Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | José Lozada-Ramírez | -- | 1882 | 2023-02-03 19:15:07 | | | |
| 2 | Rita Xu | -15 word(s) | 1867 | 2023-02-06 06:21:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
González-Peña, M.A.; Ortega-Regules, A.E.; Parrodi, C.A.D.; Lozada-Ramírez, J.D. Chemistry, Occurrence, Properties of Carotenoids. Encyclopedia. Available online: https://encyclopedia.pub/entry/40821 (accessed on 07 February 2026).
González-Peña MA, Ortega-Regules AE, Parrodi CAD, Lozada-Ramírez JD. Chemistry, Occurrence, Properties of Carotenoids. Encyclopedia. Available at: https://encyclopedia.pub/entry/40821. Accessed February 07, 2026.
González-Peña, Marco Antonio, Ana Eugenia Ortega-Regules, Cecilia Anaya De Parrodi, José Daniel Lozada-Ramírez. "Chemistry, Occurrence, Properties of Carotenoids" Encyclopedia, https://encyclopedia.pub/entry/40821 (accessed February 07, 2026).
González-Peña, M.A., Ortega-Regules, A.E., Parrodi, C.A.D., & Lozada-Ramírez, J.D. (2023, February 03). Chemistry, Occurrence, Properties of Carotenoids. In Encyclopedia. https://encyclopedia.pub/entry/40821
González-Peña, Marco Antonio, et al. "Chemistry, Occurrence, Properties of Carotenoids." Encyclopedia. Web. 03 February, 2023.
Copy Citation
Carotenoids are natural lipophilic pigments and antioxidants that are present in many fruits and vegetables. The consumption of carotenoids is correlated with positive health effects and a decreased risk of several chronic diseases. Provitamin A carotenoids (β-carotene, α-carotene, γ-carotene, and β-cryptoxanthin) are essential for the development and maintenance of sight. β-carotene, α-carotene, zeaxanthin, β-cryptoxanthin, lutein, and lycopene have high antioxidant activity and promote free radical scavenging, which helps protect against chronic diseases.
carotenoids
antioxidants
vitamin A
bioavailability
1. Introduction
Carotenoids are a group of pigments found in fruits, flowers and vegetables, such as tomato, carrot, pineapple, papaya, marigold flower, sunflower, annatto, saffron, and green leaves. They are responsible for yellow, orange, and red colors in plants, and are used commercially as natural colorants and ingredients in nutritional supplements [1].
In recent years, studies of plant pigments have increased in importance, given their provitamin A activity, and they have been classified as natural antioxidants and bioactive compounds. Studies have shown evidence of their role in the prevention of chronic degenerative diseases, cardiovascular diseases, cancer, macular degeneration and cataract formation [2]. Carotenoids are involved in immune system modulation and cell communication, embryonic development, hematopoiesis and apoptosis, and possess antioxidant, anti-inflammatory, anti-angiogenic and antiproliferative properties [3][4].
However, the use of carotenoids in the food industry is restricted due to their poor water solubility, low bioavailability, chemical instability and high sensitivity to oxidation in multiple environmental conditions, such as heat, light, oxygen, acids and metal ions [5]. To overcome this inconvenience, encapsulation techniques have been used to improve the stability, solubility and bioavailability of carotenoids. Encapsulation has been used in the food industry for more than 60 years to cover food ingredients, enzymes, cells or other functional compounds in small capsules, to protect them from environmental conditions, increase their shelf life, or to mask component attributes such as undesirable flavors [6].
2. Chemistry of Carotenoids
Carotenoids are a group of pigments, mostly of plant origin, responsible for the yellow, orange and red colors in fruits and vegetables. All have antioxidant activity, and some are precursors of vitamin A. Moreover, carotenoids have a role in intercellular communication, immune system activation and disease prevention [3][7], and hence they promote human health.
Carotenoids comprise eight repetitive units of isoprene, with cyclic or linear structures at both ends of the carbon chains, resulting in multiple cis and trans isomers, with the latter being more abundant in nature [8][9]. Carotenoids are classified into carotenes and xanthophylls. Carotenes, such as α-carotene, β-carotene, γ-carotene, and lycopene [8], are highly soluble in organic solvents and insoluble in polar solvents. In contrast, xanthophylls are soluble in polar solvents (e.g., alcohols) and organic solvents (e.g., ether and hexane). They are oxygenated derivatives of carotenes, forming alcohols, aldehydes, ketones, and acids. Examples of xanthophylls are fucoxanthin, lutein and violaxanthin [8][9].
Carotenoids are stored in plant tissues (plastids), such as chromoplasts (colored plastids), amyloplasts (starch storage plastids) and elaioplasts (lipid storage plastids). In fruits, flowers and roots, carotenoids are located in the chromoplast, whereas in grains and oilseeds they are located in amyloplasts and elaioplasts, respectively [10]. In contrast, xanthophylls are freely found in green plant tissues, whereas in fruits and flowers they are found as esters of fatty acids [11].
The biosynthesis of carotenoids (Figure 1) takes place in the chloroplasts. Two molecules of geranylgeranyl diphosphate (GGPP) are produced from isopentenyl pyrophosphate (IPP) and dimethylallyl diphosphate (DMAPP), catalyzed by geranylgeranyl pyrophosphate synthase (GGPS). After this, two molecules of GGPP are condensed into phytoene by phytoene synthase (PSY). Subsequently, phytoene is desaturated into lycopene by ζ-carotene desaturase (ZDS) and phytoene desaturase (PDS). Lycopene is cyclized into α-carotene (α pathway) and β-carotene (β pathway), in reactions catalyzed by lycopene-ε (LYC-ε) and β-cyclase (LYC-β), respectively. Xanthophylls are synthesized from carotenes; lutein is formed by the action α-carotene ring-ε hydroxylase (CHY-ε) via the α pathway; β-carotene is converted into β-cryptoxanthin via the β pathway, in a reaction catalyzed by β-carotene hydroxylase (CHY-β), which also catalyzes its conversion into zeaxanthin. In turn, zeaxanthin can be converted into violaxanthin by zeaxanthin epoxidase (ZEP); conversely, violaxanthin can be converted into zeaxanthin by violaxanthin de-epoxidase (VDE). Finally, violaxanthin is converted into neoxanthin by neoxanthin synthase (NXS) [5][10][12].
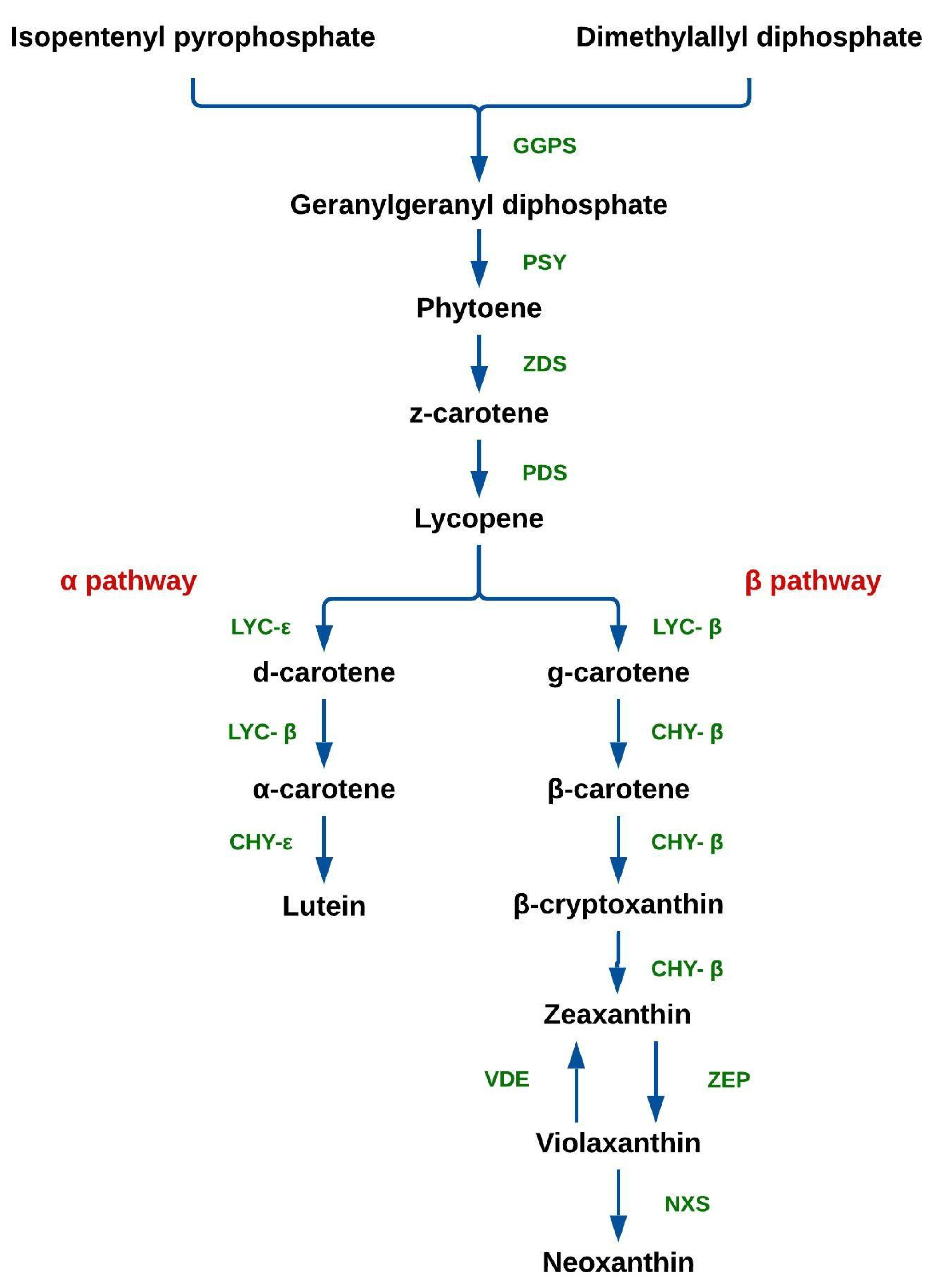
Figure 1. Carotenoid biosynthesis pathway in plants. Geranyl-geranyl pyrophosphate synthase (GGPS), phytoene synthase (PSY), ζ-carotene desaturase (ZDS), phytoene desaturase (PDS), lycopene ε-cyclase (LYC-ε), lycopene β-cyclase (LYC-β), α-carotene ring-ε hydroxylase (CHY-ε), β-carotene hydroxylase (CHY-β), zeaxanthin epoxidase (ZEP), violaxanthin de-epoxidase (VDE), and neoxanthin synthase (NXS).
3. Natural Occurrence of Carotenoids
More than 700 carotenoids have been identified, yet only 40 are included in the human diet, with β-carotene, α-carotene, lycopene, β-cryptoxanthin, lutein, and zeaxanthin the most common [13]. Carotenoid intake comes primarily from foods of plant origin (fruits and vegetables). α- and β-carotene are usually found in carrot, orange, pumpkin, pepper, sweet potato, mango, and vegetable leaves, varying in color from yellow, orange, and red. Lycopene imparts a bright red color to food, and is the main carotenoid in tomatoes, although it is also present in watermelon, guava, and papaya. β-cryptoxanthin is found in citrus fruit, melon, potato, guava and apple, giving them yellow and orange colors. The isomers lutein and zeaxanthin are found together naturally in yellow corn and marigold flower, although they are also found in broccoli, pumpkin, pepper, vegetable leaves, seeds and legumes. The xanthophylls violaxanthin (yellow), capsanthin, and capsorubin (orange to red) are frequently found in paprika and pepper. Neoxanthin, characterized by its yellow color, is a natural component of vegetable leaves. Bixin is the main component of annatto and is responsible for its characteristic red-brown color. Crocin is responsible for the yellow coloration of saffron [3][8][9][10].
Some carotenoids are found only in algae and seafood. Astaxanthin is responsible for the pink-red color of shrimp, salmon, and flamingo feathers, as it is naturally found in krill and microalgae haematococcus pluvialis, which are consumed by small crustaceans (shrimp and crawfish), fish (salmon), and birds (flamingo) [14][15], in that order. Fucoxanthin has a characteristic brown color and is only found in microalgae and the chloroplasts of brown algae [16].
The most abundant carotenoids in the Western diet include β-carotene and α-carotene from carrot, pumpkin, apricot, pepper, mango, coriander and spinach [3][13][17][18]; lutein from broccoli, pumpkin, spinach, corn, mango and papaya [3][13]; and lycopene from tomato, guava, papaya and watermelon [3][13][17][18][19][20].
Britton and Khachik (2009) proposed a ranking of fruits and vegetables based on their carotenoid levels, grouping the foods into the following categories: low level (0–1 µg/g), moderate level (1–5 µg/g), high level (5–20 µg/g), and very high level (>20 µg/g).
Carotenoid composition and content in food are affected by many factors, such as those inherent to the plant (variety, genotype and ripening stage), external to the plant (harvest season, growth conditions, post-harvest treatment, handling, storage conditions, plant diseases, and climatic conditions) [3][13].
4. Bioavailability of Carotenoids
Carotenoid bioavailability is defined as the fraction of carotenoid released from food that is absorbed in the intestine and made available for physiological processes or storage [13].
The nature of the food matrix containing carotenoids strongly affects their bioavailability. Due to their hydrophobic nature and location in plant tissues (plastids), carotenoid bioavailability in raw fruits and vegetables is limited. Therefore, carotenoids must be released from the cellular matrix and incorporated into a lipid fraction (micelles) during digestion to be absorbed [2].
Carotenoids are released from food mechanically by chewing, and chemically by the action of digestive enzymes (amylases, lipases, pepsin) and hydrochloric acid in the stomach [21]. These processes contribute to particle size reduction, thus increasing contact area for pancreatic lipases, bile salts, and enzymes, such as pancreatic amylases, nucleosidases, trypsinogen, chymotrypsinogen, carboxypeptidase, elastases, phospholipases, and carboxyl lipase ester, and the release of carotenoids and their incorporation into micelles [3][22]. Bile salts act as emulsifiers assisting with micelles formation and stabilization, whereas lipases break down lipids into fatty acids and monoglycerides, encouraging emulsification [22]. Micelles are absorbed into the enterocytes through passive diffusion or by binding to receptor proteins in the apical membrane for easy diffusion [2].
After absorption, carotenoids are enclosed in chylomicrons and transported to the bloodstream through the lymphatic system [7][17]. Once they have entered the bloodstream, the carotenoids are transported to the liver, where they are either stored or metabolized into vitamin A (only provitamin A carotenoids) by β-carotene binding oxygenases (BCO1 and BCO2). The rest are released back into the circulation and integrated into very low density (VLDL), low density (LDL), and high-density (HDL) lipoproteins to be distributed to other tissues, such as adipose tissue (vitamin A storage), skin and subcutaneous tissues (carotenes and xanthophylls reserve), macula lutea in the retina (lutein, zeaxanthin, and mesozeaxanthin), pancreas and vascular endothelium [22].
Carotenoid bioavailability is influenced by dietary factors, such as content and nature of carotenoids, fat content in the diet, and the interaction between carotenoids and other food components; and physiological factors, such as the rate of carotenoid absorption, nutritional state, genetic factors, and metabolism [2][17]. For instance, dietary fiber, especially soluble fiber, has been found to limit carotenoid availability as it affects the viscosity of the gastrointestinal content, size of lipid droplets, availability of bile salts, and enzymatic lipolysis of triglycerides [21]. Furthermore, Gul et al. [17] and Saini et al. [3] reported that the bioavailability of β-carotene in plants is low because carotenoids are bound to protein complexes, fibers, and cell walls, rendering them resistant to digestion and degradation, thus limiting their release. In contrast, several researchers have demonstrated the effect of minerals on the bioavailability of carotenoids. Borel et al. [23] found that the bioavailability of lycopene found in tomato paste is limited when ingested with 500 mg of calcium, although the mechanism is not completely understood, and micelle formation may be the limiting factor. Corte-Real et al. [24] found that the bioavailability of spinach carotenoids is not affected by 500 mg and 1000 mg of calcium.
Adding fat to the food improves the bioavailability of carotenoids as lipids favor micelle formation through the release of bile salts [2]. In this sense, Marriage et al. [25] showed that plasma concentrations of lycopene and zeaxanthin are higher when carotenoids are ingested with mono-and diacylglycerides (safflower oil) than when fat is not consumed. Similarly, White et al. [26] studied the effect of soybean oil on the absorption and bioavailability of carotenoids from spinach, lettuce, carrot, and tomato. The plasma concentrations of α-carotene, β-carotene, lutein, and lycopene increased as the concentration of soybean oil increased.
Thermal treatment affects both the cell wall and carotenoid content of plants, in turn altering their bioavailability. Aschoff et al. [27] demonstrated that the bioavailability of β-cryptoxanthin, zeinoxanthin and lutein in pasteurized orange juice is higher than in fresh orange juice. In contrast, Vimala et al. [28] evaluated carotenoid content in sweet potato undergoing different treatments (cooking, frying, oven-drying, and sun-drying). Oven-drying (50–60 °C) maintained 90% of β-carotene in sweet potato compared to the fresh product, whereas all other treatments decreased carotenoid content between 15% and 30%. Odriozola-Serrano et al. [29] examined the effect of pasteurization and electrical pulses on the carotenoid content of tomato juice. They found that tomato juice treated with electrical pulses had a higher carotenoid content. Thus, pulse treatment is the most efficient method of preserving carotenoid content and increasing their bioavailability compared to the traditional treatment. In all previously cited examples, there is a decrease in total carotenoid content; nevertheless, the bioavailability of carotenoids improves by reducing dietary fiber, releasing cellular content, softening plant material, and reducing the interactions between carotenoids and other food components. Thus, promoting both the release of carotenoids and formation of micelles helps increase their absorption.
References
- Li, H.; Tsao, R.; Deng, Z. Factors Affecting the Antioxidant Potential and Health Benefits of Plant Foods. Can. J. Plant Sci. 2012, 92, 1101–1111.
- Lemmens, L.; Colle, I.; Van Buggenhout, S.; Palmero, P.; Van Loey, A.; Hendrickx, M. Carotenoid Bioaccessibility in Fruit- and Vegetable-Based Food Products as Affected by Product (Micro)Structural Characteristics and the Presence of Lipids: A Review. Trends Food Sci. Technol. 2014, 38, 125–135.
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from Fruits and Vegetables: Chemistry, Analysis, Occurrence, Bioavailability and Biological Activities. Food Res. Int. 2015, 76, 735–750.
- Yahia, E.M.; Gutiérrez-Orozco, F.; Arvizu-de Leon, C. Phytochemical and Antioxidant Characterization of Mamey (Pouteria sapota Jacq. H.E. Moore & Stearn) Fruit. Food Res. Int. 2011, 44, 2175–2181.
- Butnariu, M. Methods of Analysis (Extraction, Separation, Identification and Quantification) of Carotenoids from Natural Products. J. Ecosyst. Ecography 2016, 6, 1–9.
- Santos, D.T.; Meireles, M.A.A. Carotenoid Pigments Encapsulation: Fundamentals, Techniques and Recent Trends. Open Chem. Eng. J. 2010, 4, 42–50.
- Salter-Venzon, D.; Kazlova, V.; Izzy Ford, S.; Intra, J.; Klosner, A.E.; Gellenbeck, K.W. Evidence for Decreased Interaction and Improved Carotenoid Bioavailability by Sequential Delivery of a Supplement. Food Sci. Nutr. 2017, 5, 424–433.
- Guerrero-Legarreta, I.; López-Hernández, E.; Armenta-López, R.E.; García-Barrientos, R. Pigmentos. In Química de Alimentos; Badui-Dergal, S., Ed.; Pearson: Mexico City, Mexico, 2013; p. 379.
- Schawartz, S.J.; Cooperstone, J.L.; Cichon, M.J.; von Elbe, J.H.; Giusti, M.M. Colorants. In Fennema’s Food Chemistry; Damodaran, S., Parkin, K.L., Eds.; CRC PressTaylor & Francis: Boca Raton, FL, USA, 2017; p. 681.
- Howitt, C.A.; Pogson, B.J. Carotenoid Accumulation and Function in Seeds and Non-Green Tissues. Plant Cell Environ. 2006, 29, 435–445.
- Perera, C.O.; Yen, G.M. Functional Properties of Carotenoids in Human Health. Int. J. Food Prop. 2007, 10, 201–230.
- Kang, L.; Park, S.-C.; Ji, C.Y.; Kim, H.S.; Lee, H.-S.; Kwak, S.-S. Metabolic Engineering of Carotenoids in Transgenic Sweetpotato. Breed. Sci. 2017, 67, 27–34.
- Olmedilla-Alonso, B. Carotenoids: Content in Foods, in Diet and Bioavailability. COST Action EUROCAROTEN CA15136. Sci. Newsl. 2017, 2, 1–9.
- Food-Info Foundation E-Numbers: E100–E200 Food Colours. Available online: http://www.food-info.net/uk/e/e100-200.htm (accessed on 2 June 2020).
- Liu, X.; Luo, Q.; Cao, Y.; Goulette, T.; Liu, X.; Xiao, H. Mechanism of Different Stereoisomeric Astaxanthin in Resistance to Oxidative Stress in Caenorhabditis elegans. J. Food Sci. 2016, 81, H2280–H2287.
- Kumar, S.; Hosokawa, M.; Miyashita, K. Fucoxanthin: A Marine Carotenoid Exerting Anti-Cancer Effects by Affecting Multiple Mechanisms. Mar. Drugs 2013, 11, 5130–5147.
- Gul, K.; Tak, A.; Singh, A.K.; Singh, P.; Yousuf, B.; Wani, A.A. Chemistry, Encapsulation, and Health Benefits of β-Carotene—A Review. Cogent Food Agric. 2015, 1, 1018696.
- Yoon, G.-A.; Yeum, K.-J.; Cho, Y.-S.; Chen, C.-Y.O.; Tang, G.; Blumberg, J.B.; Russell, R.M.; Yoon, S.; Lee-Kim, Y.C. Carotenoids and Total Phenolic Contents in Plant Foods Commonly Consumed in Korea. Nutr. Res. Pract. 2012, 6, 481.
- Bagetti, M.; Facco, E.M.P.; Piccolo, J.; Hirsch, G.E.; Rodriguez-Amaya, D.; Kobori, C.N.; Vizzotto, M.; Emanuelli, T. Physicochemical Characterization and Antioxidant Capacity of Pitanga Fruits (Eugenia uniflora L.). Food Sci. Technol. 2011, 31, 147–154.
- Chandrika, U.G.; Fernando, K.S.S.P.; Ranaweera, K.K.D.S. Carotenoid Content and in Vitro Bioaccessibility of Lycopene from Guava (Psidium guajava) and Watermelon (Citrullus lanatus) by High-Performance Liquid Chromatography Diode Array Detection. Int. J. Food Sci. Nutr. 2009, 60, 558–566.
- Cervantes-Paz, B.; Victoria-Campos, C.I.; de Ornelas-Paz, J.J. Absorption of Carotenoids and Mechanisms Involved in Their Health-Related Properties. In Carotenoids in Nature; Stange, C., Ed.; Springer International Publishing: Cham, Switzerland, 2016; Volume 79, pp. 415–454. ISBN 978-3-319-39124-3.
- Bohn, T.; Desmarchelier, C.; Dragsted, L.O.; Nielsen, C.S.; Stahl, W.; Rühl, R.; Keijer, J.; Borel, P. Host-Related Factors Explaining Interindividual Variability of Carotenoid Bioavailability and Tissue Concentrations in Humans. Mol. Nutr. Food Res. 2017, 61, 1600685.
- Borel, P.; Desmarchelier, C.; Dumont, U.; Halimi, C.; Lairon, C.; Paige, C.; Sébédio, L.; Bulsson, C.; Buffiére, C.; Rémond, D. Dietary Calcium Impairs Tomato Lycopene Bioavailability in Healthy Humans. Br. J. Nutr. 2016, 116, 2091–2096.
- Corte-Real, J.; Guignard, C.; Gantenbein, M.; Weber, B.; Burgard, K.; Hoffmann, L.; Richling, E.; Bohn, T. No Influence of Supplemental Dietary Calcium Intake on the Bioavailability of Spinach Carotenoids in Humans. Br. J. Nutr. 2017, 117, 1560–1569.
- Marriage, B.J.; Williams, J.A.; Choe, Y.S.; Maki, K.C.; Vurma, M.; DeMichele, S.J. Mono- and Diglycerides Improve Lutein Absorption in Healthy Adults: A Randomised, Double-Blind, Cross-over, Single-Dose Study. Br. J. Nutr. 2017, 118, 813–821.
- White, W.S.; Zhou, Y.; Crane, A.; Dixon, P.; Quadt, F.; Flendring, L.M. Modeling the Dose Effects of Soybean Oil in Salad Dressing on Carotenoid and Fat-Soluble Vitamin Bioavailability in Salad Vegetables. Am. J. Clin. Nutr. 2017, 106, 1041–1051.
- Aschoff, J.K.; Rolke, C.L.; Breusing, N.; Bosy-Westphal, A.; Högel, J.; Carle, R.; Schweiggert, R.M. Bioavailability of β-Cryptoxanthin Is Greater from Pasteurized Orange Juice than from Fresh Oranges—A Randomized Cross-over Study. Mol. Nutr. Food Res. 2015, 59, 1896–1904.
- Vimala, B.; Nambisan, B.; Hariprakash, B. Retention of Carotenoids in Orange-Fleshed Sweet Potato during Processing. J. Food Sci. Technol. 2011, 48, 520–524.
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Hernández-Jover, T.; Martín-Belloso, O. Carotenoid and Phenolic Profile of Tomato Juices Processed by High Intensity Pulsed Electric Fields Compared with Conventional Thermal Treatments. Food Chem. 2009, 112, 258–266.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Revisions:
2 times
(View History)
Update Date:
06 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




