bodnár, B.; Schneider, G.; Kovács, I.; Ocsovszki, I.; Zupkó, I.; Wölfling, J. Synthesis and in vitro antiproliferative evaluation of d-secooxime derivatives of 13β- and 13α-estrone. Steroids 2014, 89, 47–55.
- Canário, C.; Matias, M.; Brito, V.; Santos, A.; Falcão, A.; Silvestre, S.; Alves, G. New Estrone Oxime Derivatives: Synthesis, Cytotoxic Evaluation and Docking Studies. Molecules 2021, 26, 2687.
- Cushman, M.; He, H.-M.; Katzenellenbogen, J.A.; Varma, R.K.; Hamel, E.; Lin, C.M.; Ram, S.; Sachdeva, Y.P. Synthesis of Analogs of 2-Methoxyestradiol with Enhanced Inhibitory Effects on Tubulin Polymerization and Cancer Cell Growth. J. Med. Chem. 1997, 40, 2323–2334.
- Huang, Y.; Cui, J.; Zheng, Q.; Zeng, C.; Chen, Q.; Zhou, A. 6-Hydroximino-4-aza-A-homo-cholest-3-one and related analogue as a potent inducer of apoptosis in cancer cells. Steroids 2012, 77, 829–834.
- Sánchez-Sánchez, L.; Hernández-Linares, M.G.; Escobar, M.L.; López-Muñoz, H.; Zenteno, E.; Fernández-Herrera, M.A.; Guerrero-Luna, G.; Carrasco-Carballo, A.; Sandoval-Ramírez, J. Antiproliferative, Cytotoxic, and Apoptotic Activity of Steroidal Oximes in Cervicouterine Cell Lines. Molecules 2016, 21, 1533.
- Krstić, N.M.; Bjelaković, M.S.; Žižak, Ž.; Pavlović, M.D.; Juranić, Z.D.; Pavlović, V.D. Synthesis of some steroidal oximes, lactams, thiolactams and their antitumor activities. Steroids 2007, 72, 406–414.
- Zeferino-Díaz, R.; Olivera-Castillo, L.; Dávalos, A.; Grant, G.; Kantún-Moreno, N.; Rodriguez-Canul, R.; Bernès, S.; Sandoval-Ramírez, J.; Fernández-Herrera, M.A. 22-Oxocholestane oximes as potential anti-inflammatory drug candidates. Eur. J. Med. Chem. 2019, 168, 78–86.
- Pujol, C.A.; Sepúlveda, C.S.; Richmond, V.; Maier, M.S.; Damonte, E.B. Polyhydroxylated sulfated steroids derived from 5α-cholestanes as antiviral agents against herpes simplex virus. Arch. Virol. 2016, 161, 1993–1999.
- Wei, Z.; Tan, J.; Cui, X.; Zhou, M.; Huang, Y.; Zang, N.; Chen, Z.; Wei, W. Design, Synthesis and Bioactive Evaluation of Oxime Derivatives of Dehydrocholic Acid as Anti-Hepatitis B Virus Agents. Molecules 2020, 25, 3359.
- Aditya, S.; Rattan, A. Istaroxime: A rising star in acute heart failure. J. Pharmacol. Pharmacother. 2012, 3, 353–355.
- Bringer, J. Norgestimate: A clinical overview of a new progestin. Am. J. Obstet. Gynecol. 1992, 166, 1969–1979.
- Henzl, M.R. Norgestimate. From the laboratory to three clinical indications. J. Reprod Med. 2001, 46, 647–661.




