Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Boon-Peng Puah | -- | 8187 | 2023-02-13 09:22:45 | | | |
| 2 | Camila Xu | Meta information modification | 8187 | 2023-02-13 09:45:16 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Puah, B.; Jalil, J.; Attiq, A.; Kamisah, Y. Anti-Cancer Activities and Selective Anticancer Activities of Lycopene. Encyclopedia. Available online: https://encyclopedia.pub/entry/41145 (accessed on 07 February 2026).
Puah B, Jalil J, Attiq A, Kamisah Y. Anti-Cancer Activities and Selective Anticancer Activities of Lycopene. Encyclopedia. Available at: https://encyclopedia.pub/entry/41145. Accessed February 07, 2026.
Puah, Boon-Peng, Juriyati Jalil, Ali Attiq, Yusof Kamisah. "Anti-Cancer Activities and Selective Anticancer Activities of Lycopene" Encyclopedia, https://encyclopedia.pub/entry/41145 (accessed February 07, 2026).
Puah, B., Jalil, J., Attiq, A., & Kamisah, Y. (2023, February 13). Anti-Cancer Activities and Selective Anticancer Activities of Lycopene. In Encyclopedia. https://encyclopedia.pub/entry/41145
Puah, Boon-Peng, et al. "Anti-Cancer Activities and Selective Anticancer Activities of Lycopene." Encyclopedia. Web. 13 February, 2023.
Copy Citation
Lycopene is a well-known compound found commonly in tomatoes which brings wide range of health benefits against cardiovascular diseases and cancers. From an anti-cancer perspective, lycopene is often associated with reduced risk of prostate cancer and people often look for it as a dietary supplement which may help to prevent cancer.
lycopene
tomatoes
cancer
immune system
inflammation
1. Introduction
Cancer originates from the dysregulation of vital genes involved in the cell maintenance system. This results in abnormal cell proliferation due to the imbalance between proto-oncogene and tumor suppressive gene [1]. There were more than 277 different types of cancers with different pathogenesis pathways, with genetic inheritance being the dominant factor of cancer development and progression [1]. The available effective treatments for cancer are generally chemotherapy and radiotherapy based. Chemotherapy is a type of standard cancer therapy with the use of drugs (bleomycin and cisplatin) with severe side effects such as vomiting for approximately 12 times per day after treatment [2]. Radiotherapy is a therapy which uses radiation to damage the DNA of cancerous cells, with the major limitation being the lack of specificity and thus, causing damage to the normal cells surrounding the malignant tumor. It can also lead to many side effects with respective to the types of underlying cancers such as nausea, vomiting and gastritis for liver cancer; bowel incontinence and rectal irritation for colorectal cancer; shortness of breath and radiation pneumonitis for lung cancer [3]. Though being known to be effective against cancer, they will eventually take their tolls on patients’ bodies, both mentally and physically. In the search of alternatives, some would turn to nutritional approach for cancer with the use of plant-based food which contains various nutrients and phytochemicals which could be helpful towards cancer.
Recent advances in the field of nutrition showed that bioactive compounds from plant-based food such as rutin, curcumins, tocotrienols could enhance the efficacy of chemotherapy and alleviate the side effects of chemotherapeutic drugs [4][5][6][7][8]. Naturally occurring carotenoids were also found to be associated with decreased risk of various cancer such as prostate breast, colorectal and ovarian cancer, mostly due to their antioxidant effect and effect in reducing carcinogenesis and regulating pathway which involves cell death and growth [9]. One of the bioactive compounds which was reported to possess anti-cancer activity was lycopene [10].
2. Anti-Cancer Activities of Lycopene
2.1. Cell Culture Studies
All reviewed articles reported positive outcome in terms of the anti-cancer property of lycopene. The first published study on the topic of interest occurred in 2005, when SK-Hep-1 cells, a highly invasive hepatoma cell were treated with 1, 2.5, 5, 10, 20 µmol/L of lycopene. The result came out whereby lycopene was found to be able to suppress the cancerous cell migration and invasion, in a bell-shaped manner. Such suppression was negatively correlated with the expression of nm23-H1, a metastasis suppressor which was enhanced by lycopene. The researchers explained that the bell-shaped manner of lycopene could be due to the possibility of autooxidation of lycopene at high concentration and thus, searching for the correct concentration was crucial [16]. Two years later, by studying using the same cell line, treatment with 1–10 µM of lycopene significantly inhibited cell invasion, MMP-9, NF-κB, Sp1, IGF-1R and ROS production in cells. One important message from this research was that the antioxidant property of lycopene was only responsible for a minor role in its anti-cancer property as the anti-cancer property of lycopene was retained even after incubation with hydrogen peroxide [17]. A similar outcome was observed when the same cell line was treated with 0.1–5 µM of lycopene, induced with TGF-β for metastasis, whereby there was a reduction in NOX4 gene expression, NOX, ROS, MMP-9, MMP-2 and inhibition of cancerous cell migration, invasion and adhesion activity [19].
The anti-cancer property of lycopene was not restricted to only hepatoma cell, but also breast cancer cell. Highly aggressive breast cancer cell (H-Ras MCF10A, MDA-MB231) which had undergone treatment with lycopene exhibited inhibition of cell invasion, migration and proliferation, accompanied by a reduction in ERK and Akt, suggesting that both signaling pathways might play roles in the anti-cancer property of lycopene. In ER/PR+ MCF-7, HER2+ SK-BR-3 and MDA-MB-468 cell lines, treatment with lycopene for 168 h led to inhibition of cell cycle progression in G0/G1 stage, increased PARP cleavage, ERK 1/2, p21, Bax, reduced cyclin D1, phosphorylation of Akt, mTOR and no change for Bcl-xL [23]. The anti-cancer effect of lycopene on breast cancer was significant and thus, suggesting its potential in the prevention of breast cancer.
In human colon cancer cells (HT-29 cells), treatment with lycopene reduced cancerous cell invasion, expression of MMP-7, GSK-3β, ERK 1/2, AP-1, β-catenin, phosphorylation of Akt while increasing E-cadherin stabilization [21]. Lycopene was found to enhance the autophagy ability of gastric cancer cell line (HGC-27 cell lines) as observed by increment in the expression of LC3-1 accompanied by phosphorylation of ERK while decreasing tumor weight in Balb/c nude mice models carrying HGC-27 cells which were being fed with 20, 30 and 60 mg/kg lycopene per day [63].
In an investigation whether lycopene could play roles in DNA damage, it was revealed that incubation of lymphocytes from human blood with 10, 20 and 40 µM/mL lycopene before X-irradiation did cause significant decrement in DNA damage. However, such effect was only restricted to treatment with lycopene before induction of DNA damage via X-irradiation as lycopene treatment after irradiation failed to show such DNA protective effect. The researchers did emphasize that low doses are useful as compared to high doses of lycopene in this research [64]. The anti-cancer effect of lycopene was also observed in pancreatic cancer cells (PANC-1 cell line) whereby significant reduction of ROS, NF-κB and anti-apoptotic biomarkers (cIAP1, cIAP2 and survivin) was detected while an increment of caspase-3 and Bax:Bcl-2 ratio was noticed [24]. These evidences pointed out that via regulation of apoptosis, lycopene could reduce cell viability of cancerous cells and thereby, exhibiting its potential as a useful compound in reducing the incidence of pancreatic cancer.
In mouse epidermal cell line, JBG P+ (JB6 C1 41-5a), pretreatment with lycopene for 5 days, followed by incubation with TPA, with or without lycopene for 14 days significantly reduced colony formation and KEAP1 mRNA, while upregulating mRNA of SOD1, GSR, GPX1, CAT, GCLC, GCLM, NQO-1, HMOX1, nuclear NRF2 localization, LC3 and p62 protein levels. For in vivo part of the study, mice which were subjected to DMBA and TPA faced lower incidence rate, multiplicity of cutaneous papillomas, lower degree of increment in epidermal thickness, invasion of benign papillomas, 8-OHdG and 4HNE levels when lycopene was administered. The mice also exhibited higher survival rate, GSH/GSSG ratio, SOD, GR, GPx, CAT and mRNA of SOD1, GSR, GPX1, CAT, GCLC, GCLM, NQO-1 and HMOX1. The researcher concluded that lycopene was more effective as a pretreatment, especially during the promotion phase of induced tumors and NRF2 was required for the observed effect of lycopene-induced prevention against tumors. The activation of NRF2 signaling pathway might be related to the degradation of KEAP1 by p62 via autophagy-lysosomal pathway [65].
There were two metabolites of lycopene which were under study for their anti-cancer effect, which include apo-10′-lycopenoic acid and apo-8′-lycopenal. Treatment of NHBE cells (human bronchial epithelial cells, BEAS-2B-immortalized normal bronchial epithelial cells and A549 (non-small cell lung cancer cells) with apo-10′-lycopenoic acid managed to increase p21, p27 protein levels and reduce cyclin E level which resulted in inhibition of cell cycle progression from G(1) phase to S phase. Apo-10′-lycopenoic acid also caused a significant increment of RAR-beta which is involved in the binding of retinoic acid (a biological active form of vitamin A). The evidence of anti-cancer activity of apo-10′-lycopenoic acid was strengthened when there was a significant reduction of tumor multiplicity observed in the A/J mouse model injected with NNK (induction) and supplemented with 10, 40, 120 mg/kg of apo-10′-lycopenoic acid [14]. In another independent study investigating the mechanism behind such anti-cancer activity, the result pointed out that apo-10′-lycopenoic acid may increase NRF2, HO-1, NAD(P)H dehydrogenase (quinone 1), GSTs, GCL and GSH levels in BEAS-2B cells, suggesting that lycopene may exhibit both anti-carcinogenic and antioxidant activity via NRF2 activation and induction of detoxifying enzymes [66]. The anti-cancer activity of apo-10′-lycopenoic acid was observed in human liver THLE-2 and HuH7 cells whereby increment in SIRT1 enzyme level, p21 and apoptosis activity accompanied by a reduction in cyclin D1 protein. Under the same study, C57BI/6J mice which were induced with diethylnitrosamine following a high fat diet, supplemented with 10 mg/kg apo-10′-lycopenoic acid for 24 weeks showed significant reduction in tumor multiplicity, volume and incidence. In terms of biomarkers, caspase-1, TNF-α, IL-6, NF-κB p65 protein expression, STAT3, Akt activation and cyclin D1 were suppressed significantly while an increment in SIRT1, deacetylation of SIRT1 and PARP cleavage were observed. In vivo experiment exhibited similar outcome as shown in the in vitro test and thus, the researcher concluded that apo-10′-lycopenoic acid had the potential in inhibiting high fat diet induced hepatic tumorigenesis via suppression of inflammation and SIRT1 signaling activation [25].
In a study investigating both lycopene and apo-8′-lycopenal (lycopene metabolite) using SK-Hep-1 cell line, treatment with 1, 2.5, 5, 10 µM apo-8′-lycopenal and 10 µM of lycopene significantly inhibited the invasion and migration of cancerous cell. Apo-8′-lycopenal reduced the expression of MMP-2, MMP-9, Rho GTPase via inhibition of ERK/p38 and PI3K-Akt pathway while increased expression of metastasis suppressor nm23-H1, TIMP-1, TIMP-2. One interesting point was mentioned by the researcher whereby the anti-cancer activity of apo-8′-lycopenal was higher than lycopene, suggesting that this metabolite could play an essential role in the observed anti-cancer property exhibited by lycopene [18]. In human HepG2 cells, treatment with 1, 5, 10 µM of apo-8′-lycopenal resulted in inhibition of cancerous cell invasion, migration and changes in biomarkers (increment in NRF2 accumulation, HO-1 and NQO-1, decrement in KEAP1). The researcher discovered that ERK/p38-NRF2 pathway may be involved in activation of phase II detoxifying enzyme expression (HO-1, NQO-1) and the time taken for NRF2 accumulation for lycopene was longer than apo-8′-lycopenal, indicating that this metabolite might partially be involved in chemopreventive effects of lycopene due to its relatively short NRF2 accumulation time [15] (Table 1).
Table 1. Summary of cell culture studies evaluating anti-cancer properties of lycopene.
| Compound | Subject | Experiment Design | Outcome | Reference |
|---|---|---|---|---|
| Lycopene (Lyc) | SK-Hep-1 cells (highly invasive hepatoma cell line) | Treatment with 1, 2.5, 5, 10, 20 µmol/L Lyc | ↓cell migration, invasion (bell-shaped manner) ↑nm23-H1 (bell-shaped manner) nm23-H1 and cell migration and invasion (-ve r) |
[16] |
| Treatment with 1–10 µM Lyc | ↓cell invasion, MMP-9, NF-κB, Sp1, IGF-1R, ROS | [17] | ||
| Treatment with 1, 2.5, 5, 10 µM Apo-8′-lycopenal (Lyc metabolite), 10 µM Lyc | (Lyc, Apo-8′-lyc)↓cell invasion, migration (Apo-8′-lyc) ↓MMP-2, -9, Rho GTPase, ERK/p38, PI3K-Akt ↑nm23-H1, TIMP-1,-2 |
[18] | ||
| Treatment with Lyc (0.1–5 µM), induced with TGF-β | ↓NOX4 mRNA, NOX, ROS, cell migration, invasion, adhesion activity, MMP-9, MMP-2 | [19] | ||
| H-Ras MCF10A, MDA-MB231 (highly aggressive breast cancer cell) | Treatment with Lyc | ↓cell invasion, migration, proliferation ↓ERK, Akt |
[20] | |
| HT-29 cells (human colon cancer cells) | Treatment with Lyc | ↓cell invasion, MMP-7, phosphorylation of Akt, GSK-3β, ERK ½, AP-1, β-catenin ↑E-cadherin stabilization |
[21] | |
| ER/PR+ MCF-7, HER2+ SK-BR-3, MDA-MB-468 cell lines | Treatment with Lyc (168 h) | inhibition of cell cycle progression G0/G1 ↑PARP cleavage, ERK1/2, p21, Bax ↓cyclin D1, Akt, mTOR ↔Bcl-xL |
[23] | |
| HGC-27 cell lines | Incubated with various conc of Lyc for 24, 48 or 72 h | ↑LC3-I, p-ERK | [63] | |
| Balb/c nude mice model | Injected with HGC-27 cells, fed with 20, 30, 60 mg/kg Lyc per d, oral | ↓tumour weight | ||
| Lymphocytes from human blood |
Incubation with 10, 20, 40 µM/mL Lyc, before and after X-irradiation at doses of 0.5, 1 and 2 Gy | ↓DNA damage Note: Lyc administration after irradiation, no effect Note: Low doses are useful |
[64] | |
| (Pancreatic cancer) PANC-1 cells | Treatment with 0.25, 0.5 µM for 24 h | ↓ROS, NF-κB, cIAP1, cIAP2, survivin ↑caspase-3, Bax:Bcl-2 |
[24] | |
| Mouse epidermal cell line, JBG P+ (JB6 Cl 41-5a) | Pretreatment with Lyc for 5 days, incubation with TPA, with or without Lyc for 14 days | ↓colony formation, (mRNA) KEAP1 ↑(mRNA) SOD1, GSR, GPX1, CAT, GCLC, GCLM, NQO-1, HMOX1, nuclear NRF2 localization, LC3, p62 |
[65] | |
| Mice | Subjected to DMBA (60 µg) dissolved in 0.2 mL topically on back, after 1 week, TPA (4 µg) twice a week for 32 weeks; Control group (1), 8 µmol Lyc/d since first week (2), 8 µmol Lyc/d from first wk to 4th week only (3), 8 µmol Lyc/d since fourth week (4), Acetone/d since fourth week (5): 32 weeks experiment | ↓incidence rate, multiplicity of cutaneous papillomas, increased in epidermal thickness, invasion of benign papillomas, 8-OHdG, 4HNE ↑survival rate, GSH/GSSG ratio, SOD, GR, GPx, CAT, (mRNA) SOD1, GSR, GPX1, CAT, GCLC, GCLM, NQO-1, HMOX1 Note: lycopene was more effective as a pretreatment and during promotion phase of induced tumors.NRF2 was required for the effect of lycopene-induced prevention against tumor |
||
| Apo-10′-lycopenoic acid, A-10-LA (Lyc metabolite) | NHBE cells (human bronchial epithelial cells), BEAS-2B-immortalized normal bronchial epithelial cells, A549 (non-small cell lung cancer cells) | Treatment with apo-10′-lycopenoic acid | ↓cyclin E inhibition of cell cycle progression G(1)→S ↑p21, p27, RAR beta |
[14] |
| A/J mouse model | NNK injection (induction) and supplemented (10, 40, 120 mg/kg of A-10-LA | ↓tumor multiplicity | ||
| BEAS-2B cells | Treatment with apo-10′-lycopenoic acid | ↑NRF2, HO-1, NAD(P)H dehydrogenase (quinone 1), GSTs, GCL, GSH | [66] | |
| Human liver THLE-2, HuH7 cells | Treatment with apo-10′-lycopenoic acid | ↑SIRT1, p21, apoptosis ↓cyclin D1 |
||
| C57BI/6J mice | Supplementation with A-10-LA (10 mg/kg) for 24 wks, high fat diet, induced with diethylnitrosamine | ↓tumor multiplicity, volume, incidence, caspase-1, TNF-α, IL-6, NF-κB p65, STAT3, Akt, cyclin D1 ↑SIRT1, PARP cleavage |
[25] | |
| Apo-8′-lycopenal (Lyc metabolite) | Human HepG2 cells | Treatment with 1, 5, 10 µM Apo-8′-lycopenal (Lyc metabolite), 10 µM Lyc | ↓cell invasion, migration ↑NRF2, HO-1, NQO-1 ↓KEAP1 |
[15] |
Note: ‘↑’ indicates increment; ‘↓’ indicates decrement; ‘↔’ indicates no change.
2.2. Animal Studies
Most of the reviewed articles reported a positive the outcome on anti-cancer activity of lycopene while only two studies reported that lycopene had no significant chemopreventive effect. The first study of anti-cancer activity of lycopene on animals took place as early in 1995, whereby high mammary tumor strain of SHN virgin mice were used. In this research, the mice were fed with either control diet or diet containing 5 × 10−5% of lycopene. It was discovered that mice fed with lycopene had suppressed mammary tumor development and reduced level of TYMS, serum FFA and prolactin [67]. Similar outcome was also reported in a study using rat mammary tumor model but the major difference was the application of lycopene-enriched tomato oleoresin (LETO) instead of pure lycopene. First, rats were injected with 10 mg/kg of LETO twice per week for two weeks, followed by induction with 7, 12-dimethyl-benz[a]anthracene (DMBA) and the injection of 10 mg/kg of LETO lasted for a total of 16 weeks. The administration of LETO managed to increase both plasma and hepatic lycopene and thus, rats which received such supplementation developed less and smaller tumor, proving the effectiveness of lycopene in protecting against mammary cancer [68].
Lycopene supplementation (1, 20 mg/kg BW; 2 times per week for 12 weeks) in nude mice injected with SK-Hep-1 cells managed to significantly reduce MMP-2, VEGF, PCNA, MMP-9, suppress tumor metastasis, mean number of tumors, tumor cross-sectional area while increase nm23-H1. A dose-dependent relationship between lycopene and the observed effect was reported [22]. In Lewis lung carcinoma cells, the in vitro experiment on treatment with 10, 20 or 40 µM of lycopene caused increased IFNβ, IFNγ, IRF1, IRF7, CXCL9, CXCL10, pJAK and pSTAT3 mRNA expression while suppressed mRNA expression of DMNT3a, methylation levels of promoters (IRF1, IRF7) and PD-1 as induced by IFNγ via suppression in phosphorylation of Akt. There was no observable change in gene expression of DNMT1 and DNMT3b. Under the same study, an experiment on C57BL/6 mice with lycopene (40 mg/kg) administered intraperitoneally for 3 days resulted in the reduction of tumor volume, weight, IL-4, IL-10, gene expression of DMNT3a and methylation levels of promoters (IRF1, IRF7). There was a significant increment observed in IL-2, IFNγ, CD4+:CD8+ T cells ratio, percentage of IFNγ+/CD8+ T cells, percentage of perforin+/CD8+ T cell, percentage of granzyme B+/CD8+ T cell, gene expression of IFNβ, IFNγ, IRF1, IRF7, CXCL9 and CXCL10. Similar to the in vitro study, no change was observed in gene expression of DNMT1, DNMT3b, IRF3 and IRF8 [69].
The anti-cancer effect of lycopene was also observed in liver whereby significant reduction of GGT+, GST+ foci size and liver volume fraction occupied by foci were noticed in male weanling rats which had undergone induction using diethylnitrosamine (DEN) or 2-nitropropane (2-NP), followed by lycopene (300 mg/kg) administered intraperitoneally for 3 to 4 weeks. No change in the number or size of preneoplastic liver foci was detected and the researcher found out that such activity was unrelated to the antioxidant property of lycopene, but rather its effect in modulating CYP2E1 [70]. In resistant hepatocyte model of hepatocarcinogenesis Wistar rats, 70 mg/kg BW of lycopene increased liver carotenoid concentration and reduced number, size and area of GST+ preneoplastic lesions and hepatic DNA strand breakage. However, no effect was seen on incidence, total number and multiplicity of hepatocyte nodules [71]. In another similar study, lycopene was fed together with high fat diet after injection with DEN and lycopene was able to reduce the number of GST+ hepatic foci and PCNA, cyclin D1 levels, via inhibition of ERK pathways and NF-κB transcription. A significant increment was observed for HO-1 and NRF2 level whereby no change was detected for TNF-α, IL-1β, IL-12 and CYP2E1. Surprisingly, high fat diet feeding with tomato extract caused reduction in proinflammatory cytokines TNF-α, IL-1β, IL-12 and CYP2E1 enzyme, which were unaffected by lycopene. Thus, the researcher concluded that a combination of both tomato extract and lycopene was able to prevent hepatocarcinogenesis via a different mechanism [72]. In BCO2-knockout male mice, lycopene supplementation for 24 weeks in combination with high fat diet resulted in an increment in hepatic lycopene, miR-199a/b, miR214 and decrement in hepatocellular carcinoma incidence, multiplicity, endoplasmic reticulum stress-mediated unfolded protein response, ER(UPR) and oncogenic biomarkers such as Met mRNA, β-catenin and mTOR1. With the same experimental design but in wild type mice, lycopene significantly exhibited anti-cancer property, mainly by inhibition of proinflammatory signaling as seen in a significant reduction in phosphorylation of NF-κB p65, STAT3, IL-6 and suppressed inflammatory foci. The difference in terms of the observed anti-cancer outcome suggested that BCO2 expression may cause a difference in terms of the pathway taken to exhibit anti-cancer property [62].
In gastric cancer, male Wistar rats induced with N-methyl-N′-nitrosoguanidine (MNNG) + saturated NaCl, followed by lycopene administration resulted in reduced gastric carcinomas accompanied by increase in GSH and antioxidant enzymes GPx, GST and GR. The researcher proposed that the ability of lycopene in modulating antioxidant enzymes might be the major contributor to its anti-cancer activity [73]. In another independent experiment using the same animal model and similar experimental design, lycopene managed to reduce tumor burden, Bcl-2 levels and increase caspase-8, liver GSH, stomach GPx and GSH and GPx activities in liver and erythrocytes. No change was detected in stomach and erythrocytes GSH, liver and erythrocytes GPx, GPx activities in stomach, Bax and Bim levels [74]. The new found capability of lycopene in the modulation of apoptosis-associated protein, aside from its antioxidant property suggested that the mechanism behind its anti-cancer activity could be a combination of simultaneous activation of various pathways [26]. Recent discoveries pointed out that modulation of the immune system via cytokines and antibodies might play roles in the anti-cancer property of lycopene. Such claim arose from a study focusing on MNNG gastric cancer rat model whereby administration of 50, 100 or 150 mg/kg body weight of lycopene caused an increment in antioxidant enzymes as expected (SOD, CAT, GSH-Px) and increment in cytokines (IL-2, IL-4, IL-10, TNF-α) and antibodies (IgG, IgA, IgM). Noticeable reduction in MDA and IL-6 were detected under the same study. The researcher suggested that the upregulation of both antioxidant status and the immune system might play roles in the anti-cancer activity of lycopene [61].
In terms of ovarian cancer, in vitro study using OV-MZ-6 cells with lycopene treatment (2, 5 µM) caused a significant reduction in ITGA5 and pERK 1/2 and no change in ITGB1, total ERK and vimentin. Under the same study, there was an in vivo experiment using ovarian cancer-bearing mice which were separated to lycopene prevention and lycopene treatment group. In the prevention group, lycopene (0.75 mg/mL) was fed 2 weeks before implantation of cell-seeded hydrogels while in the treatment group, lycopene was fed 4 weeks after the implantation surgery. In the lycopene prevention group, lycopene significantly reduced metastatic load, Ki67, ITGA5B1, ITGA5, ILK, ITGB1, FAK, MMP-9. Reduction in serum and ascites CA125 and EMT markers in metastatic tissue were reported. The reduction in MMP-9 was restricted to only metastatic tissue but not tumor tissue. Lycopene had no effect upon tumor load, serum and ascites MMP-9. The in vitro study pointed out that the reduction of ITGA5 could be related to reduced pERK activity, suggesting that reduced activation of MAPK was the key to all these observed effects. In the lycopene treatment group, there was reduction in tumor load, Ki67, ITGA5 ITGA5B1, ascites CA125 but there was an increment in MMP-9. No significant change was reported for ITGA5, ILK, ITGB1, FAK, serum and ascites MMP-9 and serum CA125 [75]. Through this experiment, lycopene, when being consumed in a preventive manner was found to be effective towards metastatic cancerous tissue but not towards the primary tumor. On the other hand, even though lycopene treatment managed to reduce tumor load, its effect was relatively weaker to what had been observed in the prevention group. In laying hens, 200, 400 mg/kg per kg diet of lycopene was able to reduce incidence, number and size of ovarian tumor. The rate of adenocarcinoma and MDA level were lowered and these effects could be a result of the inhibition of NF-κB transcription and expression of STAT3. A noticeable increment was observed for NFE2 and HO-1. In this experiment, the antioxidant property of lycopene could contribute to its anti-cancer activity due to noticeable changes in oxidative stress markers [76].
In Sprague Dawley rats, induction using N-methylnitrosourea intrarectal for a week, followed by a week of lycopene administration resulted in reduced aberrant crypt foci development, suggesting the potential of lycopene in the prevention of colon carcinogenesis [77]. It was interesting to point out that often times, food source which was rich in lycopene such as tomato was more useful in the exhibition of anti-cancer activity. According to research done on F344/NSlc rats, feeding of diluted tomato juice with 17 ppm lycopene caused a significant reduction in colon cancer incidence while feeding of 17 ppm pure lycopene failed to exhibit such a result. In this study, the concentration of lycopene in tomato juice did make a difference as feeding with tomato juice with only 3.4 ppm resulted in non-detectable lycopene amount in colon mucosa, unlike the group fed with 17 ppm lycopene in diluted tomato juice, which had detectable amount of lycopene (0.02 µg/g) found in colon mucosa [78]. In a study using colon carcinogenesis Sprague Dawley rat model, lycopene treatment after induction by azoxymethane caused suppression of aberrant crypt foci, preneoplastic lesion and biomarkers such as COX-2 and iNOS expression [60]. Research on CD-1 mice in AOM-DSS model showed that lycopene administration (20, 50 mg/kg) could reduce inflammation incidence and positive rates of IGF-2, IGFBP3 at low dosage and IGFBP2 at high dosage. It could also increase lymphocyte infiltration but it caused variability in growth factor according to dosage. One noticeable variability was that at low dosage, even though there was a decrement in positive rates of IGF-2 and IGFBP3, a significant increment of positive rates was detected for IGF-1R, IGF2BP1 and IGFBP2. Similarly, for high dosage, there was a significant spike in positive rates of IGF-1, IGF-2 and IGFBP3 despite a significant reduction in IGFBP2. There was no change in the number of tumors or adenocarcinomas incidence but there was noticeable focal necrosis present in colonic tissue [79]. The anti-cancer effect of lycopene in this experiment could be said to be moderate as there were both positive and negative outcomes, accompanied by its ineffectiveness in reducing the number of tumors and adenocarcinomas incidence. One main point from this experiment was that lycopene could apparently help to suppress inflammation but its effect on colon tumorigenesis was still debatable.
Interestingly, in a multiorgan carcinogenesis B6C3F1 mice model, lycopene (25/50 ppm) administration for 21 weeks after combinational induction with diethylnitrosamine (DEN), N-methyl-N-nitrosourea (MNU) and 1,2-dimethylhydrazine (DMH) led to reduction in incidences and multiplicities of lung adenomas and carcinomas but the effect was restricted to male mice fed with 50 ppm lycopene and water. However, no effect was seen for female or aberrant crypt loci and tumors in both colon and kidney among groups [80]. This experiment pointed out that the chemopreventive effect of lycopene in multiorgan carcinogenesis model was limited and only effective for male, specifically for lung carcinogenesis.
There were two studies which reported the ineffectiveness of lycopene in exhibiting anti-cancer effect and one of such experiment occurred in 2001. In this experiment, hepatocellular carcinoma LEC rats were the subject under study and they were given diet containing 0.005% of lycopene from 6 weeks of age to 76 weeks age. The reported outcome of the study was that lycopene failed to cause any significant change in number, mean area, percentage area of GST-P-+ focal lesions in liver, Alpha-fetoprotein (AFP) and cumulative survival rates of rats. However, a depletion of iron concentration was noticed in liver. The researcher hence concluded that long term administration of lycopene had no effect in reducing risk of hepatocarcinogenesis in LEC rats [81]. Another research placed the focus on the difference between the chemopreventive effect of both pure lycopene and lycopene-rich tomato carotenoid oleoresin (TCO) in rat mammary tumor model. Rats were first supplemented with 250, 500 ppm lycopene or TCO, followed by initiation with N-methylnitrosourea (NMU) for 7 days, and the experiment lasted for 18 weeks. As a result, no significant change in tumor incidence, latency, multiplicity, volume or total tumors per group was reported for all groups. The researcher supported the fact that lycopene was ineffective in preventing breast cancer as reported by some epidemiological reports [82]. One point from this experiment worth mentioning was that supplementation with TCO managed to increase serum lycopene concentration in a higher manner than supplementation with pure lycopene under the same lycopene concentration (Table 2).
Table 2. Summary of animal studies evaluating anti-cancer properties of lycopene.
| Compound | Subject | Experiment Design | Outcome | Reference |
|---|---|---|---|---|
| Lycopene (Lyc) | High mammary tumor strain of SHN virgin mice | Control (1), 5 × 10−5% Lyc (2), AIN-76TM diet | ↓mammary tumor development, TYMS, serum FFA, prolactin | [67] |
| Sprague Dawley rats | N-methylnitrosourea (intrarectal, 1 wk), followed by administration of Lyc (1), lutein (2), α-carotene (3), β-carotene (4), palm carotene (5), daily gavage (wk 2 and wk 5) | ↓aberrant crypt foci development | [77] | |
| Male weanling rats | Induction of hepatocarcinogenesis by 6 × 100 mg/kg BW diethylnitrosamine (DEN)/100 mg/kg BW 2-nitropropane (2-NP), fed with 300 mg/kg β-carotene (1), canthaxanthin (2), astaxanthin (3), Lyc (4), 15,000 retinol equiv. excess vit A (5), 3-methycholanthrene (6) intraperitoneal, 3–4 wks | ↔No., size of preneoplastic liver foci ↓size of GGT+, GST+ foci, Liver volume fraction occupied by foci Note: modulate P-450 2E1, not antioxidant properties |
[70] | |
| Multiorgan carcinogenesis B6C3F1 mice model | Combined treatment with diethylnitrosamine (DEN), N-methyl-N-nitrosourea (MNU) and 1,2-dimethylhydrazine (DMH), Lyc + water: 25/50 ppm (1), Control (2), Lyc only: 25/50 ppm (3), 21 wks | ↓incidences and multiplicities of lung adenomas and carcinomas Note: restricted to male, G1 with 50ppm Lyc ↔aberrant crypt loci, tumors in colon and kidney among groups |
[80] | |
| F344/NSlc rats | 2 mg/ 4 mg N-methylnitrourea x 3 per wk (3 wks), plain water (1), 17 ppm Lyc (2), diluted tomato juice with 17 ppm Lyc (3), diluted tomato juice with 3.4 ppm Lyc (4) | (3) ↓colon cancer incidence, but not in (2) | [78] | |
| Hepatocellular carcinoma (HCC)LEC rats | Diet containing 0.005% Lyc (1), 1% TJ-9: crude extracts of 7 herbs (2), control (3) administered from 6 wks age to 76 wks age | ↔number, mean area and % area GST-P-+ focal lesions (liver, HCC); Note: TJ-9 had higher number of GST-P-+ lesion in HCC), AFP, cumulative survival rates ↓iron conc. in liver |
[81] | |
| N-methyl-N’-nitrosoguanidine (MNNG) and saturated NaCl (S-NaCl) induced Male Wistar rats | N-methyl-N’-nitrosoguanidine (MNNG) + saturated NaCl (1), MNNG + S-NaCl + Lyc (2), Lyc (3), Control (4) | ↓gastric carcinomas ↑GSH, GPx, GST, GR |
[73] | |
| MNNG + S-NaCl (1), MNNG + S-NaCl + Sallylcysteine (SAC) (2), MNNG + S-NaCl + Lyc (3), MNNG + S-NaCl + SAC + Lyc (4), chemoprevention agents (5–7), Control (8) | ↔GSH (stomach, erythrocytes), GPx (liver, erythrocytes), GPx activities (stomach), Bax, Bim ↑GSH (liver), GPx (stomach), GSH activities, GPx activities (liver, erythrocytes), caspase-8 ↓tumor burden, Bcl-2 |
[26] | ||
| Resistant hepatocyte (RH) model of hepatocarcinogenesis Wistar rats | 70 mg/kg BW lutein (1), Lyc (2), Control (3) | ↑liver carotenoid conc. ↔incidence, total number, multiplicity of hepatocyte nodules ↓No., size, area of GST+ preneoplastic lesions, hepatic DNA strand breakage |
[71] | |
| Colon carcinogenesis Sprague Dawley rat model | Induction by azoxymethane, followed by treatment with diallylsulfide (1), Lyc (2), theaflavin (3) | ↓aberrant crypt foci, preneoplastic lesion, COX-2, iNOS | [60] | |
| Nude mice | Supplementation 2× per wk (12 wks), with 1, 20 mg/kg BW Lyc, 20 mg/kg BW β-carotene; starting wk 2, injection with SK-Hep-1 cells via tail vein | ↓MMP-2, VEGF, tumor metastasis, mean no. of tumors, tumor cross-sectional area, PCNA, MMP-9 ↑nm23-H1 |
[22] | |
| Hepatocarcinogenesis in rat model | Injected with diethylnitrosamine (DEN) and fed with control diet or high fat diet (HFD) with or without Lyc or tomato extract | (HFD + Lyc) ↓no. of GST+ hepatic foci, PCNA, cyclin D1, ERKs, NF-κB ↔TNF-α, IL-1β, IL-12, CYP2E1 ↑HO-1, NRF2 |
[72] | |
| N-methyl-N′-nitrosoguanidine (MNNG) gastric cancer rat model | Control (1), 200 mg/kg BW MNNG + saturated NaCl (2), 200 mg/kg BW MNNG + saturated NaCl + 50 mg/kg BW Lyc (3) 200 mg/kg BW MNNG + saturated NaCl + 100 mg/kg BW Lyc (4) 200 mg/kg BW MNNG + saturated NaCl + 150 mg/kg BW Lyc (5) | ↑SOD, CAT, GSH-Px, IL-2, IL-4, IL-10, TNF-α, IgG, IgA, IgM ↓MDA, IL-6 |
[61] | |
| BCO2-knockout and wild-type male mice | Lyc supplementation (100 mg/kg diet, 24 wks), induced by high fat diet | (BCO2-KO) ↑hepatic Lyc, miR-199a/b, miR214 ↓hepatocellular carcinoma incidence, multiplicity, ER(UPR), Met mRNA, β-catenin, mTORC1 (Wild type) ↓NF-κB p65, STAT3, IL-6, inflammatory foci |
[62] | |
| (In vitro) OV-MZ-6 cells | Treatment with 2, 5 µM Lyc | ↓ITGA5, pERK 1/2 ↔ITGB1, tERK, vimentin |
[75] | |
| (In vivo) Ovarian cancer-bearing mice | Prevention Gp: Placebo (1), Lyc (2) Treatment Gp: Placebo (1), Lyc (2), Lyc + Taxol (3), Taxol + Platin (4), Platin (5), Lyc + Taxol + Platin (6) Concentration of Lyc: 0.75 mg/mL |
(Lyc Prevention Gp) ↓metastatic load, Ki67, ITGA5B1, ITGA5, ILK, ITGB1, FAK, MMP-9, serum and ascites CA125, EMT markers in metastatic tissue (Note: MMP-9 restricted to metastatic tissue, not tumor tissue) ↔tumor load, serum and ascites MMP-9 (Lyc Treatment Gp) ↓tumor load, Ki67, ITGA5, ITGA5B1, ascites CA125 ↑MMP-9 ↔ILK, ITGB1, FAK, serum and ascites MMP-9, serum CA125 |
||
| Laying hens | Control (1), 200 mg/kg per kg diet Lyc (2), 400 mg/kg per kg diet Lyc (3) | ↓incidence, no. and size ovarian tumor, rate of adenocarcinoma, MDA, NF-κB, STAT3 ↑NFE2, HO-1 |
[76] | |
| (In vitro) Lewis lung carcinoma (LLC) cells | Control (1), Lyc: 10 µM (2), Lyc: 20 µM (3), Lyc: 40 µM (4) | ↑(mRNA) IFNβ, IFNγ, IRF1, IRF7, CXCL9, CXCL10, pJAK, pSTAT3 ↓(mRNA) DMNT3a, methylation levels of promoters (IRF1, IRF7), PD-1 due to IFNγ, pAkt ↔(mRNA) DNMT1, DNMT3b |
[69] | |
| (In vivo) C57BL/6 mice | Control (1), Anti PD-1, 6 mg/kg (2), Lyc, 40 mg/kg (3), Anti-PD-1 + Lyc (4); intraperitoneal, 3 days, 4 times | ↓tumor volume, weight, IL-4, IL-10, (mRNA) DMNT3a, methylation levels of promoters (IRF1, IRF7) ↑IL-2, IFNγ, CD4+:CD8+, % IFNγ+/CD8+ T cell, % perforin+/CD8+ T cell, % granzyme B+/CD8+ T cell, (mRNA) IFNβ, IFNγ, IRF1, IRF7, CXCL9, CXCL10 ↔(mRNA) IRF3, IRF8, DNMT1, DNMT3b |
||
| CD-1 mice in AOM-DSS model | Normal (1), AOM+DSS control (2), (Bifidobacterium longum) BF + AOM + DSS (3), BF + Lyc 20 mg/kg + AOM + DSS (4), BF + Lyc 50 mg/kg + AOM + DSS (5), Lyc 20 mg/kg + AOM + DSS (6), Lyc 50 mg/kg + AOM + DSS (7), Metformin + AOM + DSS (8) |
↑positive rates of IGF-1, IGF-2 (high dose), IGF-1R, IGF2BP1, IGFBP2 (low dose), IGFBP3 (high dose), lymphocyte infiltration ↓inflammation incidence, positive rates of IGF-2 (low dose), IGFBP2 (high dose), IGFBP3 (low dose) ↔No. of tumors, adenocarcinomas incidence Note: presence of focal necrosis |
[79] | |
| Lycopene-Enriched Tomato Oleoresin (LETO) | Rat mammary tumor model | Induced with 7, 12-dimethyl-benz[a]anthracene (DMBA) 2 wks, followed by injection of 10mg/kg LETO (1), β-carotene (2), control (3) twice per wk, 16 wks | ↑plasma, hepatic Lyc ↓tumors, tumor area |
[68] |
| Supplementation with 250 ppm Lyc (1), 500 ppm (2), 250 ppm lycopene-rich tomato carotenoid oleoresin (TCO) (3), 500 ppm TCO (4), control (5) followed by initiation with N-methylnitrosourea (NMU) (7 days) 18 wks experimentation | ↔tumor incidence, latency, multiplicity, volume, total tumors per group Note: supplementation with TCO ↑serum Lyc conc. > supplementation with pure Lyc |
[82] |
Note: ‘↑’ indicates increment; ‘↓’ indicates decrement; ‘↔’ indicates no change.
2.3. Clinical Trials
A total of 15 articles involving the clinical trials on the anti-cancer effect of lycopene were reviewed and it was reported that 11 out of these 15 studies reported positive outcomes and four of them reported that lycopene had no effect upon reducing the risk of cancer. The first related cohort study came in 1995 whereby 47,894 human subjects who were initially free of diagnosed cancer were recruited in 1986 and given validated semiquantitative food-frequency questionnaire as a mean of dietary assessment. Follow-up questionnaires were given in 1988, 1990 and 1992 and the data collected were analyzed. Data analysis pointed out that only lycopene was found to be able to reduce the risk of prostate cancer. Four food items which were known for their high lycopene content such as tomatoes, tomato juices, tomato sauces and pizza were also able to reduce risk of prostate cancer. The combined intake of these four items managed to establish an inverse association with risk of prostate cancer [83]. The researcher then suggested that lycopene or tomato-based food might be beneficial for prostate cancer and this became the first evidence of anti-cancer property of lycopene among humans. A similar outcome had been reported in another cohort study comprised of 47,365 participants who were given dietary questionnaire for dietary assessment in 1986, 1990 and 1994. The pooled analysis showed that lycopene was able to reduce the risk of prostate cancer but the association was considered moderate after controlling for fruits and vegetable consumption and olive oil consumption (marker for Mediterranean diet) [84]. In terms of plasma lycopene, a case–control study nested within a prospective Health Professionals Follow-up Study with 450 incident prostate cancer cases reported that higher plasma lycopene could reduce risk of prostate cancer but such association was only restricted to older participants without family history [85].
In a clinical trial, 26 male patients with prostate cancer with 14 being in stage T1 and 12 being in stage T2 were recruited to participate in a trial examining the potential of lycopene as a chemopreventive agent. Fifteen mg of lycopene supplementation managed to reduce plasma prostate-specific antigen (PSA) and increase connexin 43, a component of gap junction which allows intercellular communication between cells. There was no significant change in Bcl-2 or Bax. The researchers mentioned that lycopene supplementation could possibly help in controlling the growth of prostate cancer. However, the small sample size was also the major limitation of this study, causing the reliability of the result to be questionable [86]. Another randomized placebo-controlled study shared a similar outcome whereby 30 mg of lycopene per day among 32 patients with localized prostate adenocarcinoma significantly reduced serum PSA and leukocyte 8OHdG while increased both serum, prostate lycopene concentration and apoptotic index of hyperplastic and neoplastic cells. These evidences pointed out that lycopene could reduce DNA damage occurred in both leukocyte and prostate tissue but whether such reduction could be beneficial or detrimental for cancer cells still requires further research [87]. Decrement of PSA was also observed in a nutritional intervention among 79 prostate cancer patients but instead of pure lycopene, lycopene rich tomatoes were used for the study. Subjects were fed with tomato products containing 30 mg of lycopene throughout the whole study and a significant reduction in PSA level was detected [88].
Meta-analysis of 11 cohort studies and 6 nested case–control studies pointed out that high tomato intake was negatively correlated with the incidence of prostate cancer and lycopene had a modest effect in the prevention of prostate cancer [89]. The result from this meta-analysis was supported by another meta-analysis involving 26 studies with 17,517 cases of prostate cancer from 563,299 participants whereby lycopene (9–21 mg/day) or plasma lycopene (2.17–85 µg/dL) was able to reduce the risk of prostate cancer [90].
Apart from prostate cancer, lycopene seemed to be able to help with breast cancer, as reported in a pooled analysis of 18 prospective cohort studies in 2012 using the interval collapsing method. In this study, lycopene was found to have a protective effect towards ER-/PR+ or ER−/PR− breast cancer [91]. In a study involving 521 an women with breast cancer, analysis of plasma using HPLC showed that there was inverse association between serum lycopene and risk of breast cancer among premenopausal women and all ER/PR subtypes [92]. In lung cancer, there was a nonlinear dose-dependent association between lung cancer incidence and plasma lycopene as reported in a meta-analysis analyzing 17 prospective studies with 3603 cases involving 458,434 participants [93]. The inverse association mentioned was even stronger at low plasma lycopene concentration (20 µg/100 mL) and a weaker association above this concentration.
The first study to report non-significance results occurred in 1990. It was a nested case–control study whereby serum was obtained from 25,802 persons in 1974 were used. Serum of 103 men who developed prostate cancer during the subsequent 13 years was compared with the serum of 103 control subjects matched for age and race. There was no significant association between serum lycopene and risk of prostate cancer [94]. A similar result was obtained in the study involving 209 prostate cancer cases with 228 control, both black and white men in the US aged between 40 and 79 years old. Analysis of serum carotenoids revealed that lycopene had inverse association with prostate cancer which failed to reach statistical significance. Lycopene only showed significant inverse association particularly for aggressive disease [95]. In terms of lycopene consumption, a cohort study with 6.3 years of follow-up involving 58,279 men aged 55–69 years old (642 prostate cancer cases) showed that lycopene consumption had no effect upon the risk of prostate cancer via dietary assessment using semi-quantitative food-frequency questionnaire [96]. A 3-month randomized, double blinded clinical trial involving 69 men with a a favourable risk for prostate cancer who took 30 mg of lycopene per day yielded no change in IGF-1 and COX-2 levels in their prostate tissue gene expression [97]. The main interest of this experiment was not focusing on the chemopreventive effect of lycopene, but rather the effect of lycopene in modulating biomarkers related to cancer and inflammation. Thus, the outcome of this research was insufficient to justify the fact that lycopene was not effective in exhibiting anti-cancer property as pathway taken to suppress cancer cell was not only restricted to the parameters under study (Table 3).
Table 3. Summary of clinical trials evaluating anti-cancer properties of lycopene.
| Compound | Subject | Experiment Design | Outcome | Ref |
|---|---|---|---|---|
| Lycopene (Lyc) | 47,894 human subjects initially free of diagnosed cancer | Validated semiquantitative food-frequency questionnaire | ↓risk of non-stage A1 prostate cancer | [83] |
| 26 male patients with prostate cancer, 14 stage T1, 12 stage T2 | Control (1), 15 mg Lyc (2) | ↓plasma prostate-specific antigen (PSA) ↑connexin 43 ↔Bcl-2, Bax Note: sample size is relatively small |
[86] | |
| 47,365 participants | Dietary questionnaires | ↓risk of prostate cancer Note: moderate association |
[84] | |
| 32 patients with localized prostate adenocarcinoma | Randomized placebo-controlled study: 30 mg Lyc/day | ↑serum and prostate Lyc conc., apoptotic index (hyperplastic and neoplastic cells) ↓serum PSA, leukocyte 8OHdG |
[87] | |
| 58,279 men aged 55–69 yrs: 642 prostate cancer cases | Cohort study, 6.3 yrs follow-up, semi-quantitative food-frequency questionnaire | ↔risk of prostate cancer | [96] | |
| 69 men with favourable risk prostate cancer | 3-month randomized, double blinded clinical trial: 30 mg/day Lyc (1), 3 g/day fish oil (2), placebo (3) | ↔IGF-1, COX-2 | [97] | |
| 11 cohort studies, 6 nested case–control studies | Meta-analysis | OR < 1 (high tomato intake and incidence of prostate cancer) Modest effect in prevention of prostate cancer |
[89] | |
| 26 studies with 17,517 cases of prostate cancer, from 563,299 participants | Meta-analysis | ↓risk of prostate cancer (Lyc: 9–21 mg/day; plasma Lyc: 2.17–85 µg/dL) | [90] | |
| 18 prospective cohort studies in 2012 | Pooled analysis (interval collapsing method) | Protective effect towards ER−/PR+ or ER−/PR− breast cancer | [91] | |
| Plasma Lycopene | 25,802 persons: 103 men with prostate cancer, 103 men as control | Analysis of serum | ↔risk of prostate cancer | [94] |
| 209 prostate cancer cases, 228 control, Black and white men in US (40–79 yrs old) | Analysis of serum carotenoids | ↔risk of prostate cancer, only useful particularly for aggressive disease Note: insignificant inverse association (serum Lyc and prostate cancer) |
[95] | |
| 450 incident prostate cancer cases | Case-control study nested within prospective Health Professionals Follow-up Study | ↓risk of prostate cancer Note: restricted to older participants, without family history |
[85] | |
| 521 women with breast cancer | Analysis of serum using HPLC | ↓risk of breast cancer among premenopausal women and all ER/PR subtypes | [92] | |
| 17 prospective studies with 3603 cases, 458,434 participants | Meta-analysis | Nonlinear dose-dependent (lung cancer and plasma Lyc) Note: stronger inverse association at low plasma Lyc conc.) |
[93] | |
| Lycopene-rich tomato | 79 prostate cancer patients | Nutritional intervention: tomato products with 30 mg Lyc (1), tomato products + selenium, omega-3 fatty acids, soy isoflavones, grape/pomegranate juice and green/black tea (2), Control (3) | ↓PSA level | [88] |
Note: ‘↑’ indicates increment; ‘↓’ indicates decrement; ‘↔’ indicates no change.
Results from cohort studies had been conflicting whereby reports from studies with large sample sizes inclined towards a direction whereby lycopene was not able to reduce risk of cancer or lycopene could only have moderate effect on cancer risk reduction [83][84][94][95][96]. The outcome from meta-analysis of cohort and case–control studies was positive whereby it was reported that lycopene could reduce risk of prostate cancer, lung cancer and breast cancer, especially at low plasma lycopene concentration [89][90][91][93]. High level of evidence from randomized controlled trials suggested that lycopene could be beneficial for cancer as seen in increment in apoptotic index among hyperplastic and neoplastic cells and suppression of PSA in prostate cancer patients [86][87][88]. However, in randomized controlled trials, lycopene failed to cause any significant change towards Bax protein and IGF-1, as opposed to what had been shown in cell culture and animal studies [86][97]. Such limited evidence from randomized controlled trials could not help to deduce whether lycopene was effective in exhibiting anti-cancer activity among human.
3. Selective Anticancer Activities of Lycopene
3.1. Carotenoids of the Same Kind with Different Fate (Lycopene and Beta-Carotene)
Both lycopene and beta-carotene are classified under the same carotenoid family. Lycopene is an aliphatic hydrocarbon with molecular formula C40H56. It is dark red in color with waxy consistency found commonly in tomatoes. It is an open chain polyene with 13 double bonds which has the longest chain length as compared to other carotenoids due to the linear array arrangement of its 11 conjugated carbon-carbon double bonds. Unlike beta carotene, it does not possess any vitamin A activity due to its symmetrical planarity [28]. Beta-carotene is also a member of carotenoid family which is often seen as a red and orange colored pigment. It shares the same chemical formula with lycopene and it is the major carotenoids in human diet. It is a significant source of vitamin A [115] with the highest provitamin A activity and its deficiency is highly similar to that of vitamin A deficiency, such as blindness and xerophthalmia [116].
In comparison, both lycopene and beta-carotene are antioxidants, with lycopene being the best of carotenoids in an in vitro experiment setting. However, in vitro experiment setting showed that beta-carotene could be prooxidative, given at a high concentration and partial pressure but there was no reported evidence of lycopene being prooxidative. Thus, beta-carotene was found to be able to contribute to smoking oxidation products while there was no reported data for lycopene. Lycopene does not possess provitamin A activity and therefore, there was no evidence of the conversion of lycopene to retinoids, unlike beta-carotene. One interesting point worth mentioning is that beta carotene was often involved with a bad reputation of increasing the risk of lung cancer [117].
Beta-carotene first gained its bad reputation in lung cancer when several large intervention trials (ATBC, CARET) reported that beta-carotene supplementation was correlated with adverse effects in smokers. In heavy smokers, beta-carotene supplementation seemed to increase lung cancer incidences among them while having no protective effects towards nonsmokers and former smokers [118][119]. Two large prospective cohort studies focusing on epidemiological data reached similar consensus whereby beta-carotene was not associated with lung cancer risk in never smokers [120] while beta-carotene showed dual association within subjects whereby beta-carotene actually increased risk of lung cancer in smokers [121]. One research pointed out that although there was inverse risk association of lung cancer for beta-carotene in nonsmokers, it was statistically insignificant.
A few hypotheses were proposed for the situation observed in smokers. Oxidative stress was one of them whereby it was speculated that beta-carotene whenever present in high concentration in lungs may have the tendency to have prooxidative activity, which eventually leads to oxidative DNA damage. This could set off the co-carcinogenic action of beta-carotene via epigenetic mechanism which were discovered in rats whereby high doses of beta-carotene induced several cytochrome p450 (CYP) enzymes. Beta-carotene is a well-known precursor of retinoic acid, which is the main component behind retinoic acid signaling pathways which governs cell proliferation and differentiation. Another hypothesis came in whereby it was proposed that in places with high oxygen concentration such as lungs, high concentration of beta carotene could lead to oxidative cleavage or degradation products such as apocarotenals. This could cause disruption towards retinoic acid signaling pathways which sets off the carcinogenesis process [122].
The same situation surprisingly could not be applied to lycopene. For lycopene, increased intake of lycopene led to a significant reduction of cancer risk, even in smokers [123] Another study revealed one interesting point whereby smoking failed to alter the concentration of lycopene but most of the other carotenoids’ concentrations were affected [124]. Thus, it was deduced that the anti-cancer of lycopene would be more robust when it comes to lung cancer prevention and may play a special role in lung cancer prevention [125].
3.2. Lycopene Is Selective against Lung Adenomas and Carcinomas
The ability of lycopene in terms of anti-cancer activity could be selective as observed in an experiment using a multiorgan carcinogenesis mice model. In this model, only male mice administered with 50 ppm of lycopene had lower incidence and multiplicities of lung adenomas and carcinomas while having no significant change found in tumors present in colon and kidney. Such evidence suggested that the anti-cancer activity behind lycopene could be highly effective and selective towards tumor cells in lungs, rather than any other part of the bodies but such a hypothesis required more research and scientific evidence. As compared to beta-carotene, which is classified under the same carotenoids family as lycopene, lycopene seemed to be able to suppress cancerous cells in lungs instead of increasing the incidence of lung cancer like how beta-carotene did [118][121][126]. Observed effects on males could indirectly provide a direction whereby lycopene may modulate testosterone levels, or more specifically, suppress serum testosterone [127].With regard to the reason behind the selective ability of lycopene towards lungs, it could be related to the role of retinoic acid in lungs and the types of epithelial cells present. Retinoic acid had long been known to be beneficial for epithelial cells as it is essential for epithelial cell differentiation. Severe deficiency in vitamin A was related to the formation of squamous metaplastic lesions [128] and retinoic acid was found to play a major role in lung development as retinoic acid signaling influences lung specification, branching, morphogenesis and alveolarization. Developing lungs are highly sensitive to retinoic acid levels as retinoid acid signaling could affect mesenchymal gene expression [129]. This could be related to the selective ability of both lycopene and beta-carotene towards lungs as retinoic acid plays major role in lungs development and slight change in retinoic acid could impact lungs greatly. In terms of epithelial cells, the distinct epithelial cells found in alveoli, specifically squamous epithelial cells which become the barrier for gaseous exchange, had greater potential for proliferation and thus, higher possibility for being cancerous as one of the most common cell origins of lung cancers are squamous cell carcinomas [130]. Combining these information led to a hypothesis whereby the selective ability of lycopene towards lung cells is a combinational result of the high possibility of carcinogenesis of squamous cells in lungs and the high sensitivity of these cells towards retinoic acid change.
One of the metabolites of lycopene, acycloretinoic acid is actually an analog of retinoic acid and it was found to be able to activate retinoic acid receptor (RAR). However, it was considered as a weak activator of RAR as the concentration required for activation needs to be much higher than that of all-trans retinoic acid, ATRA (the common activator of RAR) [131][132]. Researchers found that testosterone may have an effect on RAR signaling in prostate carcinoma cells whereby testosterone could increase RAR alpha and gamma expression, which could be attenuated by retinoic acid [133]. The complex interaction behind lycopene, its metabolites, RAR and testosterone may be the reason be related to selective ability of lycopene towards prostate cancer. The attenuation of RAR expression by retinoic acid was considered abnormal as retinoic acid was known for its anti-cancer activity via activation of RAR or RXR [134]. Though RAR had been known for its tumor suppressive ability, such a statement could not be applied to all cancerous cell lines as high expression of RAR was correlated with the progression of breast cancer and colorectal cancer [135][136]. Such an observation could be related to the difference in terms of the involvement of different signaling pathways behind the pathogenesis of various cancer. One of the hypotheses proposed would be RAR expression may be beneficial for some cancerous cells and detrimental at the same time, depending on the nature of pathogenesis of cancer as RAR and RXR were the main receptors behind RA response elements (RARE), which is essential for cell growth and development, regardless of whether the cell is normal or cancerous [137]. The difference observed between lycopene and beta-carotene could be related to the nature of lycopene metabolite, acycloretinoic acid being a weak activator of RAR as compared to ATRA (metabolite of beta-carotene). In hypothesis, given the same concentration of both metabolites, ATRA would be more likely to cause high expression of RAR, which could be beneficial for certain cancerous cells. In contrast, acycloretinoic acid would be less likely to behave as such, leading to an average expression of RAR which could neither be beneficial nor detrimental to cancerous cells.
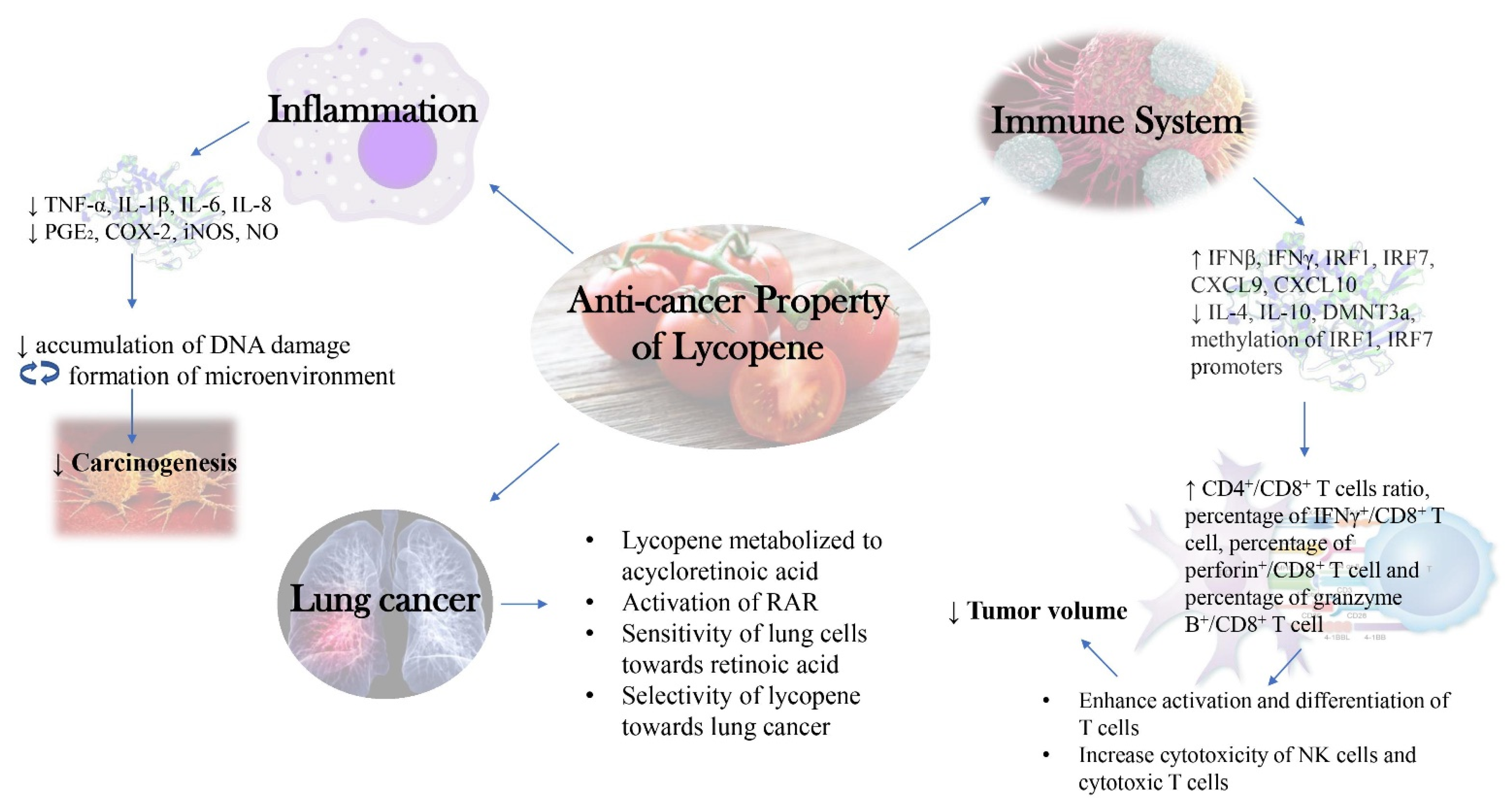
A summarized diagram depicting the new insights into the molecular mechanism behind anti-cancer activity of lycopene as discussed in this research was presented below (Figure 1).

Figure 1. New Insights into Molecular Mechanism behind Anti-cancer Activity of Lycopene.
References
- Hassanpour, S.H.; Dehghani, M. Review of cancer from perspective of molecular. J. Cancer Res. Pract. 2017, 4, 127–129, doi.:10.1016/j.jcrpr.2017.07.001.
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2019, 54, 407–419, doi:10.3892/ijo.2018.4661.
- Mohan, G.; T. P., A.H.; A. J., J.; K. M., S.D.; Narayanasamy, A.; Vellingiri, B. Recent advances in radiotherapy and its associated side effects in cancer—A review. J. Basic Appl. Zool. 2019, 80, doi:10.1186/s41936-019-0083-5.
- Subramaniam, S.; Selvaduray, K.R.; Radhakrishnan, A.K. Bioactive compounds: Natural defense against cancer? Biomolecules 2019, doi:10.3390/biom9120758.
- Siti, H.N.; Jalil, J.; Asmadi, A.Y.; Kamisah, Y. Roles of rutin in cardiac remodeling. J. Funct. Foods 2020, 64, 103606, doi:10.1016/j.jff.2019.103606.
- Gui, J.S.; Jalil, J.; Jubri, Z.; Kamisah, Y. Parkia speciosa empty pod extract exerts anti-inflammatory properties by modulating NFκB and MAPK pathways in cardiomyocytes exposed to tumor necrosis factor-α. Cytotechnology 2019, 71, 79–89, doi:10.1007/s10616-018-0267-8.
- Jalil, J.; Attiq, A.; Hui, C.C.; Yao, L.J.; Zakaria, N.A. Modulation of inflammatory pathways, medicinal uses and toxicities of uvaria species: Potential role in the prevention and treatment of inflammation. Inflammopharmacology 2020, 1–24, doi:10.1007/s10787-020-00734-2.
- Mohd Aluwi, M.F.F.; Rullah, K.; Haque, M.A.; Yamin, B.M.; Ahmad, W.; Amjad, M.W.; Leong, S.W.; Fahmizar, N.A.; Jalil, J.; Abas, F.; et al. Suppression of PGE2 production via disruption of MAPK Phosphorylation by unsymmetrical dicarbonyl curcumin derivatives. Med. Chem. Res. 2017, 26, 3323–3335, doi:10.1007/s00044-017-2025-4.
- Rowles, J.L., III; John, W.Erdman, J. Carotenoids and their role in cancer prevention. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158613.
- Mehta, N.; Patani, P.; Singhvi, I. A review on tomato lycopene. Int. J. Pharm. Sci. Res. 2018, 9, 916–923, doi:10.13040/IJPSR.0975-8232.9(3).916-23.
- Zechmeister, L.; LeRosen, A.L.; Went, F.W.; Pauling, L. Prolycopene, a naturally occuring stereoisomer of lycopene. Proc. Natl. Acad. Sci. 1941, 27, 468–474, doi:10.1073/pnas.27.10.468.
- Nguyen, M.L.; Schwartz, S.J. Lycopene: Chemical and biological properties. Food Technol. 1999, 53, 38–45.
- Arathi, B.P.; Sowmya, P.R.-R.; Vijay, K.; Baskaran, V.; Lakshminarayana, R. Biofunctionality of carotenoid metabolites: An insight into qualitative and quantitative analysis. In Metabolomics Fundamentals and Applications; IntechOpen: London, UK, 2016. doi:10.5772/66210.
- Lian, F.; Smith, D.E.; Ernst, H.; Russell, R.M.; Wang, X.D. Apo-10′-lycopenoic acid inhibits lung cancer cell growth in vitro, and suppresses lung tumorigenesis in the A/J mouse model in vivo. Carcinogenesis 2007, 28, 1567–1574, doi:10.1093/carcin/bgm076.
- Yang, C.M.; Huang, S.M.; Liu, C.L.; Hu, M.L. Apo-8′-lycopenal induces expression of HO-1 and NQO-1 via the ERK/P38-Nrf2-ARE pathway in human HepG2 Cells. J. Agric. Food Chem. 2012, 60, 1576–1585, doi:10.1021/jf204451n.
- Huang, C.-S.; Shih, M.-K.; Chuang, C.-H.; Hu, M.-L. Lycopene inhibits cell migration and invasion and upregulates Nm23-H1 in a highly invasive hepatocarcinoma, SK-Hep-1 Cells. J. Nutr. 2005, 135, 2119–2123, doi:10.1093/jn/135.9.2119.
- Huang, C.S.; Fan, Y.E.; Lin, C.Y.; Hu, M.L. Lycopene inhibits matrix metalloproteinase-9 expression and down-regulates the binding activity of nuclear factor-kappa B and stimulatory protein-1. J. Nutr. Biochem. 2007, 18, 449–456, doi:10.1016/j.jnutbio.2006.08.007.
- Yang, C.M.; Hu, T.Y.; Hu, M.L. Antimetastatic effects and mechanisms of apo-8ʹ-lycopenal, an enzymatic metabolite of lycopene, against human hepatocarcinoma SK-Hep-1 Cells. Nutr. Cancer 2012, 64, 274–285, doi:10.1080/01635581.2012.643273.
- Jhou, B.Y.; Song, T.Y.; Lee, I.; Hu, M.L.; Yang, N.C. Lycopene inhibits metastasis of human liver adenocarcinoma SK-Hep-1 cells by downregulation of NADPH oxidase 4 protein expression. J. Agric. Food Chem. 2017, 65, 6893–6903, doi:10.1021/acs.jafc.7b03036.
- Koh, M.S.; Hwang, J.S.; Moon, A. Lycopene inhibits proliferation, invasion and migration of human breast cancer cells. Biomol. Ther. 2010, 18, 92–98, doi:10.4062/biomolther.2010.18.1.092.
- Lin, M.C.; Wang, F.Y.; Kuo, Y.H.; Tang, F.Y. Cancer chemopreventive effects of lycopene: Suppression of MMP-7 expression and cell invasion in human colon cancer cells. J. Agric. Food Chem. 2011, 59, 11304–11318, doi:10.1021/jf202433f.
- Huang, C.-S.; Liao, J.-W.; Hu, M.-L. Lycopene inhibits experimental metastasis of human hepatoma SK-Hep-1 cells in athymic nude mice. J. Nutr. 2008, 138, 538–543, doi:10.1093/jn/138.3.538.
- Takeshima, M.; Ono, M.; Higuchi, T.; Chen, C.; Hara, T.; Nakano, S. Anti-proliferative and apoptosis-inducing activity of lycopene against three subtypes of human breast cancer cell lines. Cancer Sci. 2014, 105, 252–257, doi:10.1111/cas.12349.
- Jeong, Y.; Lim, J.W.; Kim, H. Lycopene Inhibits reactive oxygen species-mediated Nf-Kb signaling and induces apoptosis in pancreatic cancer cells. Nutrients 2019, 11, doi:10.3390/nu11040762.
- Ip, B.C.; Hu, K.Q.; Liu, C.; Smith, D.E.; Obin, M.S.; Ausman, L.M.; Wang, X.D. Lycopene metabolite, apo-10′-lycopenoic acid, inhibits diethylnitrosamine-initiated, high fat diet-promoted hepatic inflammation and tumorigenesis in mice. Cancer Prev. Res. 2013, 6, 1304–1316, doi:10.1158/1940-6207.CAPR-13-0178.
- Velmurugan, B.; Nagini, S. Combination chemoprevention of experimental gastric carcinogenesis by S-allylcysteine and lycopene: Modulatory effects on glutathione redox cycle antioxidants. J. Med. Food 2005, 8, 494–501, doi:10.1089/jmf.2005.8.494.
- Wang, X.D. Lycopene metabolism and its biological significance. Am. J. Clin. Nutr. 2012, 96, 1214S–1222S, doi:10.3945/ajcn.111.032359.
- Srivastava, S.; Srivastava, A.K. Lycopene; Chemistry, Biosynthesis, Metabolism and Degradation under Various Abiotic Parameters. J. Food Sci. Technol. 2015, 41–53, doi:10.1007/s13197-012-0918-2.
- Stahl, W.; Sies, H. Uptake of lycopene and its geometrical isomers is greater from heat- processed than from unprocessed tomato juice in humans. J. Nutr. 1992, 122, 2161–2166, doi:10.1093/jn/122.11.2161.
- Gärtner, C.; Stahl, W.; Sies, H. Lycopene is more bioavailable from tomato paste than from fresh tomatoes. Am. J. Clin. Nutr. 1997, 66, 116–122, doi:10.1093/ajcn/66.1.116.
- Riedl, J.; Linseisen, J.; Hoffmann, J.; Wolfram, G. Some dietary fibers reduce the absorption of carotenoids in women. J. Nutr. 1999, 129, 2170–2176, doi:10.1093/jn/129.12.2170.
- Aggarwal, B.B.; Vijayalekshmi, R.V.; Sung, B. Targeting inflammatory pathways for prevention and therapy of cancer: Short-term friend, long-term foe. Clin. Cancer Res. 2009, 425–430, doi:10.1158/1078-0432.CCR-08-0149.
- Giroux, V.; Rustgi, A.K. Metaplasia: Tissue injury adaptation and a precursor to the dysplasia-cancer sequence. Nat. Rev. Cancer 2017, 594–604, doi:10.1038/nrc.2017.68.
- Cordon-Cardo, C.; Prives, C. At the crossroads of inflammation and tumorigenesis. J. Exp. Med. 1999, 1367–1370, doi:10.1084/jem.190.10.1367.
- Maeda, H.; Akaike, T. Nitric oxide and oxygen radicals in infection, inflammation, and cancer. Biokhimiya 1998, 63, 1007–1019.
- Smyth, M.J.; Cretney, E.; Kershaw, M.H.; Hayakawa, Y. Cytokines in cancer immunity and immunotherapy. Immunol. Rev. 2004, 275–293, doi:10.1111/j.0105-2896.2004.00199.x.
- Wang, X.; Lin, Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol. Sin. 2008, 1275–1288. doi:10.1111/j.1745-7254.2008.00889.x.
- Kumari, N.; Dwarakanath, B.S.; Das, A.; Bhatt, A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumor Biol. 2016, 11553–11572, doi:10.1007/s13277-016-5098-7.
- Negus, R.P.M.; Stamp, G.W.H.; Relf, M.G.; Burke, F.; Malik, S.T.A.; Bernasconi, S.; Allavena, P.; Sozzani, S.; Mantovani, A.; Balkwill, F.R. The detection and localization of monocyte chemoattractant protein-1 (MCP-1) in human ovarian cancer. J. Clin. Investig. 1995, 95, 2391–2396, doi:10.1172/JCI117933.
- Singh, N.; Baby, D.; Rajguru, J.; Patil, P.; Thakkannavar, S.; Pujari, V. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126, doi:10.4103/aam.aam_56_18.
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 1–10, doi:10.1016/j.immuni.2013.07.012.
- Dunn, G.P.; Koebel, C.M.; Schreiber, R.D.; Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 2006, 836–848, doi:10.1038/nri1961.
- Pio, R.; Corrales, L.; Lambris, J.D. The role of complement in tumor growth. In Advances in Experimental Medicine and Biology; Springer: Berlin, Germany, 2014; Volume 772, pp. 229–262, doi:10.1007/978-1-4614-5915-6_11.
- Waldhauer, I.; Steinle, A. NK cells and cancer immunosurveillance. Oncogene 2008, 27, 5932–5943, doi:10.1038/onc.2008.267.
- Warrington, R.; Watson, W.; Kim, H.L.; Antonetti, F.R. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2011, 7, doi:10.1186/1710-1492-7-s1-s1.
- Harris, T.J.; Drake, C.G. Primer on tumor immunology and cancer immunotherapy. J. ImmunoTher. Cancer 2013, doi:10.1186/2051-1426-1-12.
- Minami, Y.; Kono, T.; Miyazaki, T.; Taniguchi, T. The IL-2 receptor complex: Its structure, function, and target genes. Annu. Rev. Immunol. 1993, 11, 245–268, doi:10.1146/annurev.iy.11.040193.001333.
- NORMAN, P. Immunobiology: The immune system in health and disease. J. Allergy Clin. Immunol. 1995, 96, 274–274, doi:10.1016/s0091-6749(95)70025-0.
- Schroeder, H.W.; Cavacini, L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 2010, 125, doi:10.1016/j.jaci.2009.09.046.
- Sun, J.C.; Lanier, L.L. Natural killer cells remember: An evolutionary bridge between innate and adaptive immunity? Eur. J. Immunol. 2009, 39, 2059–2064, doi:10.1002/eji.200939435.
- Jiang, L.N.; Liu, Y. Bin; Li, B.H. Lycopene exerts anti-inflammatory effect to inhibit prostate cancer progression. Asian J. Androl. 2019, 21, 80–85, doi:10.4103/aja.aja_70_18.
- Li, C.C.; Liu, C.; Fu, M.; Hu, K.Q.; Aizawa, K.; Takahashi, S.; Hiroyuki, S.; Cheng, J.; von Lintig, J.; Wang, X.D. Tomato powder inhibits hepatic steatosis and inflammation potentially through restoring SIRT1 activity and adiponectin function independent of carotenoid cleavage enzymes in mice. Mol. Nutr. Food Res. 2018, 62, doi:10.1002/mnfr.201700738.
- Sun, X.; Jia, H.; Xu, Q.; Zhao, C.; Xu, C. Lycopene alleviates H2O2-induced oxidative stress, inflammation and apoptosis in bovine mammary epithelial cells: Via the NFE2L2 signaling pathway. Food Funct. 2019, 10, 6276–6285, doi:10.1039/c9fo01922g.
- Zhao, Q.; Yang, F.; Meng, L.; Chen, D.; Wang, M.; Lu, X.; Chen, D.; Jiang, Y.; Xing, N. Lycopene attenuates chronic prostatitis/chronic pelvic pain syndrome by inhibiting oxidative stress and inflammation via the interaction of NF-ΚB, MAPKs, and Nrf2 signaling pathways in rats. Andrology 2020, 8, 747–755, doi:10.1111/andr.12747.
- Cha, J.H.; Kim, W.K.; Ha, A.W.; Kim, M.H.; Chang, M.J. Anti-inflammatory effect of lycopene in SW480 human colorectal cancer cells. Nutr. Res. Pract. 2017, 11, 90–96, doi:10.4162/nrp.2017.11.2.90.
- Quagliariello, V.; Vecchione, R.; Coppola, C.; Di Cicco, C.; De Capua, A.; Piscopo, G.; Paciello, R.; Narciso, V.; Formisano, C.; Taglialatela-Scafati, O.; et al. Cardioprotective effects of nanoemulsions loaded with anti-inflammatory nutraceuticals against doxorubicin-induced cardiotoxicity. Nutrients 2018, 10, doi:10.3390/nu10091304.
- Ricciotti, E.; Fitzgerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000, doi:10.1161/ATVBAHA.110.207449.
- Attiq, A.; Jalil, J.; Husain, K.; Ahmad, W. Raging the war against inflammation with natural products. Front. Pharmacol. 2018, doi:10.3389/fphar.2018.00976.
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 252–259, doi:10.1007/s10787-007-0013-x.
- Sengupta, A.; Ghosh, S.; Das, R.K.; Bhattacharjee, S.; Bhattacharya, S. Chemopreventive potential of diallylsulfide, lycopene and theaflavin during chemically induced colon carcinogenesis in rat colon through modulation of cyclooxygenase-2 and inducible nitric oxide synthase pathways. Eur. J. Cancer Prev. 2006, 15, 301–305, doi:10.1097/00008469-200608000-00005.
- Luo, C.; Wu, X.G. Lycopene enhances antioxidant enzyme activities and immunity function in N-Methyl-N′-Nitro-N-nitrosoguanidine-induced gastric cancer rats. Int. J. Mol. Sci. 2011, 12, 3340–3351, doi:10.3390/ijms12053340.
- Ip, B.C.; Liu, C.; Ausman, L.M.; Von Lintig, J.; Wang, X.D. Lycopene attenuated hepatic tumorigenesis via differential mechanisms depending on carotenoid cleavage enzyme in mice. Chest 2014, 146, 1219–1227, doi:10.1158/1940-6207.CAPR-14-0154.
- Zhou, S.K.; Zhang, R.L.; Bi, T.N.; Lu, Y.; Jiang, L.X. Inhibitory effect of lycopene against the growth of human gastric cancer cells. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 184–190, doi:10.21010/ajtcam.v13i4.24.
- Gajowik, A.; Dobrzyńska, M.M. The evaluation of protective effect of lycopene against genotoxic influence of X-irradiation in human blood lymphocytes. Radiat. Environ. Biophys. 2017, 56, 413–422, doi:10.1007/s00411-017-0713-6.
- Wang, S.; Wu, Y.Y.; Wang, X.; Shen, P.; Jia, Q.; Yu, S.; Wang, Y.; Li, X.; Chen, W.; Wang, A.; et al. Lycopene prevents carcinogen-induced cutaneous tumor by enhancing activation of the Nrf2 pathway through P62-triggered autophagic keap1 degradation. Aging 2020, 12, 8167–8190, doi:10.18632/aging.103132.
- Lian, F.; Wang, X.D. Enzymatic metabolites of lycopene induce Nrf2-mediated expression of phase II detoxifying/antioxidant enzymes in human bronchial epithelial cells. Int. J. Cancer 2008, 123, 1262–1268, doi:10.1002/ijc.23696.
- Nagasawa, H.; Mitamura, T.; Sakamoto, S.; Yamamoto, K. Effects of lycopene on spontaneous mammary tumour development in SHN virgin mice. Anticancer Res. 1995, 15, 1173–1178.
- Sharoni, Y.; Giron, E.; Rise, M.; Levy, J. Effects of lycopene-enriched tomato oleoresin on 7,12-dimethyl-benz[a]anthracene-induced rat mammary tumors. Cancer Detect. Prev. 1997, 21, 118–123.
- Jiang, X.; Wu, H.; Zhao, W.; Ding, X.; You, Q.; Zhu, F.; Qian, M.; Yu, P. Lycopene improves the efficiency of anti-PD-1 therapy via activating IFN signaling of lung cancer cells. Cancer Cell Int. 2019, 19, doi:10.1186/s12935-019-0789-y.
- Astorg, P.; Gradelet, S.; Bergès, R.; Suschetet, M. Dietary lycopene decreases the initiation of liver preneoplastic foci by diethylnitrosamine in the rat. Nutr. Cancer 1997, 29, 60–68, doi:10.1080/01635589709514603.
- Passos Toledo, L.; Prates Ong, T.; Galvão Pinho, A.L.; Jordão, A.; Vanucchi, H.; Salvador Moreno, F. Inhibitory effects of lutein and lycopene on placental glutathione s-transferase-positive preneoplastic lesions and DNA strand breakage induced in wistar rats by the resistant hepatocyte model of hepatocarcinogenesis. Nutr. Cancer 2003, 47, 62–69, doi:10.1207/s15327914nc4701_8.
- Wang, Y.; Ausman, L.M.; Greenberg, A.S.; Russell, R.M.; Wang, X.D. Dietary lycopene and tomato extract supplementations inhibit nonalcoholic steatohepatitis-promoted hepatocarcinogenesis in rats. Int. J. Cancer 2010, 126, 1788–1796, doi:10.1002/ijc24689.
- Velmurugan, B.; Bhuvaneswari, V.; Burra, U.K.; Nagini, S. Prevention of N-Methyl-N′-Nitro-N-nitrosoguanidine and saturated sodium chloride-induced gastric carcinogenesis in wistar rats by lycopene. Eur. J. Cancer Prev. 2002, 11, 19–26, doi:10.1097/00008469-200202000-00004.
- Ucci, M.; Di Tomo, P.; Tritschler, F.; Cordone, V.G.P.; Lanuti, P.; Bologna, G.; Di Silvestre, S.; Di Pietro, N.; Pipino, C.; Mandatori, D.; et al. Anti-inflammatory role of carotenoids in endothelial cells derived from umbilical cord of women affected by gestational diabetes mellitus. Oxid. Med. Cell. Longev. 2019, 2019, doi:10.1155/2019/8184656.
- Holzapfel, N.P.; Shokoohmand, A.; Wagner, F.; Landgraf, M.; Champ, S.; Holzapfel, B.M.; Clements, J.A.; Hutmacher, D.W.; Loessner, D. Lycopene reduces ovarian tumor growth and intraperitoneal metastatic load. Am. J. Cancer Res. 2017, 7, 1322–1336.
- Sahin, K.; Yenice, E.; Tuzcu, M.; Orhan, C.; Mizrak, C.; Ozercan, I.H.; Sahin, N.; Yilmaz, B.; Bilir, B.; Ozpolat, B.; et al. Lycopene protects against spontaneous ovarian cancer formation in laying hens. J. Cancer Prev. 2018, 23, 25–36, doi:10.15430/jcp.2018.23.1.25.
- Narisawa, T.; Fukaura, Y.; Hasebe, M.; Ito, M.; Aizawa, R.; Murakoshi, M.; Uemura, S.; Khachik, F.; Nishino, H. Inhibitory effects of natural carotenoids, α-carotene, β-carotene, lycopene and lutein, on colonic aberrant crypt foci formation in rats. Cancer Lett. 1996, 107, 137–142, doi:10.1016/0304-3835(96)04354-6.
- Narisawa, T.; Fukaura, Y.; Hasebe, M.; Nomura, S.; Oshima, S.; Sakamoto, H.; Inakuma, T.; Ishiguro, Y.; Takayasu, J.; Nishino, H. Prevention of N-methylnitrosourea-induced colon carcinogenesis in F344 rats by lycopene and tomato juice rich in lycopene. Jpn. J. Cancer Res. 1998, 89, 1003–1008, doi:10.1111/j.1349-7006.1998.tb00488.x.
- Valadez-Bustos, N.; Escamilla-Silva, E.M.; García-Vázquez, F.J.; Gallegos-Corona, M.A.; Amaya-Llano, S.L.; Ramos-Gómez, M. Oral Administration of Microencapsulated, B. Longum BAA-999 and lycopene modulates IGF-1/IGF-1R/IGFBP3 protein expressions in a colorectal murine model. Int. J. Mol. Sci. 2019, 20, doi:10.3390/ijms20174275.
- Kim, D.J.; Takasuka, N.; Kim, J.M.; Sekine, K.; Ota, T.; Asamoto, M.; Murakoshi, M.; Nishino, H.; Nir, Z.; Tsuda, H. Chemoprevention by lycopene of mouse lung neoplasia after combined initiation treatment with DEN, MNU and DMH. Cancer Lett. 1997, 120, 15–22, doi:10.1016/S0304-3835(97)00281-4.
- Watanabe, S.; Kitade, Y.; Masaki, T.; Nishioka, M.; Satoh, K.; Nishino, H. Effects of lycopene and sho-saiko-to on hepatocarcinogenesis in a rat model of spontaneous liver cancer. Nutr. Cancer 2001, 39, 96–101, doi:10.1207/S15327914nc391_13.
- Cohen, L.A.; Zhao, Z.; Pittman, B.; Khachik, F. Effect of dietary lycopene on N-methylnitrosourea-induced mammary tumorigenesis. Nutr. Cancer 1999, 34, 153–159, doi:10.1207/S15327914NC3402_5.
- Giovannucci, E.; Ascherio, A.; Rimm, E.B.; Stampfer, M.J.; Colditz, G.A.; Willett, W.C. Intake of carotenoids and retino in relation to risk of prostate cancer. J. Natl. Cancer Inst. 1995, 87, 1767–1776, doi:10.1093/jnci/87.23.1767.
- Giovannucci, E. A prospective study of tomato products, lycopene, and prostate cancer risk. CancerSpectrum Knowl. Environ. 2002, 94, 391–398, doi:10.1093/jnci/94.5.391.
- Wu, K.; Erdman, J.W.; Schwartz, S.J.; Platz, E.A.; Leitzmann, M.; Clinton, S.K.; DeGroff, V.; Willett, W.C.; Giovannucci, E. Plasma and dietary carotenoids, and the risk of prostate cancer: A nested case-control study. Cancer Epidemiol. Biomark. Prev. 2004, 13, 260–269, doi:10.1158/1055-9965.EPI-03-0012.
- Kucuk, O.; Sarkar, F.H.; Sakr, W.; Djuric, Z.; Pollak, M.N.; Khachik, F.; Li, Y.W.; Banerjee, M.; Grignon, D.; Bertram, J.S.; et al. Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer Epidemiol. Biomark. Prev. 2001, 10, 861–868.
- Bowen, P.; Chen, L.; Stacewicz-Sapuntzakis, M.; Duncan, C.; Sharifi, R.; Ghosh, L.; Kim, H.S.; Christov-Tzelkov, K.; Van Breemen, R. Tomato sauce supplementation and prostate cancer: Lycopene accumulation and modulation of biomarkers of carcinogenesis. Exp. Biol. Med. 2002, 227, 886–893, doi:10.1177/153537020222701008.
- Paur, I.; Lilleby, W.; Bøhn, S.K.; Hulander, E.; Klein, W.; Vlatkovic, L.; Axcrona, K.; Bolstad, N.; Bjøro, T.; Laake, P.; et al. Tomato-based randomized controlled trial in prostate cancer patients: Effect on PSA. Clin. Nutr. 2017, 36, 672–679, doi:10.1016/j.clnu.2016.06.014.
- Chen, J.; Song, Y.; Zhang, L. Lycopene/tomato consumption and the risk of prostate cancer: A systematic review and meta-analysis of prospective studie. J. Nutr. Sci. Vitaminol. 2013, 59, 213–223, doi:10.3177/jnsv.59.213.
- Chen, P.; Zhang, W.; Wang, X.; Zhao, K.; Negi, D.S.; Zhuo, L.; Qi, M.; Wang, X.; Zhang, X. Lycopene and risk of prostate cancer. Medicine 2015, 94, e1260, doi:10.1097/MD.0000000000001260.
- Bae, J.M. Reinterpretation of the results of a pooled analysis of dietary carotenoid intake and breast cancer risk by using the interval collapsing method. Epidemiol. Health 2016, 38, e2016024, doi:10.4178/epih.e2016024.
- Yan, B.; Lu, M.S.; Wang, L.; Mo, X.F.; Luo, W.P.; Du, Y.F.; Zhang, C.X. Specific serum carotenoids are inversely associated with breast cancer risk among chinese women: A case-control study. Br. J. Nutr. 2016, 115, 129–137, doi:10.1017/S000711451500416X.
- Abar, L.; Vieira, A.R.; Aune, D.; Stevens, C.; Vingeliene, S.; Navarro Rosenblatt, D.A.; Chan, D.; Greenwood, D.C.; Norat, T. Blood concentrations of carotenoids and retinol and lung cancer risk: An update of the WCRF–AICR Systematic review of published prospective studies. Cancer Med. 2016, 5, 2069–2083, doi:10.1002/cam4.676.
- Hsing, A.W.; Comstock, G.W.; Abbey, H.; Polk, B.F. Serologic precursors of cancer. Retinol, carotenoids, and tocopherol and risk of prostate cancer. J. Natl. Cancer Inst. 1990, 82, 941–946, doi:10.1093/jnci/82.11.941.
- Vogt, T.M.; Mayne, S.T.; Graubard, B.I.; Swanson, C.A.; Sowell, A.L.; Schoenberg, J.B.; Swanson, G.M.; Greenberg, R.S.; Hoover, R.N.; Hayes, R.B.; et al. Serum lycopene, other serum carotenoids, and risk of prostate cancer in US blacks and whites. Am. J. Epidemiol. 2002, 155, 1023–1032, doi:10.1093/aje/155.11.1023.
- Schuurman, A.G.; Goldbohm, R.A.; Brants, H.A.M.; Van Den Brandt, P.A. A Prospective cohort study on intake of retinol, vitamins C and E, and carotenoids and prostate cancer risk (Netherlands). Cancer Causes Control. 2002, 13, 573–582, doi:10.1023/A:1016332208339.
- Chan, J.M.; Weinberg, V.; Magbanua, M.J.; Sosa, E.; Simko, J.; Shinohara, K.; Federman, S.; Mattie, M.; Hughes-Fulford, M.; Haqq, C.; et al. Nutritional supplements, COX-2 and IGF-1 expression in men on active surveillance for prostate cancer. Cancer Causes Control 2011, 22, 141–150, doi:10.1007/s10552-010-9684-5.
- Kim, G.Y.; Kim, J.H.; Ahn, S.C.; Lee, H.J.; Moon, D.O.; Lee, C.M.; Park, Y.M. Lycopene suppresses the lipopolysaccharide-induced phenotypic and functional maturation of murine dendritic cells through inhibition of mitogen-activated protein kinases and nuclear factor-ΚB. Immunology 2004, 113, 203–211, doi:10.1111/j.1365-2567.2004.01945.x.
- Manabe, Y.; Hirata, T.; Sugawara, T. Suppressive effects of carotenoids on the antigeninduced degranulation in RBL-2H3 rat basophilic leukemia cells. J. Oleo Sci. 2014, 63, 291–294, doi:10.5650/jos.ess13169.
- Fachinello, M.R.; Fernandes, N.L.M.; de Souto, E.R.; dos Santos, T.C.; da Costa, A.E.R.; Pozza, P.C. Lycopene affects the immune responses of finishing pigs. Ital. J. Anim. Sci. 2018, 17, 666–674, doi:10.1080/1828051X.2017.1401438.
- Zaidi, M.R. The interferon-gamma paradox in cancer. J. Interf. Cytokine Res. 2019, 39, 30–38, doi:10.1089/jir.2018.0087.
- Ren, W.; Gao, L.; Song, J. Structural basis of DNMT1 and DNMT3A-Mediated DNA methylation. Genes 2018, 9, 620, doi:10.3390/genes9120620.
- Alsamman, K.; El-Masry, O.S. Interferon regulatory factor 1 inactivation in human cancer. Biosci. Rep. 2018, 38, BSR20171672, doi:10.1042/BSR20171672.
- Yang, Z.; Chen, W.; Zhu, W.; Meng, H.; Chen, J.; Zhang, J. Overexpression of interferon regulatory factor 7 (IRF7) reduces bone metastasis of prostate cancer cells in mice. Oncol. Res. 2017, 25, 511–522, doi:10.3727/096504016X14756226781802.
- Makon-Sébastien, N.; Francis, F.; Eric, S.; Henri, V.P.; François, L.J.; Laurent, P.; Yves, B.; Serge, C. Lycopene modulates THP1 and Caco2 cells inflammatory state through transcriptional and nontranscriptional processes. Mediat. Inflamm. 2014, 2014, doi:10.1155/2014/507272.
- Liu, W.J.; Ye, L.; Huang, W.F.; Guo, L.J.; Xu, Z.G.; Wu, H.L.; Yang, C.; Liu, H.F. P62 links the autophagy pathway and the ubiqutin-proteasome system upon ubiquitinated protein degradation. Cell. Mol. Biol. Lett. 2016, doi:10.1186/s11658-016-0031-z.
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 585–597, doi:10.1016/j.bbadis.2016.11.005.
- Zheng, Z.; Yin, Y.; Lu, R.; Jiang, Z. Lycopene ameliorated oxidative stress and inflammation in Type 2 diabetic rats. J. Food Sci. 2019, 84, 1194–1200, doi:10.1111/1750-3841.14505.
- Bignotto, L.; Rocha, J.; Sepodes, B.; Eduardo-Figueira, M.; Pinto, R.; Chaud, M.; De Carvalho, J.; Moreno, H.; Mota-Filipe, H. Anti-inflammatory effect of lycopene on carrageenan-induced paw oedema and hepatic ischaemia-reperfusion in the rat. Br. J. Nutr. 2009, 102, 126–133, doi:10.1017/S0007114508137886.
- Denniss, S.G.; Haffner, T.D.; Kroetsch, J.T.; Davidson, S.R.; Rush, J.W.E.; Hughson, R.L. Effect of short-term lycopene supplementation and postprandial dyslipidemia on plasma antioxidants and biomarkers of endothelial health in young, healthy individuals. Vasc. Health Risk Manag. 2008, 4, 213–222, doi:10.2147/vhrm.2008.04.01.213.
- Markovits, N.; Amotz, A. Ben; Levy, Y. The effect of tomato-derived lycopene on low carotenoids and enhanced systemic inflammation and oxidation in severe obesity. Isr. Med. Assoc. J. 2009, 11, 598–601.
- Petyaev, I.M.; Dovgalevsky, P.Y.; Klochkov, V.A.; Chalyk, N.E.; Pristensky, D.V.; Chernyshova, M.P.; Udumyan, R.; Kocharyan, T.; Kyle, N.H.; Lozbiakova, M.V.; et al. Effect of lycopene supplementation on cardiovascular parameters and markers of inflammation and oxidation in patients with coronary vascular disease. Food Sci. Nutr. 2018, 6, 1770–1777, doi:10.1002/fsn3.734.
- Li, W.W.; Wang, T.Y.; Cao, B.; Liu, B.; Rong, Y.M.; Wang, J.J.; Wei, F.; Wei, L.Q.; Chen, H.; Liu, Y.X. Synergistic protection of matrine and lycopene against lipopolysaccharide-induced acute lung injury in mice. Mol. Med. Rep. 2019, 20, 455–462, doi:10.3892/mmr.2019.10278.
- Veeramachaneni, S.; Ausman, L.M.; Choi, S.W.; Russell, R.M.; Wang, X.-D. High dose lycopene supplementation increases hepatic cytochrome P4502E1 protein and inflammation in alcohol-fed rats. J. Nutr. 2008, 138, 1329–1335, doi:10.1093/jn/138.7.1329.
- Johnson, E.J. The role of carotenoids in human health. Nutr. Clin. Care Off. Publ. Tufts Univ. 2002, 56–65, doi:10.1046/j.1523-5408.2002.00004.x.
- Mayne, S.T. Beta‐carotene, carotenoids, and disease prevention in humans. FASEB J. 1996, 10, 690–701, doi:10.1096/fasebj.10.7.8635686.
- Arab, L.; Steck-Scott, S.; Bowen, P. Participation of lycopene and beta-carotene in carcinogenesis: Defenders, aggressors, or passive bystanders? Epidemiol. Rev. 2001, 23, 211–230, doi:10.1093/oxfordjournals.epirev.a000803.
- The Alpha-Tocopherol, B.C.C.P.S.G. The effect of vitamin e and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 1994, 330, 1029–1035, doi:10.1056/nejm199404143301501.
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L.; Valanis, B.; Williams, J.H.; et al. Risk factors for lung cancer and for intervention effects in CARET, the beta-carotene and retinol efficacy trial. J. Natl. Cancer Inst. 1996, 88, 1550–1559, doi:10.1093/jnci/88.21.1550.
- Männistö, S.; Smith-Warner, S.A.; Spiegelman, D.; Albanes, D.; Anderson, K.; Van Den Brandt, P.A.; Cerhan, J.R.; Colditz, G.; Feskanich, D.; Freudenheim, J.L.; et al. Dietary carotenoids and risk of lung cancer in a pooled analysis of seven cohort studies. Cancer Epidemiol. Biomark. Prev. 2004, 13, 40–48, doi:10.1158/1055-9965.EPI-038-3.
- Touvier, M.; Kesse, E.; Clavel-Chapelon, F.; Boutron-Ruault, M.C. Dual association of β-carotene with risk of tobacco-related cancers in a cohort of french women. J. Natl. Cancer Inst. 2005, 97, 1338–1344, doi:10.1093/jnci/dji276.
- Goralczyk, R. Beta-carotene and lung cancer in smokers: Review of hypotheses and status of research. Nutr. Cancer 2009, 61, 767–774, doi:10.1080/01635580903285155.
- Michaud, D.S.; Feskanich, D.; Rimm, E.B.; Colditz, G.A.; Speizer, F.E.; Willett, W.C.; Giovannucci, E. Intake of specific carotenoids and risk of lung cancer in 2 prospective US cohorts. Am. J. Clin. Nutr. 2000, 72, 990–997, doi:10.1093/ajcn/72.4.990.
- Rao, A.V.; Agarwal, S. Effect of diet and smoking on serum lycopene and lipid peroxidation. Nutr. Res. 1998, 18, 713–721, doi:10.1016/s0271-5317(98)00057-8.
- Heber, D. Colorful cancer prevention: α-carotene, lycopene, and lung cancer. Am. J. Clin. Nutr. 2000, 72, 901–902.
- Satia, J.A.; Littman, A.; Slatore, C.G.; Galanko, J.A.; White, E. Long-term use of β-carotene, retinol, lycopene, and lutein supplements and lung cancer risk: Results from the vitamins and lifestyle (vital) study. Am. J. Epidemiol. 2009, 169, 815–828, doi:10.1093/aje/kwn409.
- Ford, N.A.; Smith, J.W.; Clinton, S.K.; Erdman, J.W. Tomato powder or lycopene reduces serum and testicular testosterone and enzymes controlling androgen and estrogen metabolism in mice lacking carotene-15,15’-monooxygenase. Exp. Biol. 2011, doi:10.1096/fasebj.25.1_supplement.975.6.
- Luca, L.M.D.; Darwiche, N.; Celli, G.; Kosa, K.; Jones, C.; Ross, S.; Chen, L. ‐C. Vitamin A in epithelial differentiation and skin carcinogenesis. Nutr. Rev. 1994, 52, S45–S52, doi:10.1111/j.1753-4887.1994.tb01386.x.
- Fernandes-Silva, H.; Araújo-Silva, H.; Correia-Pinto, J.; Moura, R.S. Retinoic acid: A key regulator of lung development. Biomolecules 2020, 10, 152, doi:10.3390/biom10010152.
- National Research Council (US) Panel on Dosimetric Assumptions Affecting the Application of Radon Risk Estimates. Comparative Dosimetry of Radon in Mines and Homes; National Academy of Sciences: Washington, DC, USA, 1991; doi:10.17226/1799.
- Ben-Dor, A.; Nahum, A.; Danilenko, M.; Giat, Y.; Stahl, W.; Martin, H.D.; Emmerich, T.; Noy, N.; Levy, J.; Sharoni, Y. Effects of acyclo-retinoic acid and lycopene on activation of the retinoic acid receptor and proliferation of mammary cancer cells. Arch. Biochem. Biophys. 2001, 391, 295–302, doi:10.1006/abbi.2001.2412.
- Stahl, W.; Von Laar, J.; Martin, H.D.; Emmerich, T.; Sies, H. Stimulation of gap junctional communication: Comparison of acyclo- retinoic acid and lycopene. Arch. Biochem. Biophys. 2000, 373, 271–274, doi:10.1006/abbi.1999.1510.
- Li, M.T.; Richter, F.; Chang, C.; Irwin, R.J.; Huang, H.F.S. Androgen and retinoic acid interaction in LNCaP cells, effects on cell proliferation and expression of retinoic acid receptors and epidermal growth factor receptor. BMC Cancer 2002, 2, doi:10.1186/1471-2407-2-16.
- Chen, M.C.; Hsu, S.L.; Lin, H.; Yang, T.Y. Retinoic acid and cancer treatment. BioMedicine 2014, 4, 1–6, doi:10.7603/s40681-014-0022-1.
- Alsafadi, S.; Even, C.; Falet, C.; Goubar, A.; Commo, F.; Scott, V.; Quidville, V.; Albiges, L.; Dieci, M.V.; Guegan, J.; et al. Retinoic acid receptor alpha amplifications and retinoic acid sensitivity in breast cancers. Clin. Breast Cancer 2013, 13, 401–408, doi:10.1016/j.clbc.2013.02.001.
- Wang, W.; Liu, S.; Jiang, C.; Wang, Y.; Zhu, H.; Wang, X.D. High expression of RARβ is a favorable factor in colorectal cancer. Dis. Markers 2019, 2019, doi:10.1155/2019/7138754.
- Ghyselinck, N.B.; Duester, G. Retinoic acid signaling pathways. Development 2019, 146, doi:10.1242/dev.167502.
More
Information
Subjects:
Oncology; Nutrition & Dietetics; Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
779
Revisions:
2 times
(View History)
Update Date:
13 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




