Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marwan Habiba | -- | 2355 | 2023-02-16 00:21:36 | | | |
| 2 | Conner Chen | -8 word(s) | 2347 | 2023-02-20 02:33:05 | | | | |
| 3 | Conner Chen | Meta information modification | 2347 | 2023-02-20 03:15:17 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Guo, S.; Habiba, M.; Benagiano, G. Aberrations in Eutopic Endometrium. Encyclopedia. Available online: https://encyclopedia.pub/entry/41267 (accessed on 08 February 2026).
Guo S, Habiba M, Benagiano G. Aberrations in Eutopic Endometrium. Encyclopedia. Available at: https://encyclopedia.pub/entry/41267. Accessed February 08, 2026.
Guo, Sun-Wei, Marwan Habiba, Giuseppe Benagiano. "Aberrations in Eutopic Endometrium" Encyclopedia, https://encyclopedia.pub/entry/41267 (accessed February 08, 2026).
Guo, S., Habiba, M., & Benagiano, G. (2023, February 16). Aberrations in Eutopic Endometrium. In Encyclopedia. https://encyclopedia.pub/entry/41267
Guo, Sun-Wei, et al. "Aberrations in Eutopic Endometrium." Encyclopedia. Web. 16 February, 2023.
Copy Citation
The “endometrial determinism” theory was proposed to account for the apparent gap between the relatively low prevalence of endometriosis and nearly universal retrograde menstruation. The theory was advanced to justify “root treatment”, intended to nip endometriosis in the bud. The theory has significant weaknesses. Critically, identified aberrations in the ectopic endometrium in endometriosis may be the consequence rather than the cause of endometriosis.
aberration
endometrium
endometriosis
eutopic endometrium
endometrial determinism
retrograde menstruation
1. Introduction
Among all theories on the pathogenesis of endometriosis, Sampson’s retrograde menstruation has the greatest supporting evidence. Written nearly a century ago, Sampson proposed that “retrograde transport of menstrual blood with the consequent implantation of exfoliated endometrial mucosa cells within the peritoneal cavity” causes what he named “endometriosis” [1]. The validity of this theory was first demonstrated in 1950 in a Macacus rhesus monkey experimentation by the successful establishment of endometriosis through artificial shunting of menstrual debris into the abdominal cavity [2] and, in 1958, through subcutaneous inoculation at “one finger breadth above the symphysis pubis in the midline” in humans [3]. Later on, it was shown that intrapelvic injection of menstrual endometrium in female baboons can induce endometriosis [4]. The baboon model was further modified by peritoneal inoculation, twice, with menstrual endometrial debris leading to the establishment of endometriotic foci [5]. In addition, there is a well-documented link between reproductive tract obstruction that enhances retrograde menstrual flux and endometriosis [2]. Thus, experimental data, as well as epidemiological findings, support Sampson’s retrograde menstruation theory.
However, among women with patent fallopian tubes, retrograde menstruation is nearly universal [6], whereas the prevalence of endometriosis is typically around 10% [7]. Hence the conundrum: If retrograde menstruation is ubiquitous and nearly universal, why is there such a vast gap in prevalence?
A comprehensive review, published over two decades ago, tallied several explanations for this apparent gap [8]. It elaborated the steps required for the establishment of endometriotic lesions through retrograde menstruation, i.e., reflux, adhesion, proteolysis, proliferation, angiogenesis, and cicatrization [8]. The possibility was raised that abnormal endometrium is the foremost predisposing factor for endometriosis. Specifically, the endometrium should have the ability to evade immune detection either through the possession of antigenic capability, harboring immune cell populations different from those of healthy women, and the synthesis and release of immune regulator; produce estrogens in excess and enhance survival, invasive and implantation propensity; reduce apoptotic tendency, carry molecules harmful to the peritoneum, or enhance angiogenic capability [8]. In other words, it is the seed, i.e., the defective endometrium, that is chiefly responsible for endometriosis.
In China, this hypothesis went much further and was elevated to a full-fledged theory, termed “endometrial determinism”, which “revamped and refined Sampson’s hypothesis” [9]. Practically unknown outside of China, but acclaimed by many as the maestro stroke in the most populous nation with likely the highest number of women with endometriosis in the world, the theory claims to be able to perfectly explain the huge gap between nearly universal retrograde menstruation and the 10% prevalence of the disease [9]. In essence, the theory postulates that the occurrence of endometriosis is mainly dependent on the characteristics of the eutopic endometrium and that retrograde menstruation may act merely as the precipitating factor [9]. In particular, endometriosis originates from eutopic endometrium through a process of “3A” (namely, attachment, aggression and angiogenesis) [10][11] and, as such, treatment should start from its root causes, or “root treatment”, aimed at rectifying the endometrium and preventing endometriosis [12]. Winning accolades and numerous awards, the “endometrial determinism” theory has gained a firm traction in gynecology in China, attracting a huge crowd of cult-like followers. Based on this theory, a recent consensus opinion by a group of elite Chinese gynecologists, published in the flagship obstetrics/gynecology journal in China, proposed that endometriosis should be treated “from its root cause”, i.e., the abnormal endometrium [13].
The credence of defective endometrium responsible for endometriosis seems to have been recently bolstered by sequencing data demonstrating increased cancer-associated mutations (CAMs) in both eutopic and ectopic endometrium, implicating that the retrograde flow of CAM-harboring endometrial cells will confer selective advantages at ectopic sites that may lead to the development of endometriosis [14]. This implies that if those women whose CAMs-carrying endometrium can be detected early and certain counter measures are taken, endometriosis could be prevented.
Indeed, one specific CAM, i.e., the KRAS (G12C) mutation, which occurs in endometrium of some women with endometriosis [14], has been shown to be linked with the reduced expression of progesterone receptors (PGR) in endometrial epithelial cells, conferring resistance to progestin treatment [15]. The idea of rectifying the endometrium can be further buoyed and emboldened by the recent US Food and Drug Administration approval of Sotorasib, a first-in-class specific small molecule that irreversibly inhibits KRAS (G12C) [16].
Thus, we could be at the dawn of a brave new world in which the “root treatment” would potentially forestall the genesis of endometriosis for good, sparing millions of women worldwide from pain and suffering, the dashed dream of having a family, endless distress, fear of uncertainty of surgery and recurrence, lost productivity, school absenteeism, strained relationships and emotional, social and financial tolls and alleviating enormous economic burden to the society.
2. Aberrations in Eutopic Endometrium: Cause or Consequence of Endometriosis?
The presence of various molecular and cellular aberrations in the endometrium of women with endometriosis has been extensively reported. Searching PubMed using the phrase “endometriosis and (endometrial or endometrium)“ yielded 7109 papers (accessed on 1 February 2023), published since 2000 when aberrations reported before were comprehensively reviewed by Vantier et al. [8]. Indeed, expression profiling studies typically report tens or hundreds of genes, miRNAs, or long non-coding RNAs differentially expressed in the endometrium between women with endometriosis and without [17][18][19][20][21][22][23]. The evaluation of changes in DNA methylome yielded similar findings [24][25]. A comprehensive review, published in 2016, of published endometrial biomarkers of endometriosis involving 2729 participants concluded that only “17βHSD2, IL-1R2, caldesmon (a binding protein capable of regulating actomyosin contraction), and a number of neural markers (VIP, CGRP, SP, NPY and combination of VIP, PGP 9.5 and SP) showed promising evidence of diagnostic accuracy, but there was insufficient or poor quality evidence for any clinical recommendations.” [26].
Thus, considering the sheer number and type of aberrations, it may not be entirely informative to tally these aberrations, especially since different studies often yield conflicting results and because such differences vanish under refined grouping [27].
The first such aberration is the increased nerve fiber density in the endometrium. First reported in 2006 [28], it was subsequently consistently observed by the same research team [29][30], which went on to demonstrate, in a double-blinded study, an impressive specificity and sensitivity of 83% and 98%, respectively, in diagnosing endometriosis [31]. More remarkably, this finding was independently corroborated by a Belgian team, reporting a nearly perfect 95% sensitivity, 100% specificity and 97.5% accuracy in predicting the presence of minimal–mild endometriosis using just three neural markers [32].
Unfortunately, subsequent studies failed to replicate the relationship between endometriosis and increased nerve fiber density in the endometrium. Instead, they found that endometrial innervation is pain-dependent, rather than endometriosis specific [33][34]. Indeed, several later studies found either no such difference [35] or unacceptable sensitivity and specificity (32% and 46%, respectively, as reported in [36], and 64% and 50%, respectively, as reported in [37]). Consistent with the observation that the endometrial hyperinnervation is pain-, but not endometriosis-related, the estimates of sensitivity and specificity are the lowest when subjects who complained of pelvic pains were included [36].
Thus, 16 years after its first report, endometrial hyperinnervation as quantitated by the nerve fiber density in endometrium has not become a diagnostic tool for endometriosis as of today. In essence, this example illustrates that an endometrial aberration may be linked with a non-specific symptom, such as dysmenorrhea or pelvic pain, which may be shared by many disorders, including endometriosis, but not endometriosis exclusively, as shown in Figure 1A.
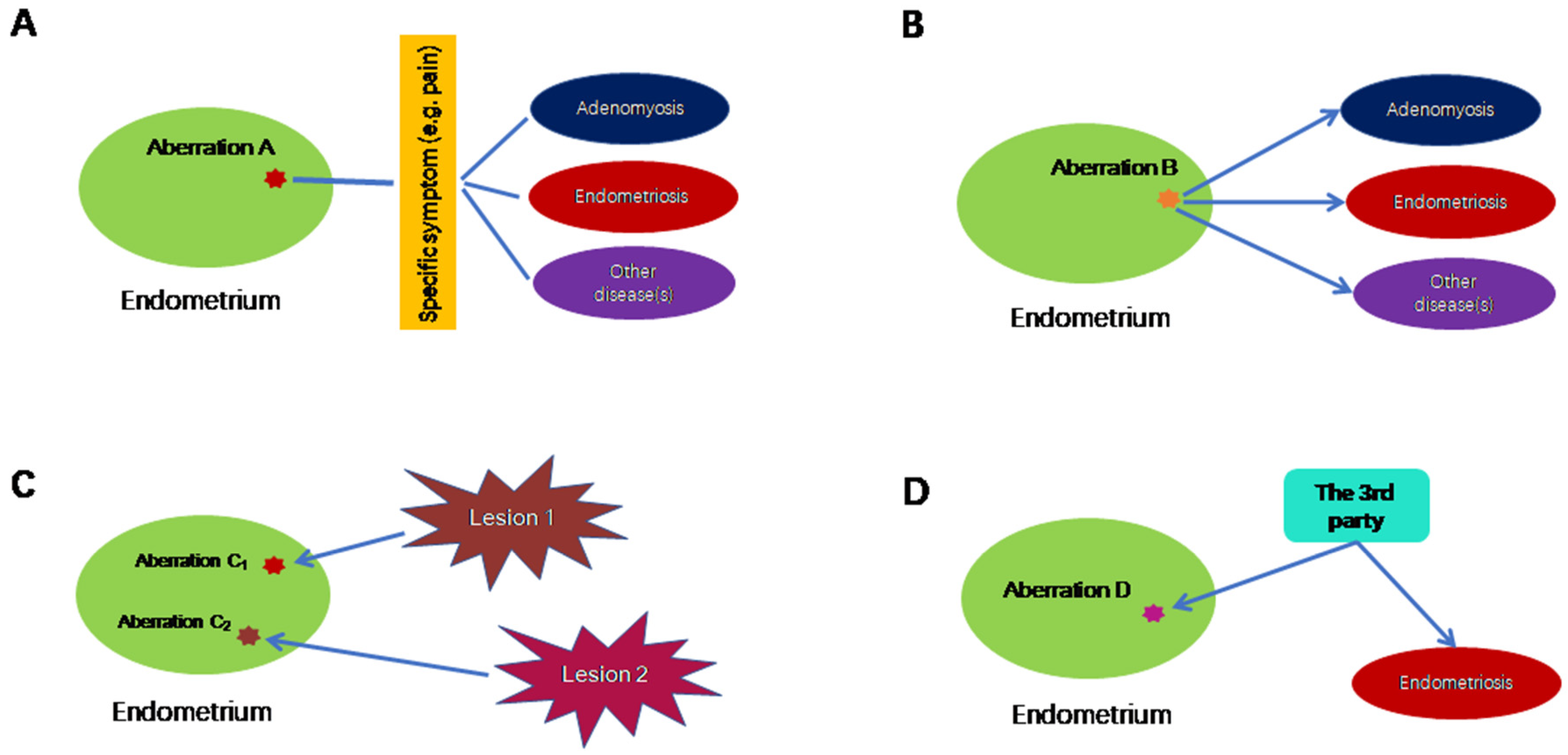
Figure 1. Possible scenarios in which endometrial aberrations do not cause endometriosis exclusively. (A) In this scenario, endometrial aberration A may be associated with a particular symptom, which is not specific to endometriosis. (B) Endometrial aberration B could lead to multiple conditions, including endometriosis. (C) Endometrial aberrations C1 and C2 are the consequences of endometriosis, where C1 and C2 are induced by different lesions of possibly different subtypes and/or their proximity to uterus. (D) Endometrial aberration D, along with endometriosis, is the result of a third, unknown factor. Lines without arrows indicate an association. The directional arrows indicate the causal relationship.
The second aberration is the gene or protein expression levels of PGRs. Progesterone is known to be a critical sex steroid hormone for the endometrium, and it also exerts a therapeutic effect on ectopic endometrium. Progesterone, as well as progestins, mediates their effects through two isoforms of PGR, namely PR-A and PR-B. Both PR-A and PR-B are encoded by the same gene, PGR, and, as such, both isoforms are identical in sequence, except PR-A is short of 164 amino acids at the N terminus, since both are transcribed from the same gene via two independent specific promoter regions and translation start sites [38]. Since progestins constitute an important class of drugs in the limited armamentarium of medical treatment of endometriosis, and since progesterone resistance (manifesting itself clinically as refractoriness to progestin treatment) is well-documented in endometriosis, PGR expression in both eutopic and ectopic endometrium is of major interest in endometriosis [39].
In endometriotic lesions, the reduced expression of both PR-A and PR-B, especially the latter, has been well-documented [40][41][42]. In fact, promoter hypermethylation of PR-B, which is responsible for its silence in endometriosis as well as in adenomyosis, has been reported as early as 2006 [43][44], and is likely caused by persistent inflammation [45]. Suppressed expression of PR-B in endometriosis apparently endows enhanced proliferative propensity [46].
In eutopic endometrium, the tendency for progesterone resistance has been also well-established [17][18]. Published data predominantly show PGR, especially PR-B, expression is either lost or reduced in the eutopic endometrium from women with endometriosis [42][47][48][49][50][51][52]. There is evidence for PR-B promoter hypermethylation, which may account for its loss [43][53]. However, negative reports also have been reported [54][55][56]. These discrepancies have been attributed to possible differences in experimental methods, endometriosis subtypes and cell types analyzed, and resolution of PGR isoforms [57]. It is also attributble to “patchy” endometrium [58], i.e., endometrial heterogeneity within the same patient.
However, reduced PGR expression, or even PR-B promoter hypermethylation in the endometrium is not exclusively confined to patients with endometriosis. Endometrial PR-B expression also is reduced in patients with adenomyosis [59], and its reduction may also be attributed to its promoter hypermethylation [44]. In poorly differentiated endometrial cancer, PR-B expression is reduced as well [60]. In women wearing a levonorgestrel-releasing intrauterine system (LNG-IUS), total PGR and PR-B staining in the endometrium are significantly reduced [61]. Therefore, reduced endometrial PGR or PR-B expression is not a sufficient condition for endometriosis. This scenario, called “one-to-many”, is depicted in Figure 1B.
The last example is promoter hypermethylation of HOXA10 in eutopic endometrium from women with endometriosis. First reported in 2005 [62], it was later independently confirmed in baboon and mouse models of endometriosis [63][64], as well as in humans [65][66]. The silencing of endometrial HOXA10 because of promoter hypermethylation is associated with decreased fertility, implantation defects and/or the reproductive wastage seen in certain disease states that affect the female reproductive tract [67]. However, promoter hypermethylation of HOXA10 in the endometrium turns out to occur not only in patients with endometriosis but also those with Asherman’s syndrome, intramural and submucosal uterine myoma, as well as endometrial polyps [67]. The endometrium of women wearing intrauterine devices also carry HOXA10 methylation [68]. Therefore, again, this molecular aberration is not exclusively confined to patients with endometriosis, and, as such, does not qualify as a sufficient condition (Figure 1C).
Through the above three examples, it can be concluded that many molecular/cellular aberrations in the endometrium of women with endometriosis are either not universal to all patients with endometriosis (as in endometrial hyperinnervation restricted to those complaining of pain), or not exclusively confined to those with endometriosis. In other words, these aberrations are not a sufficient condition, and in some cases not even a necessary condition. Most importantly, however, since these aberrations are found, post hoc, in the endometrium from women who have been already diagnosed with endometriosis, there is no way to tell whether these aberrations are the cause, or merely the consequences, of endometriosis. Of course, for a complex disease such as endometriosis, a linear cause-and-effect relationship seldom exists during the entire course of the disease progression. Many factors could initially be the consequence of the disease but later become part of the causal complex. However, to truly qualify for “endometrial determinism”, the endometrial aberration must occur first, endowing the aberration-carrying menstrual debris the ability to invade, adhere to ectopic sites and then evade immune surveillance, detection and clearance, and cause symptoms. That is, it predisposes its bearer to endometriosis. Therefore, two additional requirements are needed to establish “endometrial determinism” (Figure 1C,D). In scenario C (the consequence), the endometrial aberration is the consequence of endometriosis, and the aberration may vary depending on the subtype of endometriotic lesion and its proximity to the uterus, as reviewed below. In scenario D (the third party), both endometrial aberration and endometriosis could be caused by a third party, which could be linked to in utero exposure of high levels of estrogens [69][70] or some inborn errors or other extraneous factors yet to be identified.
When exploring the hypothesis that endometriosis originates from the endometrium harboring molecular aberrations, it should be considered that differences between ectopic and the eutopic endometrium could be secondary to the vast differences in the microenvironment existing between the two locations. The ectopic endometrium in endometriosis is influenced by the peritoneal fluid, or by local microenvironments in the case of ovarian endometrioma [71]. In turn, ectopic endometrium could influence gene expression in the eutopic endometrium, since endometrial tissue implanted at sites proximal and distal to the uterus in the mouse model of endometriosis alters gene expression in the eutopic endometrium. Furthermore, the changes in gene expression differ in relation to the site of implantation [72].
In addition to aberrations in eutopic endometrium, emerging data also indicate that endometriosis may cause systemic changes. In mice with induced endometriosis, dysregulated hepatic metabolic genes have been demonstrated [73]. This may be responsible for the low body mass index observed in women with endometriosis [74]. Recently, it has been reported that endometriosis promotes atherosclerosis in mice [75].
References
- Sampson, J.A. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am.J. Obstet Gynecol. 1927, 14, 422–469.
- Te Linde, R.W.; Scott, R.B. Experimental endometriosis. Am.J. Obstet Gynecol. 1950, 60, 1147–1173.
- Ridley, J.H.; Edwards, I.K. Experimental endometriosis in the human. Am.J. Obstet Gynecol. 1958, 76, 783–789.
- D’Hooghe, T.M.; Bambra, C.S.; Raeymaekers, B.M.; De Jonge, I.; Lauweryns, J.M.; Koninckx, P.R. Intrapelvic injection of menstrual endometrium causes endometriosis in baboons (Papio cynocephalus and Papio anubis). Am.J. Obstet Gynecol. 1995, 173, 125–134.
- Fazleabas, A.T. A baboon model for inducing endometriosis. Methods Mol. Med. 2006, 121, 95–99.
- Liu, D.T.; Hitchcock, A. Endometriosis: Its association with retrograde menstruation, dysmenorrhoea and tubal pathology. Br.J. Obstet Gynaecol. 1986, 93, 859–862.
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799.
- Vinatier, D.; Cosson, M.; Dufour, P. Is endometriosis an endometrial disease? Eur.J. Obstet Gynecol. Reprod. Biol. 2000, 91, 113–125.
- Lang, J.H. Cornerstone of study on endometriosis. Zhonghua Fu Chan Ke Za Zhi 2005, 40, 3–4.
- Lang, J.H. Promotion and enhancement of research on endometriosis. Zhonghua Fu Chan Ke Za Zhi 2010, 45, 241–242.
- Lang, J. Endometriosis: Its re-acquaintance and significance. Chin. Eng. Sci. 2009, 11, 137–142. (In Chinese)
- Lang, J. Facilitating endometriosis research based on the principles of evidence-based medicine. J. Int. Obstet. Gynecol. 2011, 38, 261–262. (In Chinese)
- Leng, J.o.b.o.t.E.S.; Gynecologists and Obstetrician Branch; Association, C.P. . Chin.J. Obstet. Gynecol. 2018, 53, 836–840. (In Chinese)
- Suda, K.; Nakaoka, H.; Yoshihara, K.; Ishiguro, T.; Tamura, R.; Mori, Y.; Yamawaki, K.; Adachi, S.; Takahashi, T.; Kase, H.; et al. Clonal Expansion and Diversification of Cancer-Associated Mutations in Endometriosis and Normal Endometrium. Cell Rep. 2018, 24, 1777–1789.
- Inoue, S.; Yoshida, E.; Fukui, Y.; Ueno, T.; Kawazu, M.; Takeyama, R.; Ikemura, M.; Osuga, Y.; Terao, Y.; Hirota, Y.; et al. KRAS mutations in uterine endometrium are associated with gravidity and parity. Cell Death Dis. 2020, 11, 347.
- Canon, J.; Rex, K.; Saiki, A.Y.; Mohr, C.; Cooke, K.; Bagal, D.; Gaida, K.; Holt, T.; Knutson, C.G.; Koppada, N.; et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature 2019, 575, 217–223.
- Kao, L.C.; Germeyer, A.; Tulac, S.; Lobo, S.; Yang, J.P.; Taylor, R.N.; Osteen, K.; Lessey, B.A.; Giudice, L.C. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology 2003, 144, 2870–2881.
- Burney, R.O.; Talbi, S.; Hamilton, A.E.; Vo, K.C.; Nyegaard, M.; Nezhat, C.R.; Lessey, B.A.; Giudice, L.C. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 2007, 148, 3814–3826.
- Burney, R.O.; Hamilton, A.E.; Aghajanova, L.; Vo, K.C.; Nezhat, C.N.; Lessey, B.A.; Giudice, L.C. MicroRNA expression profiling of eutopic secretory endometrium in women with versus without endometriosis. Mol. Hum. Reprod 2009, 15, 625–631.
- Fassbender, A.; Verbeeck, N.; Bornigen, D.; Kyama, C.M.; Bokor, A.; Vodolazkaia, A.; Peeraer, K.; Tomassetti, C.; Meuleman, C.; Gevaert, O.; et al. Combined mRNA microarray and proteomic analysis of eutopic endometrium of women with and without endometriosis. Hum. Reprod. 2012, 27, 2020–2029.
- Laudanski, P.; Charkiewicz, R.; Tolwinska, A.; Szamatowicz, J.; Charkiewicz, A.; Niklinski, J. Profiling of Selected MicroRNAs in Proliferative Eutopic Endometrium of Women with Ovarian Endometriosis. BioMed Res. Int. 2015, 2015, 760698.
- Wang, Y.; Li, Y.; Yang, Z.; Liu, K.; Wang, D. Genome-Wide Microarray Analysis of Long Non-Coding RNAs in Eutopic Secretory Endometrium with Endometriosis. Cell Physiol. Biochem. 2015, 37, 2231–2245.
- Herndon, C.N.; Aghajanova, L.; Balayan, S.; Erikson, D.; Barragan, F.; Goldfien, G.; Vo, K.C.; Hawkins, S.; Giudice, L.C. Global Transcriptome Abnormalities of the Eutopic Endometrium From Women With Adenomyosis. Reprod. Sci. 2016, 23, 1289–1303.
- Kukushkina, V.; Modhukur, V.; Suhorutsenko, M.; Peters, M.; Magi, R.; Rahmioglu, N.; Velthut-Meikas, A.; Altmae, S.; Esteban, F.J.; Vilo, J.; et al. DNA methylation changes in endometrium and correlation with gene expression during the transition from pre-receptive to receptive phase. Sci. Rep. 2017, 7, 3916.
- Barjaste, N.; Shahhoseini, M.; Afsharian, P.; Sharifi-Zarchi, A.; Masoudi-Nejad, A. Genome-wide DNA methylation profiling in ectopic and eutopic of endometrial tissues. J. Assist. Reprod. Genet. 2019, 36, 1743–1752.
- Gupta, D.; Hull, M.L.; Fraser, I.; Miller, L.; Bossuyt, P.M.; Johnson, N.; Nisenblat, V. Endometrial biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2016, 4, CD012165.
- Da Broi, M.G.; Meola, J.; Placa, J.R.; Peronni, K.C.; Rocha, C.V.; Silva, W.A.; Ferriani, R.A.; Navarro, P.A. Is the profile of transcripts altered in the eutopic endometrium of infertile women with endometriosis during the implantation window? Hum. Reprod. 2019, 34, 2381–2390.
- Tokushige, N.; Markham, R.; Russell, P.; Fraser, I.S. High density of small nerve fibres in the functional layer of the endometrium in women with endometriosis. Hum. Reprod. 2006, 21, 782–787.
- Tokushige, N.; Markham, R.; Russell, P.; Fraser, I.S. Effects of hormonal treatment on nerve fibers in endometrium and myometrium in women with endometriosis. Fertil. Steril. 2008, 90, 1589–1598.
- Tokushige, N.; Markham, R.; Russell, P.; Fraser, I.S. Different types of small nerve fibers in eutopic endometrium and myometrium in women with endometriosis. Fertil. Steril. 2007, 88, 795–803.
- Al-Jefout, M.; Dezarnaulds, G.; Cooper, M.; Tokushige, N.; Luscombe, G.M.; Markham, R.; Fraser, I.S. Diagnosis of endometriosis by detection of nerve fibres in an endometrial biopsy: A double blind study. Hum. Reprod. 2009, 24, 3019–3024.
- Bokor, A.; Kyama, C.M.; Vercruysse, L.; Fassbender, A.; Gevaert, O.; Vodolazkaia, A.; De Moor, B.; Fulop, V.; D’Hooghe, T. Density of small diameter sensory nerve fibres in endometrium: A semi-invasive diagnostic test for minimal to mild endometriosis. Hum. Reprod. 2009, 24, 3025–3032.
- Zhang, X.; Lu, B.; Huang, X.; Xu, H.; Zhou, C.; Lin, J. Endometrial nerve fibers in women with endometriosis, adenomyosis, and uterine fibroids. Fertil. Steril. 2009, 92, 1799–1801.
- Zhang, X.; Lu, B.; Huang, X.; Xu, H.; Zhou, C.; Lin, J. Innervation of endometrium and myometrium in women with painful adenomyosis and uterine fibroids. Fertil. Steril. 2010, 94, 730–737.
- Newman, T.A.; Bailey, J.L.; Stocker, L.J.; Woo, Y.L.; Macklon, N.S.; Cheong, Y.C. Expression of neuronal markers in the endometrium of women with and those without endometriosis. Hum. Reprod. 2013, 28, 2502–2510.
- Ellett, L.; Readman, E.; Newman, M.; McIlwaine, K.; Villegas, R.; Jagasia, N.; Maher, P. Are endometrial nerve fibres unique to endometriosis? A prospective case-control study of endometrial biopsy as a diagnostic test for endometriosis in women with pelvic pain. Hum. Reprod. 2015, 30, 2808–2815.
- Liutkeviciene, R.; Mecejus, G.; Zilovic, D.; Bumbuliene, Z. Endometrial biopsy and density of nerve fibers in eutopic endometrium. Looking for easier ways to diagnose endometriosis. Gynecol. Endocrinol. 2019, 35, 1107–1110.
- Gronemeyer, H.; Meyer, M.E.; Bocquel, M.T.; Kastner, P.; Turcotte, B.; Chambon, P. Progestin receptors: Isoforms and antihormone action. J. Steroid Biochem. Mol. Biol. 1991, 40, 271–278.
- McKinnon, B.; Mueller, M.; Montgomery, G. Progesterone Resistance in Endometriosis: An Acquired Property? Trends Endocrinol. Metab. 2018, 29, 535–548.
- Attia, G.R.; Zeitoun, K.; Edwards, D.; Johns, A.; Carr, B.R.; Bulun, S.E. Progesterone receptor isoform A but not B is expressed in endometriosis. J.Clin. Endocrinol. Metab. 2000, 85, 2897–2902.
- Bukulmez, O.; Hardy, D.B.; Carr, B.R.; Word, R.A.; Mendelson, C.R. Inflammatory status influences aromatase and steroid receptor expression in endometriosis. Endocrinology 2008, 149, 1190–1204.
- Bedaiwy, M.A.; Dahoud, W.; Skomorovska-Prokvolit, Y.; Yi, L.; Liu, J.H.; Falcone, T.; Hurd, W.W.; Mesiano, S. Abundance and Localization of Progesterone Receptor Isoforms in Endometrium in Women With and Without Endometriosis and in Peritoneal and Ovarian Endometriotic Implants. Reprod. Sci. 2015, 22, 1153–1161.
- Wu, Y.; Strawn, E.; Basir, Z.; Halverson, G.; Guo, S.W. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics 2006, 1, 106–111.
- Jichan, N.; Xishi, L.; Guo, S.W. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in adenomyosis and its rectification by a histone deacetylase inhibitor and a demethylation agent. Reprod. Sci. 2010, 17, 995–1005.
- Wu, Y.; Starzinski-Powitz, A.; Guo, S.W. Prolonged stimulation with tumor necrosis factor-alpha induced partial methylation at PR-B promoter in immortalized epithelial-like endometriotic cells. Fertil. Steril. 2008, 90, 234–237.
- Wu, Y.; Shi, X.; Guo, S.W. The knockdown of progesterone receptor isoform B (PR-B) promotes proliferation in immortalized endometrial stromal cells. Fertil. Steril. 2008, 90, 1320–1323.
- Igarashi, T.M.; Bruner-Tran, K.L.; Yeaman, G.R.; Lessey, B.A.; Edwards, D.P.; Eisenberg, E.; Osteen, K.G. Reduced expression of progesterone receptor-B in the endometrium of women with endometriosis and in cocultures of endometrial cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fertil. Steril. 2005, 84, 67–74.
- Shen, F.; Yan, C.; Liu, M.; Feng, Y.; Chen, Y. Decreased expression of mucin-1 in endometriosis endometrium correlated with progesterone receptor B involved in infertility. Arch. Gynecol. Obstet. 2015, 291, 439–445.
- Wolfler, M.M.; Kuppers, M.; Rath, W.; Buck, V.U.; Meinhold-Heerlein, I.; Classen-Linke, I. Altered expression of progesterone receptor isoforms A and B in human eutopic endometrium in endometriosis patients. Ann. Anat. 2016, 206, 1–6.
- Hou, Z.; Mamillapalli, R.; Taylor, H.S. Predictive biomarkers may allow precision therapy of endometriosis. J. Endometr. Pelvic Pain Disord. 2017, 9, 279–285.
- Pei, T.; Liu, C.; Liu, T.; Xiao, L.; Luo, B.; Tan, J.; Li, X.; Zhou, G.; Duan, C.; Huang, W. miR-194-3p Represses the Progesterone Receptor and Decidualization in Eutopic Endometrium From Women With Endometriosis. Endocrinology 2018, 159, 2554–2562.
- Colon-Caraballo, M.; Garcia, M.; Mendoza, A.; Flores, I. Human Endometriosis Tissue Microarray Reveals Site-specific Expression of Estrogen Receptors, Progesterone Receptor, and Ki67. Appl. Immunohistochem. Mol. Morphol. 2018, 27, 491–500.
- Rocha-Junior, C.V.; Da Broi, M.G.; Miranda-Furtado, C.L.; Navarro, P.A.; Ferriani, R.A.; Meola, J. Progesterone Receptor B ( PGR-B) Is Partially Methylated in Eutopic Endometrium From Infertile Women With Endometriosis. Reprod. Sci. 2019, 26, 1568–1574.
- Prentice, A.; Randall, B.J.; Weddell, A.; McGill, A.; Henry, L.; Horne, C.H.; Thomas, E.J. Ovarian steroid receptor expression in endometriosis and in two potential parent epithelia: Endometrium and peritoneal mesothelium. Hum. Reprod. 1992, 7, 1318–1325.
- Broi, M.G.D.; Rocha, C.V.J.; Meola, J.; Martins, W.P.; Carvalho, F.M.; Ferriani, R.A.; Navarro, P.A. Expression of PGR, HBEGF, ITGAV, ITGB3 and SPP1 genes in eutopic endometrium of infertile women with endometriosis during the implantation window: A pilot study. JBRA Assist. Reprod. 2017, 21, 196–202.
- Gentilini, D.; Vigano, P.; Vignali, M.; Busacca, M.; Panina-Bordignon, P.; Caporizzo, E.; Di Blasio, A.M. Endometrial stromal progesterone receptor-A/progesterone receptor-B ratio: No difference between women with and without endometriosis. Fertil. Steril. 2010, 94, 1538–1540.
- Marquardt, R.M.; Kim, T.H.; Shin, J.H.; Jeong, J.W. Progesterone and Estrogen Signaling in the Endometrium: What Goes Wrong in Endometriosis? Int.J. Mol. Sci. 2019, 20, 3822.
- Huang, Q.; Liu, X.; Critchley, H.; Fu, Z.; Guo, S.-W. How does the extent of fibrosis in adenomyosis lesions contribute to heavy menstrual bleeding? Reprod. Med. Biol. 2022, 21, e12442.
- Nie, J.; Lu, Y.; Liu, X.; Guo, S.W. Immunoreactivity of progesterone receptor isoform B, nuclear factor kappaB, and IkappaBalpha in adenomyosis. Fertil. Steril. 2009, 92, 886–889.
- Kumar, P.V.; Esfahani, F.N. Cytopathology of peritoneal endometriosis caused by ruptured ovarian cysts. Acta Cytol. 1988, 32, 523–526.
- Critchley, H.O.; Wang, H.; Kelly, R.W.; Gebbie, A.E.; Glasier, A.F. Progestin receptor isoforms and prostaglandin dehydrogenase in the endometrium of women using a levonorgestrel-releasing intrauterine system. Hum. Reprod. 1998, 13, 1210–1217.
- Wu, Y.; Halverson, G.; Basir, Z.; Strawn, E.; Yan, P.; Guo, S.W. Aberrant methylation at HOXA10 may be responsible for its aberrant expression in the endometrium of patients with endometriosis. Am. J. Obstet. Gynecol. 2005, 193, 371–380.
- Kim, J.J.; Taylor, H.S.; Lu, Z.; Ladhani, O.; Hastings, J.M.; Jackson, K.S.; Wu, Y.; Guo, S.W.; Fazleabas, A.T. Altered expression of HOXA10 in endometriosis: Potential role in decidualization. Mol. Hum. Reprod. 2007, 13, 323–332.
- Lee, B.; Du, H.; Taylor, H.S. Experimental murine endometriosis induces DNA methylation and altered gene expression in eutopic endometrium. Biol. Reprod. 2009, 80, 79–85.
- Szczepanska, M.; Wirstlein, P.; Luczak, M.; Jagodzinski, P.P.; Skrzypczak, J. Reduced expression of HOXA10 in the midluteal endometrium from infertile women with minimal endometriosis. Biomed. Pharmacother. 2010, 64, 697–705.
- Fambrini, M.; Sorbi, F.; Bussani, C.; Cioni, R.; Sisti, G.; Andersson, K.L. Hypermethylation of HOXA10 gene in mid-luteal endometrium from women with ovarian endometriomas. Acta Obstet. Gynecol. Scand. 2013, 92, 1331–1334.
- Kulp, J.L.; Mamillapalli, R.; Taylor, H.S. Aberrant HOXA10 Methylation in Patients With Common Gynecologic Disorders: Implications for Reproductive Outcomes. Reprod. Sci. 2016, 23, 455–463.
- Lu, Y.; Nie, J.; Liu, X.; Guo, S.W. Reduced expression and concomitant promoter hypermethylation of HOXA10 in endometrium from women wearing intrauterine devices. Fertil. Steril. 2010, 94, 1583–1588.
- Sanchez-Ferrer, M.L.; Mendiola, J.; Jimenez-Velazquez, R.; Canovas-Lopez, L.; Corbalan-Biyang, S.; Hernandez-Penalver, A.I.; Carmona-Barnosi, A.; Maldonado-Carceles, A.B.; Prieto-Sanchez, M.T.; Machado-Linde, F.; et al. Investigation of anogenital distance as a diagnostic tool in endometriosis. Reprod. Biomed. Online 2017, 34, 375–382.
- Dinsdale, N.L.; Crespi, B.J. Endometriosis and polycystic ovary syndrome are diametric disorders. Evol. Appl. 2021, 14, 1693–1715.
- Koninckx, P.R.; Kennedy, S.H.; Barlow, D.H. Endometriotic disease: The role of peritoneal fluid. Hum. Reprod. Update 1998, 4, 741–751.
- Naqvi, H.; Mamillapalli, R.; Krikun, G.; Taylor, H.S. Endometriosis Located Proximal to or Remote From the Uterus Differentially Affects Uterine Gene Expression. Reprod. Sci. 2016, 23, 186–191.
- Goetz, T.G.; Mamillapalli, R.; Taylor, H.S. Low Body Mass Index in Endometriosis Is Promoted by Hepatic Metabolic Gene Dysregulation in Mice. Biol. Reprod. 2016, 95, 115.
- Shah, D.K.; Correia, K.F.; Vitonis, A.F.; Missmer, S.A. Body size and endometriosis: Results from 20 years of follow-up within the Nurses’ Health Study II prospective cohort. Hum. Reprod. 2013, 28, 1783–1792.
- Mamillapalli, R.; Toffoloni, N.; Habata, S.; Qunhua, H.; Atwani, R.; Stachenfeld, N.; Taylor, H.S. Endometriosis promotes atherosclerosis in a murine model. Am. J. Obstet. Gynecol. 2022, 227, 248.e1–248.e8.
More
Information
Subjects:
Obstetrics & Gynaecology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
694
Revisions:
3 times
(View History)
Update Date:
20 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




