Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sasa Rajsic | -- | 1826 | 2023-02-09 07:24:09 | | | |
| 2 | Rita Xu | Meta information modification | 1826 | 2023-02-09 08:34:35 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rajsic, S.; Breitkopf, R.; Jadzic, D.; Krneta, M.P.; Tauber, H.; Treml, B. Extracorporeal Membrane Oxygenation. Encyclopedia. Available online: https://encyclopedia.pub/entry/41014 (accessed on 13 January 2026).
Rajsic S, Breitkopf R, Jadzic D, Krneta MP, Tauber H, Treml B. Extracorporeal Membrane Oxygenation. Encyclopedia. Available at: https://encyclopedia.pub/entry/41014. Accessed January 13, 2026.
Rajsic, Sasa, Robert Breitkopf, Dragana Jadzic, Marina Popovic Krneta, Helmuth Tauber, Benedikt Treml. "Extracorporeal Membrane Oxygenation" Encyclopedia, https://encyclopedia.pub/entry/41014 (accessed January 13, 2026).
Rajsic, S., Breitkopf, R., Jadzic, D., Krneta, M.P., Tauber, H., & Treml, B. (2023, February 09). Extracorporeal Membrane Oxygenation. In Encyclopedia. https://encyclopedia.pub/entry/41014
Rajsic, Sasa, et al. "Extracorporeal Membrane Oxygenation." Encyclopedia. Web. 09 February, 2023.
Copy Citation
Extracorporeal membrane oxygenation (ECMO) is a specialized temporary life support for patients with severe cardiac or pulmonary failure bridging the time for organ recovery, transplant, or permanent assistance.
extracorporeal life support
ECMO
inflammation
monitoring
mortality
1. Introduction
The development of extracorporeal life support modalities has added a new dimension to the care of critically ill patients who fail conventional treatment options. Extracorporeal circuits, like those used in hemodialysis, cardiopulmonary bypass, ventricular assist devices, extracorporeal membrane oxygenation (ECMO), and therapeutic apheresis, are common in modern medicine.
Extracorporeal membrane oxygenation presents specialized temporary life support for patients with severe cardiac or pulmonary failure, bridging time for organ recovery, transplant, or permanent assistance. The beginning of ECMO support dates from 1971, when the first prolonged extracorporeal oxygenation and perfusion were used in the case of a patient with severe acute respiratory distress syndrome (ARDS) [1]. Over the last decade, the indications for ECMO support have expanded beyond severe respiratory failure and refractory cardiogenic shock [2] to include an assortment of clinical presentations, including a bridge to heart or lung transplantation [3], extracorporeal cardiopulmonary reanimation (ECPR) [4], resuscitation of patients with severe traumas [5], and rewarming due to accidental deep hypothermia [6]. Recently, the use of ECMO support for out-of-hospital cardiac arrest has been popularized in some countries, with reported improvements in outcome [7][8][9][10].
The outbreak of coronavirus disease 2019 (COVID-19) led to a significant increase in ECMO use and, based on the Extracorporeal Life Support Organization (ELSO) data, almost 173,000 ECMO runs had been reported by the end of 2021, with 17,777 runs in the last year. The overall in-hospital survival was 54% [11].
The overall survival is dependent on the underlying disease, comorbidities, patient reaction to critical illness, and potential adverse events during ECMO support. The initiation of ECMO support is associated with complex inflammatory and coagulation responses, as a reaction to the blood encountering the large artificial surface of an extracorporeal system circuit [12]. These processes may further lead to endothelial injury and disrupted microcirculation with consequent end-organ dysfunction and the need for systemic anticoagulation [12][13].
Therefore, anticoagulation is considered crucial to reduce the risk of thrombosis and failure of circuit components. Unfractionated heparin (UFH) is the gold standard and most-used anticoagulant during extracorporeal life support. It achieves anticoagulatory effects by enhancing the activity of antithrombin, which results in downregulation of thrombin and activated factor X (factor Xa) [14]. However, therapeutic anticoagulation of a critically ill patient carries the risk of clinically relevant bleeding with the potential for permanent injury or death [15][16][17]. Similarly, thrombotic events may occur [18][19]. Therefore, the monitoring of anticoagulation and the balance of procoagulant and anticoagulatory factors is of immense importance [17].
2. ECMO Configurations and Circuits
There are two main ECMO configurations: venoarterial (va-ECMO), used for a refractory cardiogenic shock, and venovenous (vv-ECMO), used for a severe respiratory failure, both of which can be subject to several modifications [20]. Venoarterial ECMO combines adequate oxygen delivery and carbon dioxide removal with circulatory support [21]. Vascular access is obtained by the placement of a drainage cannula in a large central vein supplying the ECMO system with patient blood. A second cannula returns the oxygenated blood either to the venous (vv-ECMO) or the arterial system (va-ECMO).
The traditional ECMO circuit utilizes technology as a cardiopulmonary bypass, i.e., it is a closed circuit with a membrane-type gas-exchange system [21]. The main distinctions between those two extracorporeal life modalities are in the duration of support, the existence of a venous reservoir, the air–blood interface, and the cardiotomy reservoir. Cardiopulmonary bypass is usually only employed for the duration of surgery, while ECMO support may be needed for weeks or even months [21].
The ECMO circuit consists of three main components: the pump, the gas, and the heat-exchange device connected with the polyvinyl chloride tubing (usually UFH coated). The earlier roller blood pumps have now been exchanged with more advanced and magnetically actuated centrifugal pumps that control the required blood flow [22][23]. Gas-exchange devices, fulfilling the patient’s metabolic needs for oxygen and the removal of carbon dioxide, evolved from direct air–blood contact systems to membrane-type gas-exchange devices (oxygenators). Since the early 2000s, polymethylpentene hollow fiber membranes are increasingly used, where the gas is ventilated through hollow fiber bundles and the blood circulates around the fibers (Figure 1) [21][24].
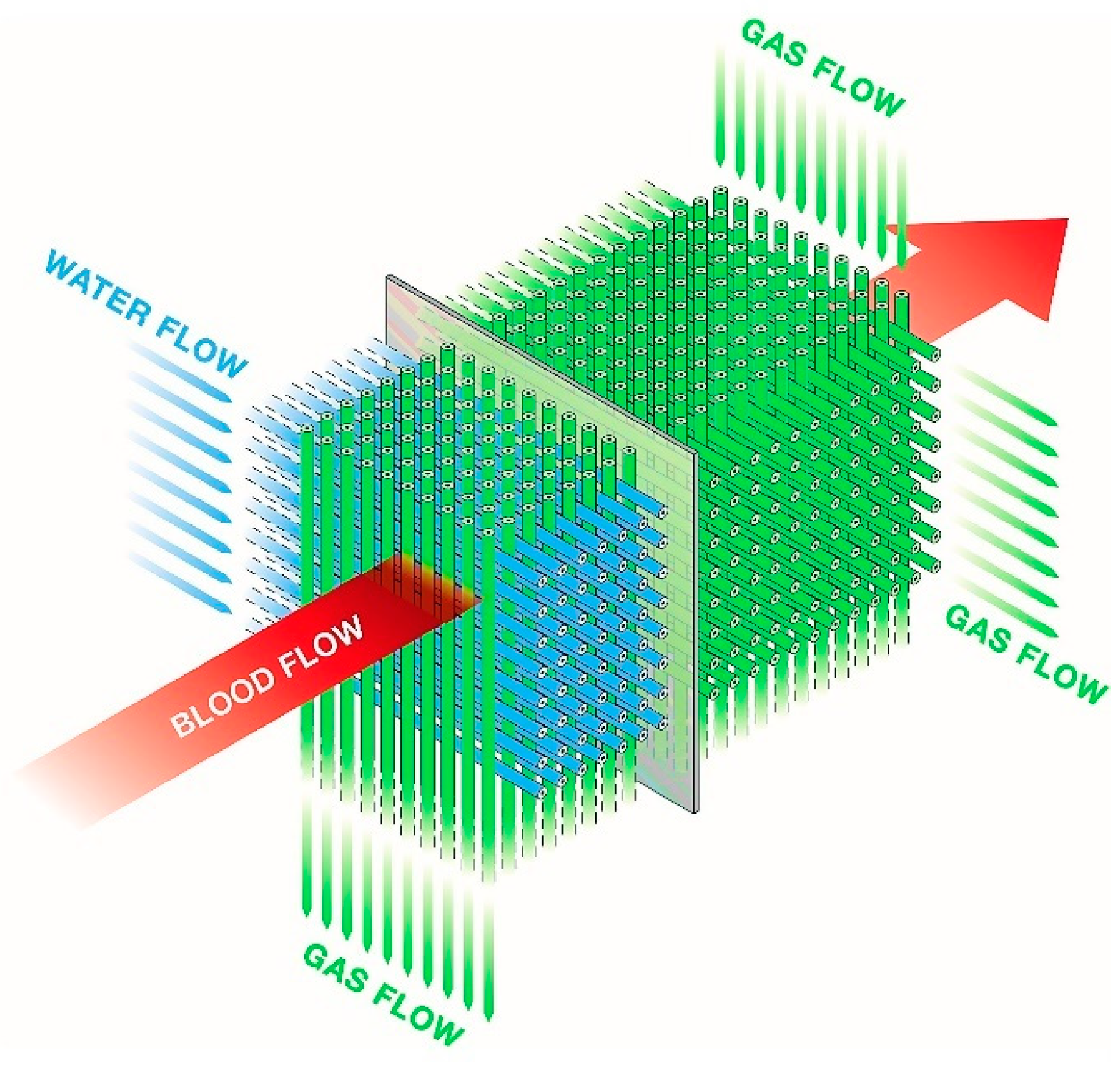
Figure 1. Schematic presentation of a diffusion membrane showing blood flow between the gas and water-filled network of hollow fibers. With permission of Maquet.
The amount of oxygen in the gas mixture depends on the metabolic need of the patient and can be increased up to 100% oxygen. The gas–blood flow ratio is typically adjusted to maintain normocapnia. Increasing the sweep gas flow will lead to an increased clearance of carbon dioxide while not altering oxygenation. Decreasing the sweep flow will result in increased partial pressure of carbon dioxide in the blood [21].
Keeping the circulating blood pressure in the oxygenator higher than the pressure of the circulating gas is of utmost importance to prevent the passage of air bubbles across the membrane, which can result in air embolism. Therefore, the oxygenator device should always be located below the level of the patient’s heart. Furthermore, insertion or removal of central catheters or interventions with the opening of blood vessels could result in aspiration of air by the ECMO system [21][25].
Finally, the third component of the ECMO system is a heat exchanger preventing circuit-related heat dispersion but also giving the option of a targeted temperature management, for example, in the treatment of sepsis, metabolic crisis, rewarming of accidental hypothermia, or as therapeutic hypothermia after cardiac arrest. The heat exchanger may be integrated into the gas-exchange device or be a separate component. It is usually based on nonpermeable hollow fiber bundles with circulating nonsterile water (Figure 1) [21].
3. Inflammation, Coagulation, and ECMO
The normal hemostasis in critically ill patients receiving ECMO support is distorted. Surgical trauma (ECMO implantation or cardiac surgery) and exposure of blood to the large surfaces of the ECMO circuits initiate and propagate immediate inflammatory response and activation of the coagulation cascade. Furthermore, the complexity of critical illness and its inflammatory response may additionally imbalance patient hemostasis [12]. The systemic inflammatory response in patients receiving cardiopulmonary bypass is well established and discussed extensively in the literature [26][27][28][29][30], but the information on this complex and multifaceted inflammatory response to ECMO support is still limited.
Several humoral and cellular systems are involved in complex interactions between inflammation and coagulation during ECMO support. Acute inflammation initiates clotting, compromises the fibrinolytic system, and reduces the activity of natural anticoagulant mechanisms. Moreover, endotoxin, IL-1β, and tumor necrosis factor-α (TNF-α) downregulate thrombomodulin and neutrophil elastase cleaves thrombomodulin from the endothelial cell surfaces [31][32]. P-selectin and E-selectin are synthesized or expressed on endothelial and platelet surfaces. Tissue factor, from the cell surface of leucocytes and monocytes, is induced by endotoxin, CD40 ligand, or TNF-α. It further binds factor VIIa and converts factor X to its activated form (Xa), which together with factor Va generates thrombin from prothrombin [33]. Additionally, inflammation reduces protein C levels, probably due to a combination of consumption and associated liver dysfunction with a consequent nonactivation of factor Va leading to the stabilization of prothrombin activation complexes [34]. Increased C-reactive protein levels facilitate monocyte–endothelial cell interactions, promote plasminogen activator inhibitor-1 and tissue factor formation, and induce complement activation [35][36][37]. The activation of platelets, key elements of hemostasis and inflammation, occurs as a result of complement activation and thrombin generation with a consequent release of a variety of mediators (proinflammatory cytokines, chemokines, adhesion factors, proteases, hemostatic factors, etc.). This all together plays a role in the development of a systemic inflammatory response [38][39][40][41].
Antithrombin is inactivated and/or consumed, while the levels of vascular heparin-like molecules may be reduced due to neutrophil activation products and inflammatory cytokines [42][43][44]. Finally, detailed information on the role of the fibrinolytic system in patients receiving ECMO support is still lacking, but recent studies reported on the association between increased fibrinolysis and bleeding complications [45][46].
Furthermore, different configurations of ECMO may also alter hemostasis. In the prospective HECTIC trial, the rate of thrombosis in va-ECMO was around 40%, with rates twice as high in vv-ECMO [47]. Moreover, in an ex vivo model, ECMO flow rates below 1.5 L/min were shown to decrease platelet aggregation, weaken clot firmness, and surprisingly increase hemolysis (despite the lower pump speed) [48]. Given the sparsity of evidence for low ECMO flows in humans, researchers establish more anticoagulation at lower flow rates (e.g., ACT 150–170 s at 2–3 L/min). Moreover, researchers use the same anticoagulation protocol per se in va- and vv-ECMO configurations but strive to tailor anticoagulation to each patient using viscoelastic monitoring on a routine basis.
Therefore, homeostasis and the balance between the procoagulant and anticoagulatory factors are crucial to avoid hemorrhagic or thromboembolic complications, and for the patency of the extracorporeal circuit and its components (see Figure 2).
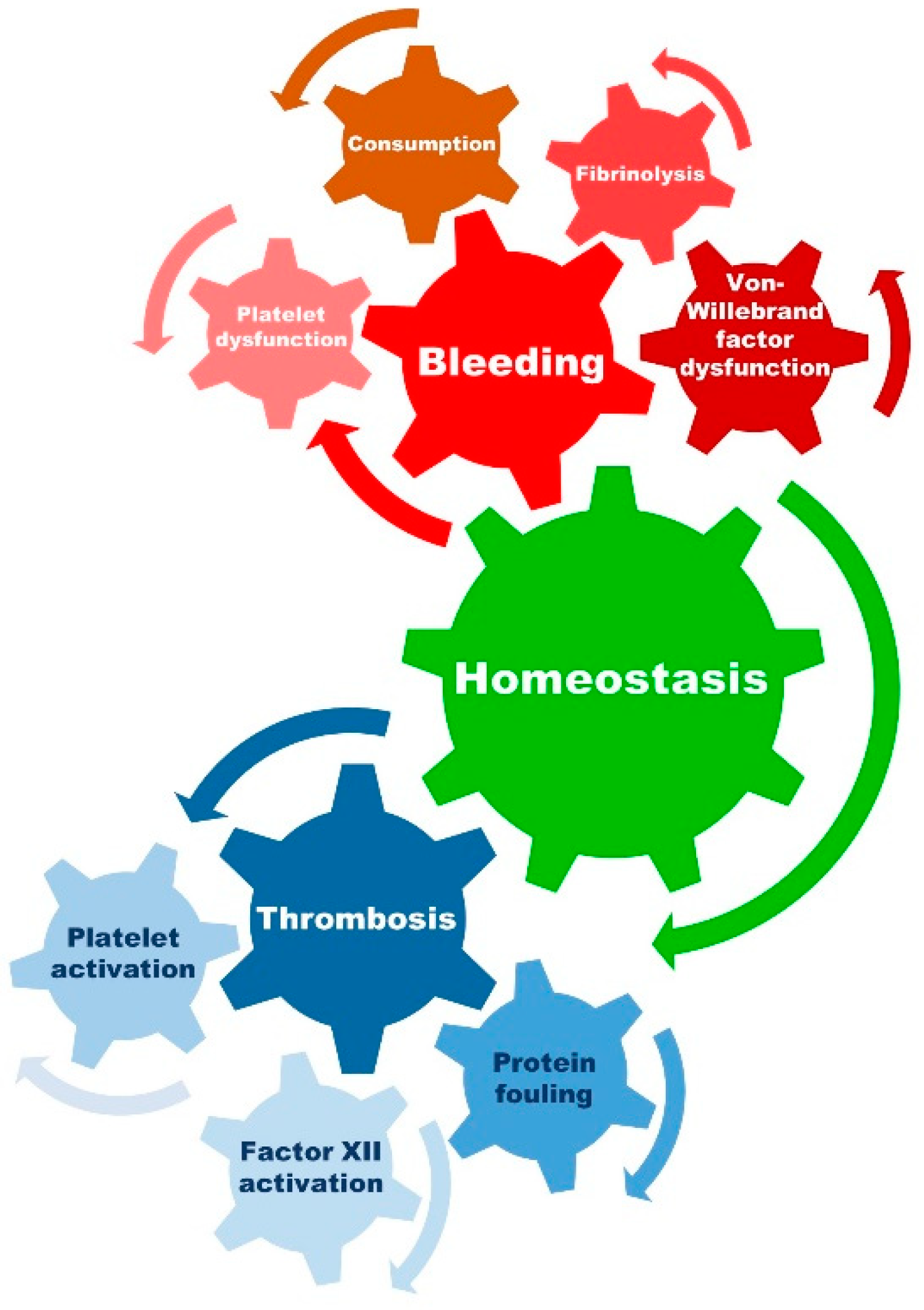
Figure 2. Presentation of prothrombotic and prohemorrhagic factors with an influence on homeostasis. Achieving a balance between the risk of bleeding and thrombosis is both critical and complex in patients receiving ECMO support. Aside from the initiation and propagation of the inflammatory response (proinflammatory state) and the activation of the coagulation cascade (prothrombotic state), ECMO may also lead to platelet dysfunction, fibrinolysis, malfunction of von Willebrand factor, and consumption of coagulation factors leading to a prohemorrhagic state.
Interestingly, within the first 10 min after ECMO support initiation and contact of blood with the artificial surfaces, factor XII cleaves into factor XIIa and XIIf. Factor XIIa has an important role in the activation of kallikrein and bradykinin, both strong drivers of inflammation and coagulation [49][50][51]. The role of bradykinin in inflammation may be even more interesting in va-ECMO with the lungs, the major site of bradykinin inactivation, being bypassed. As a response to the role of factor XII, its neutralization may lead to a reduction in inflammation, which has been recently shown in ex vivo and animal ECMO models with the use of a plasma protease factor XII function-neutralizing antibodies [52][53]. Further studies focused on potential uses in humans are warranted.
Lastly, it should be mentioned that, like any exposure to mechanical support devices, patients on ECMO support can develop increased human leukocyte antigen (HLA) sensitization, which is of relevance in bridge-to-transplant therapeutic considerations [54].
Given the above, systemic anticoagulation and coagulation monitoring are of immense importance for adverse events prevention in patients receiving ECMO support. The association of inflammation and thrombosis, known as thromboinflammation, is well reported in the literature, especially in COVID-19 patients [55][56]. Hyperinflammation may lead to a limitation of the UFH effect by decreasing antithrombin levels or increasing heparin binding to acute phase proteins [42][43][44]. A recent report on a possible association between bleeding and unintended excessive anticoagulation in ECMO patients without hyperinflammation remains to be confirmed in larger cohorts [13]. Finally, despite the extensive development of anticoagulants, ECMO pumps, oxygenators, and tubing systems, the systemic inflammatory response syndrome and distorted hemostasis remain a clinical concern. It is still unclear if the extent of inflammation may also benefit the patient, beyond its deleterious effects. To warrant a more detailed understanding of the underlying pathophysiological processes, the reporting on inflammatory response during ECMO support should be improved in forthcoming studies.
References
- Featherstone, P.J.; Ball, C.M. The early history of extracorporeal membrane oxygenation. Anaesth. Intensive Care 2018, 46, 555–557.
- Shekar, K.; Mullany, D.V.; Thomson, B.; Ziegenfuss, M.; Platts, D.G.; Fraser, J.F. Extracorporeal life support devices and strategies for management of acute cardiorespiratory failure in adult patients: A comprehensive review. Crit. Care 2014, 18, 219.
- Schechter, M.A.; Ganapathi, A.M.; Englum, B.R.; Speicher, P.J.; Daneshmand, M.A.; Davis, R.D.; Hartwig, M.G. Spontaneously Breathing Extracorporeal Membrane Oxygenation Support Provides the Optimal Bridge to Lung Transplantation. Transplantation 2016, 100, 2699–2704.
- Kim, H.; Cho, Y.H. Role of extracorporeal cardiopulmonary resuscitation in adults. Acute Crit. Care 2020, 35, 1–9.
- Wang, C.; Zhang, L.; Qin, T.; Xi, Z.; Sun, L.; Wu, H.; Li, D. Extracorporeal membrane oxygenation in trauma patients: A systematic review. World J. Emerg. Surg. 2020, 15, 51.
- Morley, D.; Yamane, K.; O’Malley, R.; Cavarocchi, N.C.; Hirose, H. Rewarming for accidental hypothermia in an urban medical center using extracorporeal membrane oxygenation. Am. J. Case Rep. 2013, 14, 6–9.
- Bougouin, W.; Dumas, F.; Lamhaut, L.; Marijon, E.; Carli, P.; Combes, A.; Pirracchio, R.; Aissaoui, N.; Karam, N.; Deye, N.; et al. Extracorporeal cardiopulmonary resuscitation in out-of-hospital cardiac arrest: A registry study. Eur. Heart J. 2020, 41, 1961–1971.
- Inoue, A.; Hifumi, T.; Sakamoto, T.; Kuroda, Y. Extracorporeal Cardiopulmonary Resuscitation for Out-of-Hospital Cardiac Arrest in Adult Patients. J. Am. Heart Assoc. 2020, 9, e015291.
- Scquizzato, T.; Bonaccorso, A.; Consonni, M.; Scandroglio, A.M.; Swol, J.; Landoni, G.; Zangrillo, A. Extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest: A systematic review and meta-analysis of randomized and propensity score-matched studies. Artif. Organs 2022, 46, 755–762.
- Downing, J.; Al Falasi, R.; Cardona, S.; Fairchild, M.; Lowie, B.; Chan, C.; Powell, E.; Pourmand, A.; Tran, Q.K. How effective is extracorporeal cardiopulmonary resuscitation (ECPR) for out-of-hospital cardiac arrest? A systematic review and meta-analysis. Am. J. Emerg. Med. 2022, 51, 127–138.
- Extracorporeal Life Support Organization (ELSO). Registry Report on Extracorporeal Life Support, International Summary. Available online: https://www.elso.org/Registry/InternationalSummaryandReports/InternationalSummary.aspx (accessed on 8 July 2022).
- Millar, J.E.; Fanning, J.P.; McDonald, C.I.; McAuley, D.F.; Fraser, J.F. The inflammatory response to extracorporeal membrane oxygenation (ECMO): A review of the pathophysiology. Crit. Care 2016, 20, 387.
- Rajsic, S.; Breitkopf, R.; Oezpeker, U.C.; Bukumirić, Z.; Dobesberger, M.; Treml, B. The Role of Excessive Anticoagulation and Missing Hyperinflammation in ECMO-Associated Bleeding. J. Clin. Med. 2022, 11, 2314.
- Hirsh, J.; Anand, S.S.; Halperin, J.L.; Fuster, V. Mechanism of action and pharmacology of unfractionated heparin. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1094–1096.
- Aubron, C.; DePuydt, J.; Belon, F.; Bailey, M.; Schmidt, M.; Sheldrake, J.; Murphy, D.; Scheinkestel, C.; Cooper, D.J.; Capellier, G.; et al. Predictive factors of bleeding events in adults undergoing extracorporeal membrane oxygenation. Ann. Intensive Care 2016, 6, 97.
- Treml, B.; Breitkopf, R.; Bukumirić, Z.; Bachler, M.; Boesch, J.; Rajsic, S. ECMO Predictors of Mortality: A 10-Year Referral Centre Experience. J. Clin. Med. 2022, 11, 1224.
- Levy, J.H.; Staudinger, T.; Steiner, M.E. How to manage anticoagulation during extracorporeal membrane oxygenation. Intensive Care Med. 2022, 48, 1076–1079.
- Abruzzo, A.; Gorantla, V.; Thomas, S.E. Venous thromboembolic events in the setting of extracorporeal membrane oxygenation support in adults: A systematic review. Thromb. Res. 2022, 212, 58–71.
- Deshpande, S.R.; Hastings, S.; Wagoner, S.; Ku, D.; Maher, K. New Insights into Thrombosis in ECMO: Circuits: Where, How and Why? J. Heart Lung Transplant. 2015, 34, S87–S88.
- Brasseur, A.; Scolletta, S.; Lorusso, R.; Taccone, F.S. Hybrid extracorporeal membrane oxygenation. J. Thorac. Dis. 2018, 10, S707–S715.
- Brogan, T.V.; Lequier, L.; Lorusso, R.; MacLaren, G.; Peek, G.J. Extracorporeal Life Support: The ELSO Red Book, 5th ed.; Extracorporeal Life Support Organization: Ann Arbor, MI, USA, 2017; p. 831.
- Khan, S.; Vasavada, R.; Qiu, F.; Kunselman, A.; Undar, A. Extracorporeal life support systems: Alternative vs. conventional circuits. Perfusion 2011, 26, 191–198.
- Palanzo, D.; Qiu, F.; Baer, L.; Clark, J.B.; Myers, J.L.; Undar, A. Evolution of the extracorporeal life support circuitry. Artif. Organs 2010, 34, 869–873.
- Iwahashi, H.; Yuri, K.; Nosé, Y. Development of the oxygenator: Past, present, and future. J. Artif. Organs 2004, 7, 111–120.
- Diehl, A.; Pellegrino, V.; Sheldrake, J. ECMO Guideline. Available online: https://ecmo.icu/#menuRoot (accessed on 9 July 2022).
- Warren, O.J.; Smith, A.J.; Alexiou, C.; Rogers, P.L.; Jawad, N.; Vincent, C.; Darzi, A.W.; Athanasiou, T. The inflammatory response to cardiopulmonary bypass: Part 1—Mechanisms of pathogenesis. J. Cardiothorac. Vasc. Anesth. 2009, 23, 223–231.
- Warren, O.J.; Watret, A.L.; de Wit, K.L.; Alexiou, C.; Vincent, C.; Darzi, A.W.; Athanasiou, T. The inflammatory response to cardiopulmonary bypass: Part 2—Anti-inflammatory therapeutic strategies. J. Cardiothorac. Vasc. Anesth. 2009, 23, 384–393.
- Landis, R.C.; Brown, J.R.; Fitzgerald, D.; Likosky, D.S.; Shore-Lesserson, L.; Baker, R.A.; Hammon, J.W. Attenuating the Systemic Inflammatory Response to Adult Cardiopulmonary Bypass: A Critical Review of the Evidence Base. J. Extra-Corpor. Technol. 2014, 46, 197–211.
- Paparella, D.; Yau, T.M.; Young, E. Cardiopulmonary bypass induced inflammation: Pathophysiology and treatment. An update. Eur. J. Cardio-Thorac. Surg. 2002, 21, 232–244.
- Warltier, D.C.; Laffey, J.G.; Boylan, J.F.; Cheng, D.C.H. The Systemic Inflammatory Response to Cardiac Surgery: Implications for the Anesthesiologist. Anesthesiology 2002, 97, 215–252.
- Conway, E.M. Thrombomodulin and its role in inflammation. Semin. Immunopathol. 2012, 34, 107–125.
- Moore, K.L.; Esmon, C.T.; Esmon, N.L. Tumor necrosis factor leads to the internalization and degradation of thrombomodulin from the surface of bovine aortic endothelial cells in culture. Blood 1989, 73, 159–165.
- Lindmark, E.; Tenno, T.; Siegbahn, A. Role of platelet P-selectin and CD40 ligand in the induction of monocytic tissue factor expression. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2322–2328.
- Van de Wouwer, M.; Collen, D.; Conway, E.M. Thrombomodulin-protein C-EPCR system: Integrated to regulate coagulation and inflammation. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1374–1383.
- Devaraj, S.; Xu, D.Y.; Jialal, I. C-reactive protein increases plasminogen activator inhibitor-1 expression and activity in human aortic endothelial cells: Implications for the metabolic syndrome and atherothrombosis. Circulation 2003, 107, 398–404.
- Wolbink, G.J.; Bossink, A.W.; Groeneveld, A.B.; de Groot, M.C.; Thijs, L.G.; Hack, C.E. Complement activation in patients with sepsis is in part mediated by C-reactive protein. J. Infect. Dis. 1998, 177, 81–87.
- Han, K.H.; Hong, K.H.; Park, J.H.; Ko, J.; Kang, D.H.; Choi, K.J.; Hong, M.K.; Park, S.W.; Park, S.J. C-reactive protein promotes monocyte chemoattractant protein-1--mediated chemotaxis through upregulating CC chemokine receptor 2 expression in human monocytes. Circulation 2004, 109, 2566–2571.
- Cheung, P.Y.; Sawicki, G.; Salas, E.; Etches, P.C.; Schulz, R.; Radomski, M.W. The mechanisms of platelet dysfunction during extracorporeal membrane oxygenation in critically ill neonates. Crit. Care Med. 2000, 28, 2584–2590.
- Hamad, O.A.; Bäck, J.; Nilsson, P.H.; Nilsson, B.; Ekdahl, K.N. Platelets, complement, and contact activation: Partners in inflammation and thrombosis. Adv. Exp. Med. Biol. 2012, 946, 185–205.
- Whiteheart, S.W. Platelet granules: Surprise packages. Blood 2011, 118, 1190–1191.
- Kraft, F.; Schmidt, C.; Van Aken, H.; Zarbock, A. Inflammatory response and extracorporeal circulation. Best Pract. Research. Clin. Anaesthesiol. 2015, 29, 113–123.
- Esmon, C.T. The interactions between inflammation and coagulation. Br. J. Haematol. 2005, 131, 417–430.
- Mulloy, B.; Hogwood, J.; Gray, E.; Lever, R.; Page, C.P. Pharmacology of Heparin and Related Drugs. Pharmacol. Rev. 2016, 68, 76–141.
- Levy, J.H.; Connors, J.M. Heparin Resistance-Clinical Perspectives and Management Strategies. N. Engl. J. Med. 2021, 385, 826–832.
- Doyle, A.J.; Hunt, B.J. Current Understanding of How Extracorporeal Membrane Oxygenators Activate Haemostasis and Other Blood Components. Front. Med. 2018, 5, 352.
- Malhotra Kapoor, P.; Karanjkar, A.; Bhardwaj, V. Evaluation of coagulopathy on veno-arterial ECMO (VA) extracorporeal membrane oxygenation using platelet aggregometry and standard tests: A narrative review. Egypt. J. Crit. Care Med. 2018, 6, 73–78.
- Cartwright, B.; Bruce, H.M.; Kershaw, G.; Cai, N.; Othman, J.; Gattas, D.; Robson, J.L.; Hayes, S.; Alicajic, H.; Hines, A.; et al. Hemostasis, coagulation and thrombin in venoarterial and venovenous extracorporeal membrane oxygenation: The HECTIC study. Sci. Rep. 2021, 11, 7975.
- Ki, K.K.; Passmore, M.R.; Chan, C.H.H.; Malfertheiner, M.V.; Fanning, J.P.; Bouquet, M.; Millar, J.E.; Fraser, J.F.; Suen, J.Y. Low flow rate alters haemostatic parameters in an ex-vivo extracorporeal membrane oxygenation circuit. Intensive Care Med. Exp. 2019, 7, 51.
- Wendel, H.P.; Scheule, A.M.; Eckstein, F.S.; Ziemer, G. Haemocompatibility of paediatric membrane oxygenators with heparin-coated surfaces. Perfusion 1999, 14, 21–28.
- Rodell, T.C.; Naidoo, Y.; Bhoola, K.D. Role of Kinins in Inflammatory Responses. Clin. Immunother. 1995, 3, 352–361.
- Wachtfogel, Y.T.; Hack, C.E.; Nuijens, J.H.; Kettner, C.; Reilly, T.M.; Knabb, R.M.; Bischoff, R.; Tschesche, H.; Wenzel, H.; Kucich, U.; et al. Selective kallikrein inhibitors alter human neutrophil elastase release during extracorporeal circulation. Am. J. Physiol. 1995, 268, H1352–H1357.
- Larsson, M.; Rayzman, V.; Nolte, M.W.; Nickel, K.F.; Björkqvist, J.; Jämsä, A.; Hardy, M.P.; Fries, M.; Schmidbauer, S.; Hedenqvist, P.; et al. A factor XIIa inhibitory antibody provides thromboprotection in extracorporeal circulation without increasing bleeding risk. Sci. Transl. Med. 2014, 6, 222ra217.
- Wallisch, M.; Lorentz, C.U.; Lakshmanan, H.H.S.; Johnson, J.; Carris, M.R.; Puy, C.; Gailani, D.; Hinds, M.T.; McCarty, O.J.T.; Gruber, A.; et al. Antibody inhibition of contact factor XII reduces platelet deposition in a model of extracorporeal membrane oxygenator perfusion in nonhuman primates. Res. Pract. Thromb. Haemost. 2020, 4, 205–216.
- Hayes, D., Jr.; Preston, T.J.; Kirkby, S.; Nicol, K.K. Human leukocyte antigen sensitization in lung transplant candidates supported by extracorporeal membrane oxygenation. Am. J. Respir. Crit. Care Med. 2013, 188, 627–628.
- Ranucci, M.; Ballotta, A.; Di Dedda, U.; Baryshnikova, E.; Dei Poli, M.; Resta, M.; Falco, M.; Albano, G.; Menicanti, L. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J. Thromb. Haemost. JTH 2020, 18, 1747–1751.
- Lim, M.S.; McRae, S. COVID-19 and immunothrombosis: Pathophysiology and therapeutic implications. Crit. Rev. Oncol./Hematol. 2021, 168, 103529.
More
Information
Subjects:
Critical Care Medicine
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
892
Revisions:
2 times
(View History)
Update Date:
09 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




