
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Olga Senko | -- | 4252 | 2023-02-13 10:52:02 | | | |
| 2 | Camila Xu | Meta information modification | 4252 | 2023-02-14 02:28:42 | | |
Video Upload Options
Organophosphorus hydrolase, containing a genetically introduced hexahistidine sequence (His6-OPH) can hydrolyze various substrates, such as organophosphorus pesticides and chemical warfare agents, mycotoxins, and N-acyl homoserine lactones. The application of various carrier materials (metal-organic frameworks, polypeptides, bacterial cellulose, polyhydroxybutyrate, succinylated gelatin, etc.) for the immobilization and stabilization of His6-OPH by various methods, enables creation of biocatalysts with various properties and potential uses, in particular, as antidotes, recognition elements of biosensors, in fibers with chemical and biological protection, dressings with antimicrobial properties, highly porous sorbents for the degradation of toxicants, including in flow systems, etc. Immobilized variants of His6-OPH are characterized by increased stability, and the hydrolytic process of destruction of many substrates can be carried out in wider temperature and pH ranges than when using a free form of the enzyme. The variation in methods and carriers for the immobilization of His6-OPH makes it possible to create a wide palette of biocatalysts, significantly expanding the boundaries of enzyme use.
1. Introduction
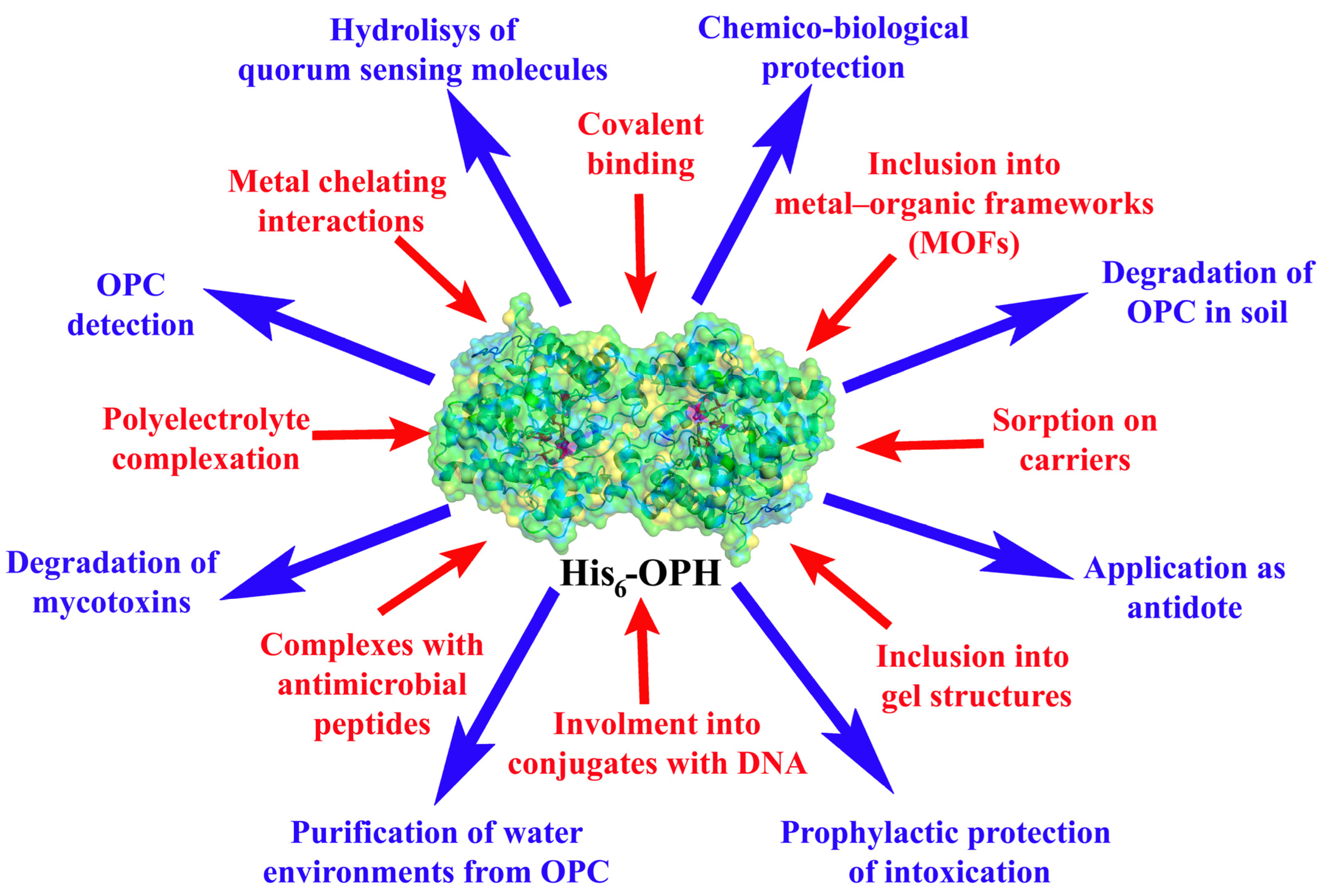
2. Stabilization of Hexa-Histidine-Containing OPH for OPC Hydrolysis
| Variants of His-Tagged OPH or PTE [Reference] | Immobilization Technique and Carrier |
Properties | Application |
|---|---|---|---|
| Use of metalorganic interactions and frameworks | |||
| OpdA@Ni-NTA-VMSN [21] | Ni2+-nitrilotriacetic acid (NTA)-modified, virus-like, mesoporous silica nanoparticles | The affine capacity of substrates became higher for the enzyme after its oriented immobilization | Hydrolysis of methyl parathion in a plug-flow reactor |
| OpdA@MIL-88A [22] |
MOF synthesized on the basis of fumaric acid and FeCl3 | The catalytic activity is 5 times higher as compared to the free form of the enzyme. The enzyme possessed improved organic detergent and solvent tolerance and thermal and storage stability | Degradation of organophosphorus pesticides on grapes and cucumbers |
| BSA-Cu@CaPs-OPH [23] |
A hybrid organic–inorganic, calcium phosphate-containing nanocrystal, based on the use of bovine serum albumin modified by Cu2+ ions | Improved multiple usages with up to 56% retainment of activity after 10 working cycles; advanced thermal and pH stability | Hydrolysis of methyl parathion on fruits or vegetables |
| OPH6His/UiO-66-NH2 [24] |
MOF, synthesized using amino-terephthalic acid and zirconium chloride with further pre-activation by N,N′-dicyclohexylcarbodiimide, was covalently bonded with the enzyme over the surface via UiO-66-NH2 | Improved storage stability (up to 60 days), reusability (in 9 working cycles), and 37% improved catalytic activity | Degradation of methyl parathion |
| OPH6His@Tb-BTC [25] |
Encapsulation of the enzyme in MOF, formed by terbium nitrate and 1,2,4-benzenetricarboxylic acid (BTC) | Improved storage stability and a 30% increase in activity | Sensing of methyl parathion |
| OpdA@Co/C@SiO2@Ni/C [26] | An enzyme created multiple affine coordination bonds with Co and Ni introduced into carbonized hybrid nanocomposite ZIF-67@ SiO2 with a yolk-shell structure | Increased pH, thermal and storage stability, and SDS resistance; long reusability (in 7 working cycles) with 60% of residual activity | Chemo-enzymatic cascade degradation of methyl parathion |
| Co/MnHF@PTE [27] |
An enzyme was involved in the multimetallic nanoflower structure during its formation in a mixture of metal salt solutions and protein: Co-PTE (CoHF@PTE) and Mn-PTE (MnHF@PTE) hybrid | The improved catalytic activity of 4 times due to the use of two metals (Co and Mn) in the frame of the nanoflower-like structure of MOF | Hydrolysis of methyl parathion, VX and soman |
| OPH@H-Au-TiO2 [28] |
An enzyme was adsorbed on the hollow-structured nanoparticles, Au-TiO2 | Increased reusability in the destruction of OPCs and very stable activity due to the synergistic combination of photo- and enzymatic catalysis | Degradation of methyl parathion |
| OPH@0.8CoZIF [29] |
An enzyme was encapsulated to Zn-doped Co-based ZIF via biomimetic mineralization | The enzyme is involved in a cascade of chemical reactions where the 4-aminophenol is the last product | Conversion of methyl parathion |
| YT-PTE on FE [30] |
A mutant enzyme was immobilized by sorption on the Fuller’s Earth (FE) | The temperature and storage stability were improved, but there were no notable changes in the activity of the immobilized enzyme compared to its free form; several bivalent ions (Co, Ni, Cu, Fe, Zn) exhibited significantly higher increases in the activity of the immobilized enzyme | Hydrolysis of paraoxon |
| Use of fusion proteins and bioconjugations | |||
| [scGFP-arPTE][S−] [31] |
Fusion proteins, containing green fluorescent protein (GFP) and PTE, were involved in an electrolytic interaction with monomeric S− with further drying and cross-linking to obtain a catalytically active porous film or cross-linking in the presence of cotton fiber to obtain composite textiles | Improved thermal stability and higher catalytic constant (kcat) | Introduction into cotton fibers for the creation of personalized protective composite material useful for the recyclable hydrolysis of paraoxon |
| [arPTE][S+][S−] [32] |
Obtainment of bioconjugates through the subsequent introduction of the enzyme into contact with cationic (Ethoquad) and anionic (oxidized IGEPAL) polymer surfactants (S+ or S−), resulting in the formation of such a structure as corona encapsulating the enzyme | Enhancement of the efficiency of catalytic action by 3 times | Hydrolysis of paraoxon |
| [arPTE][S+][S−] –ABC, [arPTE][S+][S−] –PCL [33] |
An enzyme, in the presence of cationic or anionic polymer surfactants (S+ or S−), was lyophilized with further melting of the obtained powder and used in co-dispersion with curtain polymers (ABC, acrylonitrile butadiene styrene; PCL, polycaprolactone) for 3D-printing on the surface of stainless steel rings | Improved stability of enzyme action | Hydrolysis of paraoxon-ethyl by composite enzyme plastics developed for use in self-decontaminating surfaces |
| PoOPHM9-CLEPC [34] |
An enzyme containing 10 residues of genetically introduced phenylalanine was conjugated with Pluronic F127 to form a cross-linked enzyme–polymer conjugate (CLEPC) | Increased optimal temperature (50 °C) and pH stability in the range of 7–11 | Hydrolysis of malathion |
| OPH@pID-MSN [35] |
An enzyme was covalently immobilized on mesoporous silica nanoparticles (MSNs) coated with a zwitterionic polymer containing short hydrophobic chains, which is a product of the ring-opening reaction between poly (isobutylene-alt-maleic anhydride) and N,N-dimethylethylenediamine (pID) | Significantly improved stability |
Hydrolysis of methyl parathion |
| Sorption and covalent immobilization on natural carriers | |||
| OPH/PCD [36] |
An enzyme was adsorbed onto the microparticles of poly-β-cyclodextrin (PCD) with further freeze-drying |
Increased sorption capacity of the substrate and product of the enzymatic hydrolysis; possible regeneration of activity via new enzyme sorption and the self-decontamination of the biocatalyst | Hydrolysis of methyl paraoxon |
| OpdA-PHB [37] |
An enzyme was immobilized on non-porous poly(hydroxyl butyrate) (PHB) microspheres | The carrier increased the sorption of the hydrophobic substrate from the medium | Hydrolysis of coumaphos |
| SpOpdA-SPS-S [38] |
An enzyme was immobilized via the covalent binding of His-tag as SpyTag (Sp) to such fusion proteins as SpyCatcher (SPS)-coated poly(hydroxy alkanoates) (PHAs) spheres (S) | Improved catalytic activity and stability | Hydrolysis of coumaphos |
| Conjugation using DNA molecules | |||
| QD-DNA-PTE [39] |
An enzyme was attached, via the DNA-containing linker, to PEGylated quantum dots (QDs) | Enhanced efficiency and rate of catalytic reaction (kcat) | Hydrolysis of paraoxon |
| DNA cage-QD-PTE [40] |
Molecules of DNA were conjugated into a cage by His5-peptide and modified by quantum dots (QDs) of ZeS with further immobilization of PTE | Enhanced catalytic activity by 12.5 times | Hydrolysis of paraoxon |
| PTEpAzF -DNA [41] |
An enzyme was conjugated to a DNA scaffold modified by dibenzocyclooctyl via a site-specific incorporated azido-group to the enzyme molecule structure |
Improved catalytic constants | Hydrolysis of paraoxon |
| Formation of complexes | |||
| OPT−PIMs [42] |
An enzyme (OPT) was involved in the formation of pollen-inspired microparticles (PIMs), prepared based on complexation between CaCO3, gelatin, and the enzyme |
Improved stability at 50 °C and low pH 4.8 (citric acid/sodium citrate buffer) | Detoxification of pollen contaminated by paraoxon or malathion |
| His6-OPH/PLE50, His6-OPH/PLD50 [43] |
Enzyme was involved in polyelectrolyte complexes with poly-l-glutamic acid (PLE50) or poly-l-aspartic acid (PLD50) | Increased stability of catalytic action in the soil | Destruction of chlorpyrifos in different types of soil |
| His6OPH/PEG113PLE10, His6OPH/PEG113PLE50, His6OPH/PEG113PLE100, His6OPH/PEG113PLD50, His6OPH/PEG22PLE50, His6OPH/PLE50PEG113PLE50 His6OPH/Hydroxyethyl starch His6OPH/Succinylated gelatin [44] |
An enzyme was involved in polyelectrolyte complexes with PEGylated poly-l-glutamic acid (PLE10-100), poly-l-aspartic acid (PLD50) of a different polymerization degree, hydroxyethyl starch, or succinylated gelatin |
20–40% increased catalytic efficiency of enzyme action | Hydrolysis of methyl parathion and paraoxon |
| PCL-RHP-OPH [45] |
An enzyme was immobilized through electrospinning in poly-ε-caprolactone fibrous mats in the form of a lyophilized complex dissolved in toluene with random heteropolymers (RHPs) |
Enhanced stability and reusability (40% residual activity after everyday use for 3 months) | Hydrolysis of methyl parathion and paraoxon |
References
- Alejo-González, K.; Hanson-Viana, E.; Vazquez-Duhalt, R. Enzymatic detoxification of organophosphorus pesticides and related toxicants. J. Pestic. Sci. 2018, 43, 1–9.
- Varfolomeev, S.D.; Efremenko, E.N. Organophosphorus Neurotoxins; RIOR: Moscow, Russia, 2020; p. 380. ISBN 978-5-369-02026-5.
- Zhao, S.; Xu, W.; Zhang, W.; Wu, H.; Guang, C.; Mu, W. Overview of a bioremediation tool: Organophosphorus hydrolase and its significant application in the food, environmental, and therapy fields. Appl. Microbiol. Biotechnol. 2021, 105, 8241–8253.
- Lyagin, I.; Efremenko, E. Enzymes, reacting with organophosphorus compounds as detoxifiers: Diversity and functions. Int. J. Mol. Sci. 2021, 22, 1761.
- Lyagin, I.V.; Andrianova, M.S.; Efremenko, E.N. Extensive hydrolysis of phosphonates as unexpected behaviour of the known His6-organophosphorus hydrolase. Appl. Microbiol. Biotechnol. 2016, 100, 5829–5838.
- Lyagin, I.; Efremenko, E. Theoretical evaluation of suspected enzymatic hydrolysis of Novichok agents. Catal. Commun. 2019, 120, 91–94.
- Latip, W.; Knight, V.F.; Abdul Halim, N.; Ong, K.K.; Mohd Kassim, N.A.; Wan Yunus, W.M.Z.; Mohd Noor, S.A.; Mohamad Ali, M.S. Microbial phosphotriesterase: Structure, function, and biotechnological applications. Catalysts 2019, 9, 671.
- Efremenko, E.N.; Lyagin, I.V.; Klyachko, N.L.; Bronich, T.; Zavyalova, N.V.; Jiang, Y.; Kabanov, A.V. A simple and highly effective catalytic nanozyme scavenger for organophosphorus neurotoxins. J. Control. Release 2017, 247, 175–181.
- Zhang, P.; Liu, E.J.; Tsao, C.; Kasten, S.A.; Boeri, M.V.; Dao, T.L.; DeBus, S.J.; Cadieux, C.L.; Baker, C.A.; Otto, T.C.; et al. Nanoscavenger provides long-term prophylactic protection against nerve agents in rodents. Sci. Transl. Med. 2019, 11, eaau7091.
- Jain, M.; Yadav, P.; Joshi, A.; Kodgire, P. Advances in detection of hazardous organophosphorus compounds using organophosphorus hydrolase based biosensors. Crit. Rev. Toxicol. 2019, 49, 387–410.
- Xu, W.; Zhao, S.; Zhang, W.; Wu, H.; Guang, C.; Mu, W. Recent advances and future prospective of organophosphorus-degrading enzymes: Identification, modification, and application. Crit. Rev. Biotechnol. 2021, 41, 1096–1113.
- Chi, M.C.; Liao, T.Y.; Lin, M.G.; Lin, L.L.; Wang, T.F. Expression and physiochemical characterization of an N-terminal polyhistidine-tagged phosphotriesterase from the soil bacterium Brevundimonas diminuta. Biocatal. Agric. Biotechnol. 2020, 29, 101811.
- Mali, H.; Shah, C.; Patel, D.; Trivedi, U.; Subramanian, R. Bio-catalytic system of metallohydrolases for remediation of neurotoxin organophosphates and applications with a future vision. J. Inorg. Biochem. 2022, 231, 111771.
- Manco, G.; Porzio, E.; Suzumoto, Y. Enzymatic detoxification: A sustainable means of degrading toxic organophosphate pesticides and chemical warfare nerve agents. J. Chem. Technol. Biot. 2018, 93, 2064–2082.
- Chi, M.-C.; Liao, T.-Y.; Lin, M.-G.; Lin, L.-L.; Wang, T.-F. Catalytic performance of a recombinant organophosphate-hydrolyzing phosphotriesterase from Brevundimonas diminuta in the presence of surfactants. Catalysts 2021, 11, 597.
- Efremenko, E.; Lyagin, I.; Votchitseva, Y.; Sirotkina, M.; Varfolomeyev, S. Polyhistidine-containing organophosphorus hydrolase with outstanding properties. Biocatal. Biotransfor. 2007, 25, 103–108.
- Bigley, A.N.; Raushel, F.M. The evolution of phosphotriesterase for decontamination and detoxification of organophosphorus chemical warfare agents. Chem. Biol. Interact. 2019, 308, 80–88.
- Katyal, P.; Chu, S.; Montclare, J. Enhancing organophosphate hydrolase efficacy via protein engineering and immobilization strategies. Ann. N. Y. Acad. Sci. 2020, 1480, 54–72.
- Aslanli, A.; Lyagin, I.; Efremenko, E. Novel approach to quorum quenching: Rational design of antibacterials in combination with hexahistidine-tagged organophosphorus hydrolase. Biol. Chem. 2018, 399, 869–879.
- Kumar, A. (Ed.) Supermacroporous Cryogels: Biomedical and Biotechnological Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019; p. 496. ISBN 9780367869472.
- Zhou, L.; Li, J.; Gao, J.; Liu, H.; Xue, S.; Ma, L.; Cao, G.; Huang, Z.; Jiang, Y. Facile oriented immobilization and purification of His-tagged organophosphohydrolase on virus-like mesoporous silica nanoparticles for organophosphate bioremediation. ACS Sustain. Chem. Eng. 2018, 6, 13588–13598.
- Xue, S.; Li, J.; Zhou, L.; Gao, J.; Liu, G.; Ma, L.; He, Y.; Jiang, Y. Simple purification and immobilization of his-tagged organophosphohydrolase from cell culture supernatant by metal organic frameworks for degradation of organophosphorus pesticides. J. Agric. Food Chem. 2019, 67, 13518–13525.
- Wang, Z.; Gao, J.; Shi, Q.; Dong, X.; Sun, Y. Facile purification and immobilization of organophosphorus hydrolase on protein-inorganic hybrid phosphate nanosheets. Chin. J. Chem. Eng. 2022; in press.
- Mehta, J.; Dhaka, S.; Paul, A.K.; Dayananda, S.; Deep, A. Organophosphate hydrolase conjugated UiO-66-NH2 MOF based highly sensitive optical detection of methyl parathion. Environ. Res. 2019, 174, 46–53.
- Mehta, J.; Dhaka, S.; Bhardwaj, N.; Paul, A.K.; Dayananda, S.; Lee, S.-E.; Kim, K.-H.; Deep, A. Application of an enzyme encapsulated metal-organic framework composite for convenient sensing and degradation of methyl parathion. Sens. Actuators B Chem. 2019, 290, 267–274.
- Li, Y.X.; Luan, P.Q.; Zhou, L.Y.; Xue, S.G.; Liu, Y.H.; Liu, Y.T.; Jiang, Y.J.; Gao, J. Purification and immobilization of His-tagged organophosphohydrolase on yolk–shell Co/2@Ni/C nanoparticles for cascade degradation and detection of organophosphates. Biochem. Eng. J. 2021, 167, 107895.
- Chen, J.; Guo, Z.; Zhang, H.; Xin, Y.; Shi, Y.; Gu, Z.; Zhang, L.; Zhong, J.; Guo, X.; Li, Y.; et al. Development of a multimetal-based phosphotriesterase hybrid nanoflowers for decontamination of environmental organophosphorus compounds pollution. Chem. Eng. J. 2022, 446, 136933.
- Zhang, Y.; Cao, X.; Yang, Y.; Guan, S.; Wang, X.; Li, H.; Zheng, X.; Zhou, L.; Jiang, Y.; Gao, J. Visible light assisted enzyme-photocatalytic cascade degradation of organophosphorus pesticides. Green Chem. Eng. 2022, in press.
- Wang, Z.; Sun, Y. A hybrid nanobiocatalyst with in situ encapsulated enzyme and exsolved Co nanoclusters for complete chemoenzymatic conversion of methyl parathion to 4-aminophenol. J. Hazard. Mater. 2022, 424, 127755.
- Latip, W.; Knight, V.F.; Khim, O.K.; Mohd Kasim, N.A.; Wan Yunus, W.M.Z.; Mohamad Ali, M.S.; Mohd Noor, S.A. Immobilization of mutant phosphotriesterase on fuller’s earth enhanced the stability of the enzyme. Catalysts 2021, 11, 983.
- Day, G.J.; Zhang, W.H.; Carter, B.M.; Xiao, W.; Sambrook, M.R.; Perriman, A.W. A rationally designed supercharged protein-enzyme chimera self-assembles in situ to yield bifunctional composite textiles. ACS Appl. Mater. Interfaces 2021, 13, 60433–60445.
- Zhang, W.H.; Carter, B.M.; Day, G.J.; Govan, N.; Jackson, C.; Perriman, A.W. Sequential electrostatic assembly of a polymer surfactant corona increases activity of the phosphotriesterase arPTE. Bioconjug. Chem. 2019, 30, 2771–2776.
- Zhang, W.H.; Day, G.J.; Zampetakis, I.; Carrabba, M.; Zhang, Z.; Carter, B.M.; Govan, N.; Jackson, C.; Chen, M.; Perriman, A.W. Three-dimensional printable enzymatically active plastics. CS Appl. Polym. Mater. 2021, 3, 6070–6077.
- Cheng, H.; Zhao, Y.; Luo, X.-J.; Xu, D.-S.; Cao, X.; Xu, J.-H.; Zhang, X.-Y.; Ge, J.; Bai, Y. Cross-linked enzyme–polymer conjugates with excellent stability and detergent-enhanced activity for efficient organophosphate degradation. Bioresour. Bioprocess. 2018, 5, 49.
- Wang, X.; Wang, Z.; Yu, L.; Shi, Q.; Dong, X.; Sun, Y. Zwitterionic polymer-mediated immobilization of organophosphorus hydrolase enhances hydrolysis of methyl parathion by substrate enrichment. Biochem. Eng. J. 2022, 184, 108491.
- Moon, Y.; Jafry, A.T.; Kang, S.B.; Seo, J.Y.; Baek, K.Y.; Kim, E.J.; Pan, J.G.; Choi, J.Y.; Kim, H.J.; Lee, K.H.; et al. Organophosphorus hydrolase-poly-β-cyclodextrin as a stable self-decontaminating bio-catalytic material for sorption and degradation of organophosphate pesticide. J. Hazard. Mater. 2019, 365, 261–269.
- Ogura, K.; Rehm, B.H.A. Alginate encapsulation of bioengineered protein-coated polyhydroxybutyrate particles: A new platform for multifunctional composite materials. Adv. Funct. Mater. 2019, 29, 1901893.
- Wong, J.X.; Gonzalez-Miro, M.; Sutherland-Smith, A.J.; Rehm, B.H.A. Covalent functionalization of bioengineered polyhydroxyalkanoate spheres directed by specific protein-protein interactions. Front. Bioeng. Biotechnol. 2020, 8, 44.
- Breger, J.C.; Buckhout-White, S.; Walper, S.A.; Oh, E.; Susumu, K.; Ancona, M.G.; Medintz, I.L. Assembling high activity phosphotriesterase composites using hybrid nanoparticle peptide-DNA scaffolded architectures. Nano Futures 2017, 1, 011002.
- Samanta, A.; Breger, J.C.; Susumu, K.; Oh, E.; Walper, S.A.; Bassim, N.; Medintz, I.L. DNA–nanoparticle composites synergistically enhance organophosphate hydrolase enzymatic activity. ACS Appl. Nano Mater. 2018, 1, 3091–3097.
- Hong, X.; Cholko, T.; Chang, C.A.; Wheeldon, I. Multiscale simulation-guided design of enzyme bioconjugates with enhanced catalysis. Chem Catal. 2022, 2, 2691–2703.
- Chen, J.; Webb, J.; Shariati, K.; Guo, S.; Montclare, J.-K.; Scott McArt, S.; Ma, M. Pollen-inspired enzymatic microparticles to reduce organophosphate toxicity in managed pollinators. Nat. Food 2021, 2, 339–347.
- Senko, O.; Maslova, O.; Efremenko, E. Optimization of the use of His6-OPH-based enzymatic biocatalysts for the destruction of chlorpyrifos in soil. Int. J. Environ. Res. Public Health 2017, 14, 1438.
- Lyagin, I.V.; Efremenko, E.N. Biomolecular engineering of biocatalysts hydrolyzing neurotoxic organophosphates. Biochimie 2018, 144, 115–121.
- DelRe, C.; Huang, C.; Dennis, P.; Drockenmuller, E.; Xu, T. Reusable enzymatic fiber mats for neurotoxin remediation in water. ACS Appl. Mater. Interfaces 2018, 10, 44216–44220.
- Zango, Z.U.; Jumbri, K.; Sambudi, N.S.; Ramli, A.; Abu Bakar, N.H.H.; Saad, B.; Rozaini, M.N.H.; Isiyaka, H.A.; Jagaba, A.H.; Aldaghri, O.; et al. A critical review on metal-organic frameworks and their composites as advanced materials for adsorption and photocatalytic degradation of emerging organic pollutants from wastewater. Polymers 2020, 12, 2648.
- Ye, N.; Kou, X.; Shen, J.; Huang, S.; Chen, G.; Ouyang, G. Metal-organic frameworks: A new platform for enzyme immobilization. ChemBioChem 2020, 21, 2585–2590.
- An, H.; Song, J.; Wang, T.; Xiao, N.; Zhang, Z.; Cheng, P.; Ma, S.; Huang, H.; Chen, Y. Metal-organic framework disintegrants: Enzyme preparation platforms with boosted activity. Angew. Chem. Int. Ed. Engl. 2020, 59, 16764–16769.
- Ryu, U.; Jee, S.; Rao, P.C.; Shin, J.; Ko, C.; Yoon, M.; Park, K.S.; Choi, K.M. Recent advances in process engineering and upcoming applications of metal-organic frameworks. Coord. Chem. Rev. 2021, 426, 213544.
- Bobbitt, N.S.; Mendonca, M.L.; Howarth, A.J.; Islamoglu, T.; Hupp, J.T.; Farha, O.K.; Snurr, R.Q. Metal-organic frameworks for the removal of toxic industrial chemicals and chemical warfare agents. Chem. Soc. Rev. 2017, 46, 3357–3385.
- Gorzkowska-Sobas, A.; Lausund, K.B.; de Koning, M.C.; Petrovic, V.; Chavan, S.M.; Smith, M.W.; Nilsen, O. Utilizing zirconium MOF-functionalized fiber substrates prepared by molecular layer deposition for toxic gas capture and chemical warfare agent degradation. Glob. Chall. 2021, 5, 2100001.
- Islamoglu, T.; Chen, Z.; Wasson, M.C.; Buru, C.T.; Kirlikovali, K.O.; Afrin, U.; Mian, M.R.; Farha, O.K. Metal-organic frameworks against toxic chemicals. Chem. Rev. 2020, 120, 8130–8160.
- Cui, J.; Jia, S. Organic–inorganic hybrid nanoflowers: A novel host platform for immobilizing biomolecules. Coord. Chem. Rev. 2017, 352, 249–263.
- Shcharbin, D.; Halets-Bui, I.; Abashkin, V.; Dzmitruk, V.; Loznikova, S.; Odabaşı, M.; Acet, Ö.; Önal, B.; Özdemir, N.; Shcharbina, N.; et al. Hybrid metal-organic nanoflowers and their application in biotechnology and medicine. Colloids Surf. B 2019, 182, 110354.
- Jafari-Nodoushan, H.; Mojtabavi, S.; Faramarzi, M.A.; Samadi, N. Organic-inorganic hybrid nanoflowers: The known, the unknown, and the future. Adv. Colloid Interface Sci. 2022, 309, 102780.
- Lyagin, I.; Stepanov, N.; Maslova, O.; Senko, O.; Aslanli, A.; Efremenko, E. Not a mistake but a feature: Promiscuous activity of enzymes meeting mycotoxins. Catalysts 2022, 12, 1095.




