You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Roberta Cassano | -- | 2888 | 2023-02-03 11:19:43 | | | |
| 2 | Rita Xu | -14 word(s) | 2874 | 2023-02-03 11:30:02 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Serini, S.; Cassano, R.; Curcio, F.; Trombino, S.; Calviello, G. Omega-3 PUFAs and OvCa. Encyclopedia. Available online: https://encyclopedia.pub/entry/40806 (accessed on 25 December 2025).
Serini S, Cassano R, Curcio F, Trombino S, Calviello G. Omega-3 PUFAs and OvCa. Encyclopedia. Available at: https://encyclopedia.pub/entry/40806. Accessed December 25, 2025.
Serini, Simona, Roberta Cassano, Federica Curcio, Sonia Trombino, Gabriella Calviello. "Omega-3 PUFAs and OvCa" Encyclopedia, https://encyclopedia.pub/entry/40806 (accessed December 25, 2025).
Serini, S., Cassano, R., Curcio, F., Trombino, S., & Calviello, G. (2023, February 03). Omega-3 PUFAs and OvCa. In Encyclopedia. https://encyclopedia.pub/entry/40806
Serini, Simona, et al. "Omega-3 PUFAs and OvCa." Encyclopedia. Web. 03 February, 2023.
Copy Citation
Different strategies have been investigated for a more satisfactory treatment of advanced breast cancer, including the adjuvant use of omega-3 polyunsaturated fatty acids (PUFAs). These nutritional compounds have been shown to possess potent anti-inflammatory and antiangiogenic activities, the capacity to affect transduction pathways/receptors involved in cell growth and to reprogram tumor microenvironment. Omega-3 PUFA-containing nanoformulations designed for drug delivery in breast cancer were shown to potentiate the effects of enclosed drugs, enhance drug delivery to target sites, and minimize drug-induced side effects.
breast cancer
in vitro studies
nanoformulations
omega-3 PUFA
1. Introduction
Over the past few decades, the potential preventive or therapeutic use of nutraceuticals as anti-inflammatory or antineoplastic agents in different pathological settings has represented an area of great interest [1][2][3][4][5][6][7]. In the past, researchers explored whether combinations of nutraceuticals, known for their anti-inflammatory and/or antineoplastic activities with conventional or innovative anticancer drugs, could potentially enhance the drug effects and ameliorate the physical conditions of patients [8][9][10][11][12]. Most recently, researchers have also investigated the possibility of improving the action of nutraceuticals or anticancer drugs through their inclusion in nanomaterials with the potential to protect and, more specifically, deliver them to tissues affected by pathological processes [13][14][15][16][17][18]. The strategy of administrating bioactive drugs and/or nutraceuticals in nanoformulations has recently attracted considerable interest worldwide [19]. This nanotherapeutic approach was initially developed since blood vessel endothelium in solid tumors shows enhanced permeability and can be easily crossed in both directions by free antineoplastic drugs and bioactive nutritional compounds having relatively low molecular weight, thus, not allowing an efficient accumulation of these compounds by tumor tissues. On the other hand, nanostructures with a diameter ranging from 10 to 500 nm go through the highly permeable tumor vascular wall but tend to accumulate in the underlying interstitium. These features of the tumor endothelium are known as the permeability and retention effect (EPR), a phenomenon allowing nanoparticles to target tumor cells effectively and passively [20]. In addition, nanoparticle build-up at tumor level has been also related to the insufficient drainage of the fluid escaped from tumor vessels by the lymphatic vessels [21].
Particularly, in recent years, there has been a remarkable upsurge of interest in the potential application of nanotechnologies for the therapy of breast cancer (BCa), which represents the world’s most prevalent cancer [22]. The high rate of frequency of BCa has favored a large-scale mammography screening prevention practice in the female population and its early detection, which has resulted in a considerable decrease in the rate of mortality for this tumor in the last few decades [23]. Nevertheless, despite the early BCa mortality rate has been reduced by modern multimodal therapy approaches, currently, this cancer remains the second-leading cause of cancer-related death in women worldwide [24], by advanced BCa with metastases being a virtually incurable disease, showing a 2/3-year median overall survival [24]. In order to obtain a satisfactory treatment for advanced BCa, many routes are being pursued, including the adjuvant use of nutraceuticals showing antioxidant, anti-inflammatory or antineoplastic properties [25]. The nutraceutical strategy should not be considered as something archaic in comparison to the currently used innovative approaches of personalized targeted therapies and immunotherapies [12]. In fact, some of these dietary compounds were demonstrated to have powerful antiangiogenic effects, the capacity to specifically affect molecular transduction pathways and receptor activities involved in cell growth, as well as to reprogram tumor microenvironments [15][26]. The most recent and relevant advancement in this field of research is the inclusion of these natural bioactive substances in nanoformulations, either alone or in combination with conventional drugs, to better and more specifically deliver them to the neoplastic lesions.
Most of the recently published reports focused on the possibility of using nutraceutical-based nanoformulations for improving the anti-cancer treatment of BCa, flavonoids (such as curcumin, quercetin, epigallocatechin gallate (EGCG), and resveratrol) [27] or garlic constituents [28] have been included in the nanomaterials under study. Starting from these results, two comprehensive reviews of the literature have been recently published [27][28]. Instead, researchers decided to critically analyze the most recent (2017–2022) literature focusing on dietary omega-3 PUFAs enclosed in newly developed nanomaterials for their possible use in BCa therapy. This represents a specific update for BCa [15], researchers already discussed the results of reports that had been published during the period July 2017–July 2018 and that were focused on omega-3 PUFA-containing formulations for the therapy of cardiovascular diseases and cancers of different origin.
Important properties of omega-3 PUFAs may explain the reasons underlying their frequent inclusion in nanoformulations designed for cancer therapy, and BCa in particular. Among them, there are their powerful and widely demonstrated anti-inflammatory and anti-cancer activities [2][29][30][31]. Their anti-inflammatory property has been often invoked to explain their cancer-preventive effects, as well as their efficacy in inhibiting the deleterious side effects frequently associated with chemotherapy [32][33]. However, plenty of preclinical studies have shown that these FAs may exert other and more direct and specific anticancer activities at molecular levels [30][34][35][36][37]. Moreover, it was also shown that they can potentially increase cancer cell sensitivity to conventional antineoplastic therapy [38]. It has been reported that these FAs are able to modulate the activities of a series of membrane-associated receptors and transporters, as well as protein kinases and phosphatases, transcription factors and factors driving and regulating apoptotic or autophagic processes [31]. There is a unifying hypothesis according to which these pleiotropic activities of omega-3 PUFAs may be related to the deep changes that they are able to induce in the lipid microenvironments of cell membranes (lipid rafts) as they become incorporated into them [31]. Consequently, changes in the activity/expression of molecular factors/signaling pathways located there may take place.
Speaking specifically of BCa, it should be underlined that, besides the vast amount of preclinical studies providing evidence for the antineoplastic efficacy of these FAs and exploring their possible mechanisms of action, there exists a consistent body of literature on the outcomes of observational and interventional human studies that substantiate and encourage the possibility of omega-3 PUFA use in the clinical setting (for a review see: [39]).
Another interesting aspect that makes these FAs ideal components of nanoformulations constructed for a more specific delivery to tumor tissues is that cancer cells grow at an abnormally high rate and need great amounts of FAs for the development of their membranes. In particular, cancer cells require and incorporate high amounts of the essential omega-3 PUFA α-linolenic acid (ALA, 18:3 ω-3), which cannot be synthesized by mammalian cells, as well as its metabolic derivatives, the long-chain omega-3 PUFAs eicosapentaenoic acid (EPA, 20:5 ω-3) and docosahexaenoic acid (DHA, 22:6 ω-3). Moreover, the presence of multiple double bonds in their moieties makes them highly peroxidable. Therefore, their inclusion in nanoformulations may help to protect them from oxidative degradation and increase their bioavailability and specific effects at tumor level.
Moreover, recently, considerable effort has been expended in finding strategies that could improve the EPR-induced selective tumor accumulation of drug-delivering nanoparticles by the highly leaky and permeable tumor blood vessels. In fact, whereas a good EPR effect is found in most solid animal tumors rich in blood flow, EPR appears to be inadequate in solid human tumors, especially in some advanced forms of cancer [20]. This has been related to the high interstitial fluid pressure (IFP) and atypical extracellular matrix of the abnormal tumor environment. Consequently, the tumor blood flow becomes heterogeneous and, besides leading to hypoxia and acidosis, it makes difficult the diffusion and uniform distribution of nanoparticles into the vascular tumor area [21]. It has been suggested that one strategy to increase EPR and make nanodrug delivery easier in human tumors is the downregulation of the VEGF signaling and the inhibition of tumor neo-angiogenesis which may produce beneficial changes to the vasculature and IFP reduction [21]. Therefore, nanoformulations able to inhibit angiogenesis, and thus, reduce IFP and improve the delivery of the nanodrugs themselves to tumor tissues have been produced [40][41]. The observation that omega-3 PUFAs possess powerful antiangiogenic properties [42] further indicates the appropriateness of their inclusion in nanoformulations carrying drugs to tumors.
2. Omega-3 PUFAs and OvCa: What Is Known from Preclinical In Vivo Studies
Over the last two decades, a consistent body of literature has been accumulated demonstrating, both in vitro and in vivo, the potential antineoplastic efficacy of omega-3 PUFAs by using preclinical models of OvCa (see below for details). On the other hand, there are limited numbers of human observational studies, especially if compared to those available for BCa, and often their outcomes are also inconsistent [43][44]. Moreover, there are no clinical interventional trials to date [39]. This may have discouraged researchers from developing omega-3 PUFA-containing nanoformulations to improve OvCa therapy. For these reasons, it may be important to have a picture of the current knowledge in this field of research to better orientate future developments of the research both in the clinical setting and in the nanotherapy area. To this aim, researchers will describe and critically analyze the most relevant findings obtained with omega-3 PUFA treatments in preclinical in vivo models of OvCa, which are better than the in vitro ones that may mimic the human condition (Figure 1).
Recently, West et al. [45] reported the ability of DHA to inhibit the growth and proliferation of OvCa developing in a transgenic mouse model of OvCa in vivo (the KpB transgenic mouse model of high-grade serous epithelial OvCa mouse model (K18-gT121 ±; p53fl/fl; Brca1fl/fl; KpB)). In this work, DHA was intraperitoneally (IP) injected at the daily dose of 15 mg/kg for 28 days. The intraperitoneal way of administration appears very appropriate since the most common way of OvCa spreading is through the direct exfoliation of cancer cells into the peritoneal cavity [46]. It was also shown that the drug intraperitoneal (IP) injection increases their half-life and concentrations in the target of OvCa cells [47]. Nanoparticles encapsulating another nutraceutical, the epigallocatechin gallate, were recently administered IP in a peritoneal metastatic murine model of human OvCa [48]. The recent findings of West et al. [45] are extremely interesting, since their work is the first preclinical work where the anticancer effects of an omega-3 PUFA were investigated in an immunocompetent mammalian OvCa model, especially considering the crucial role exerted by the tumor microenvironment immune cells in the induction of the growth and progression of tumors. Instead, another early work was performed using SCID mice transplanted with paclitaxel-sensitive human ovarian carcinoma A121 cells [49]. That report also demonstrated that DHA covalently conjugated to the chemotherapeutic second-generation taxoid paclitaxel was able to potentiate the anticancer activity of this drug, inducing the complete regression of the A121 cell xenografts. Interestingly, when these conjugates were used by the same authors [49] against drug-resistant human colon cancer cells implanted in SCID mice, they were able to overcome the resistance and, also in this case, differently from paclitaxel alone and paclitaxel-DHA, induced the total regression of the tumor xenografts. A similar report was recently published by Udumula et al. [50], who implanted the human OvCa SKOV3ip or CaOV3 cells, in the peritoneal cavity of nude mice and then dietary supplemented the mice with 100 mg/kg EPA or DHA administered by gavage per 4–5 weeks. The antineoplastic efficacy of the two FAs was different, and dependent on the cell line implanted. In the SKOV3ip xenograft model, both the FAs resulted as less active than in the CaOV3 one. EPA resulted more efficacious than DHA, and increased the median mouse survival of control mice (23.5 days, corn oil-treated mice) by 97.8%, whereas DHA induced only a 23.4% increase. Moreover, just EPA reduced markedly and significantly the size of the excised tumors, as well as the Ki-67 proliferation index of the OvCa cells grown in vivo, as compared to the cells grown in control mice.
Instead, another work evaluated the ALA antineoplastic activity. In this case, another animal model was used, the xenograft model of zebrafish [51], which is farther than the xenograft mouse models in terms of evolutionary distance and phylogenetic trees from the human species. Anyway, this model was recently considered a helpful model for the study of metastasis in vivo [52], since it has the advantage of mirroring the degree of invasion/metastasis in vitro and in mice, and it is able to furnish information in a faster and less expensive way compared to mouse models of metastasis. In fact, in the zebrafish model, the human cancer cell lines are injected into the perivitelline space of two-days old larvae, where they can survive and metastasize, since the larvae will not develop an adaptive immune system until 14 days post-fertilization [53]. In Wang et al.’s work [51], performed on the zebrafish larvae (at 1-day post-fertilization) only DHA was able to suppress the development of cell metastasis. From the in vitro experiments, the authors suggested that this effect could be related to the DHA ability to suppress the NF-κB signaling.
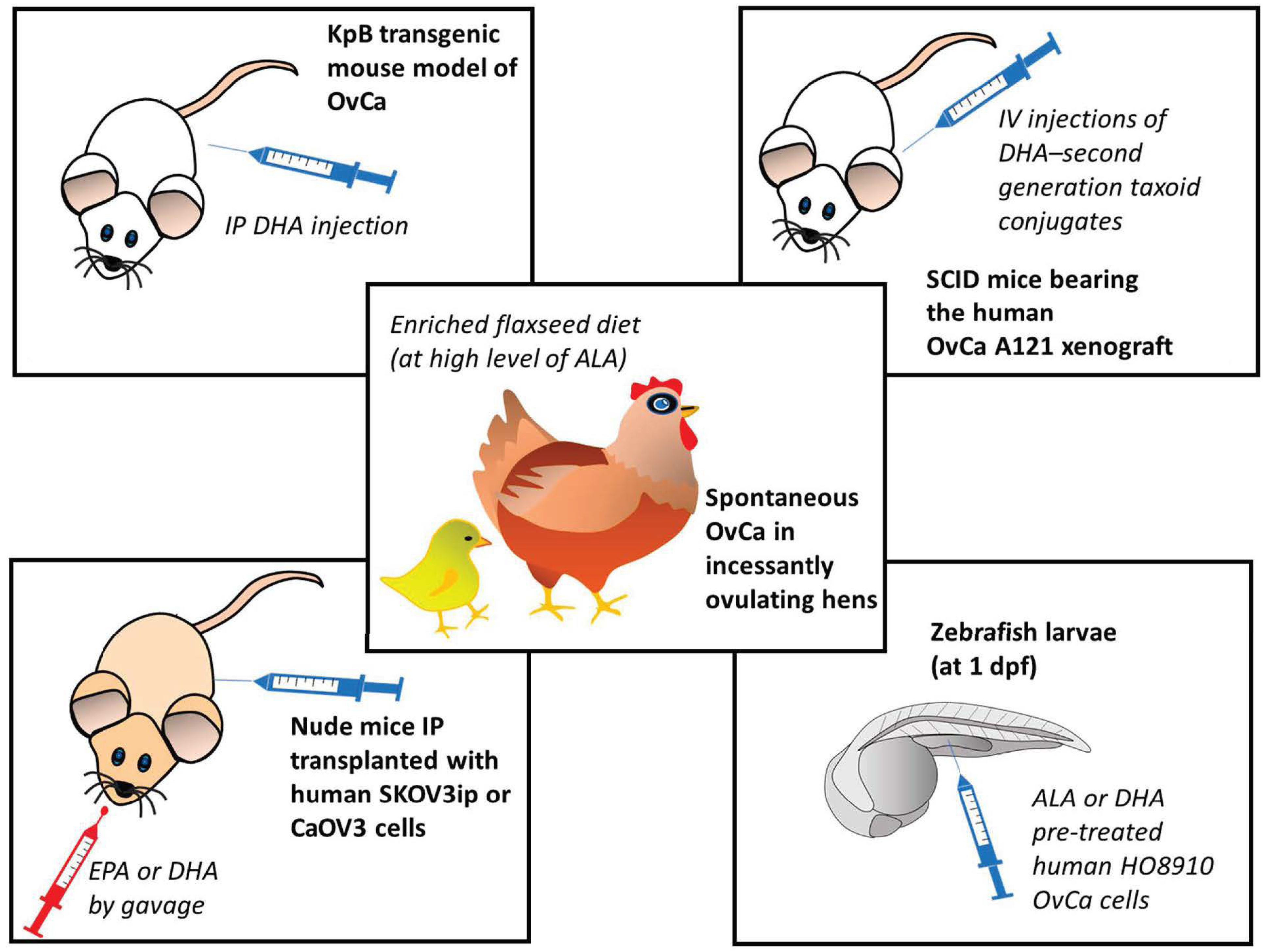
Figure 1. Preclinical in vivo models of ovarian cancer used in studies for the evaluation of the anticancer effects of omega-3 PUFAs [45][49][50][51][54]. ALA: α-linolenic acid; DHA: docosahexaenoic acid; dpf: day after fecundation; EPA: eicosapentaenoic acid; IP: intraperitoneal; IV; intravascular; OvCa: ovarian cancer. The numbers in square brackets indicate the references in which the respective animal models were used.
Over the last two decades, a series of in vivo studies had been also conducted using a model of spontaneous OvCa frequent in incessantly ovulating hens (for a review see: [54]). This model, even though again not using mammalians, has the advantage of being the only known animal model of spontaneous OvCa, and it has been so far considered useful for pre-clinical and dietary intervention studies focused on OvCa prevention, since the OvCa developed in the hen is histologically similar and shares similar features with the human OvCa considering the ascites and metastasis formation in peritoneum [55][56]. The research developed over the years by using this model has demonstrated that a flaxseed-rich diet can decrease the severity and incidence of OvCa [56]. Moreover, it was observed that this diet promotes apoptosis and suppresses angiogenesis in the OvCa, but not in the normal avian ovaries [56]. Interestingly, besides containing high levels of ALA, which can then be metabolically converted into the long-chain EPA and DHA, flaxseed is also a source of the phytoestrogen lignan secoisolaricisresinol diglucoside, which is converted to enterodiol and enterolactone, both having anti-estrogenic and antioxidant properties [56]. Moreover, the enriched flaxseed diet was shown to induce the CYP1A1 pathway, and through it, the formation of 2-hydroxy estradiol metabolites, which are then converted into 2-methoxyestradiol (2MeOE2) estrogen, which is the least powerful estrogenic metabolite as compared to the others (such as 4-hydroxy and 16-hydroxy metabolites) produced by the different CYP enzymes (CYP3A4 and CYP1B1, respectively) [57]. This will result in a higher 2-hydroxy/16-hydroxy estradiol ratio, previously shown to be protective against postmenopausal BCa [58]. On these bases, Pal et al. [56] recently also investigated the effects of individual treatments with DHA or 2MeOE2 in vitro in human OvCa cells, and in an in vivo model that mimics the neo-angiogenetic process in vivo, i.e., the chorioallantoic membrane assay [59]. Using the two models, the authors demonstrated that, whereas only 2MeOE2 induced apoptosis, both the compounds acted by inhibiting neo-angiogenesis, even though with different molecular mechanisms. These results suggest a possible 2MeOE2/DHA combinational approach for the treatment of advanced OvCa. Therefore, the inclusion of both compounds in newly developed nanoformulations to be evaluated for their potential to contrast OvCa would deserve particular attention. Overall, the in vivo studies substantiate the hypothesis that omega-3 PUFAs may represent potential preventive/therapeutic agents against not only BCa, but also OvCa. Moreover, they suggest that their anticancer potential, when enclosed in nanoformulations in combination with other nutraceuticals or chemotherapeutic drugs, would be worth to be evaluated.
A considerable number of in vitro studies were also performed to evaluate the effects of omega-3 PUFAs on OvCa cells, but researchers will not analyze them here, both for the sake of brevity and being this issue beyond the scope of this research. However, it is interesting to underline that in a comparable number of these studies the cells were treated either with EPA or DHA, and just in two of them [50][60] both the two long-chain PUFAs were separately evaluated to make a comparison between them. In one case, DHA showed more pronounced anticancer properties [60], whereas in the other one EPA resulted in being the most active [50]. Therefore, robust indications are not yet available for choosing one or the other PUFA for further OvCa in vitro studies. In this context, also the hypothesis put forward very recently by Udumula et al. is of interest [50]. Based on their in vitro findings, these authors suggested that metformin, a “repurposed” drug for OvCa, previously reported to have anticancer effects in preclinical and human observational studies [61], could exert its powerful antineoplastic effects in OvCa by enhancing the endogenous production of EPA and DHA from the precursor ALA [50]. This stimulating hypothesis could represent the rationale for the evaluation of new treatments for OvCa both in vitro and in vivo, where the anticancer properties of metformin could be enhanced by the combined treatment with omega-3 PUFAs, furnishing all these antineoplastic agents (metformin and EPA or DHA) either in their free forms or co-delivered in newly developed nanoformulations. In their opinion, the reason why studies focused on the inclusion of omega-3 PUFAs in nanoformulations designed for the therapy of OvCa have not yet been performed, even despite all the robust and consistent data obtained in the preclinical setting, may be especially related to the inconsistency of the outcomes of the human observational studies performed in this field [43][44].
References
- Fasano, E.; Serini, S.; Piccioni, E.; Innocenti, I.; Calviello, G. Chemoprevention of lung pathologies by dietary n-3 polyunsaturated fatty acids. Curr. Med. Chem. 2010, 17, 3358–3376.
- Serini, S.; Ottes Vasconcelos, R.; Fasano, E.; Calviello, G. Epigenetic regulation of gene expression and M2 macrophage polarization as new potential omega-3 polyunsaturated fatty acid targets in colon inflammation and cancer. Expert Opin. Ther. Targets 2016, 20, 843–858.
- AlAli, M.; Alqubaisy, M.; Aljaafari, M.N.; AlAli, A.O.; Baqais, L.; Molouki, A.; Abushelaibi, A.; Lai, K.S.; Lim, S.E. Nutraceuticals: Transformation of conventional foods into health promoters/disease preventers and safety considerations. Molecules 2021, 26, 2540.
- Castejón-Vega, B.; Giampieri, F.; Alvarez-Suarez, J.M. Nutraceutical compounds targeting inflammasomes in human diseases. Int. J. Mol. Sci. 2020, 21, 4829.
- Maiuolo, J.; Gliozzi, M.; Carresi, C.; Musolino, V.; Oppedisano, F.; Scarano, F.; Nucera, S.; Scicchitano, M.; Bosco, F.; Macri, R.; et al. Nutraceuticals and cancer: Potential for natural polyphenols. Nutrients 2021, 13, 3834.
- Stromsnes, K.; Correas, A.G.; Lehmann, J.; Gambini, J.; Olaso-Gonzalez, G. Anti-inflammatory properties of diet: Role in healthy aging. Biomedicines 2021, 9, 922.
- Serini, S.; Calviello, G. New Insights on the Effects of Dietary Omega-3 Fatty Acids on Impaired Skin Healing in Diabetes and Chronic Venous Leg Ulcers. Foods 2021, 10, 2306.
- Trombino, S.; Serini, S.; Di Nicuolo, F.; Celleno, L.; Andò, S.; Picci, N.; Calviello, G.; Palozza, P. Antioxidant effect of ferulic acid in isolated membranes and intact cells: Synergistic interactions with alpha-tocopherol, beta-carotene, and ascorbic acid. J. Agric. Food Chem. 2004, 52, 2411–2420.
- Calviello, G.; Di Nicuolo, F.; Serini, S.; Piccioni, E.; Boninsegna, A.; Maggiano, N.; Ranelletti, F.O.; Palozza, P. Docosahexaenoic acid enhances the susceptibility of human colorectal cancer cells to 5-fluorouracil. Cancer Chemother. Pharmacol. 2005, 55, 12–20.
- Calviello, G.; Serini, S.; Piccioni, E.; Pessina, G. Antineoplastic effects of n-3 polyunsaturated fatty acids in combination with drugs and radiotherapy: Preventive and therapeutic strategies. Nutr. Cancer 2009, 61, 287–301.
- Fasano, E.; Serini, S.; Mondella, N.; Trombino, S.; Celleno, L.; Lanza, P.; Cittadini, A.; Calviello, G. Antioxidant and anti-inflammatory effects of selected natural compounds contained in a dietary supplement on two human immortalized keratinocyte lines. BioMed Res. Int. 2014, 2014, 327452.
- Ottes Vasconcelos, R.; Serini, S.; de Souza Votto, A.P.; Santos Trindade, G.; Fanali, C.; Sgambato, A.; Calviello, G. Combination of ω-3 fatty acids and cisplatin as a potential alternative strategy for personalized therapy of metastatic melanoma: An in-vitro study. Melanoma Res. 2019, 29, 270–280.
- Serini, S.; Cassano, R.; Corsetto, P.A.; Rizzo, A.M.; Calviello, G.; Trombino, S. Omega-3 PUFA loaded in resveratrol-based solid lipid nanoparticles: Physicochemical properties and antineoplastic activities in human colorectal cancer cells in vitro. Int. J. Mol. Sci. 2018, 19, 586.
- Trombino, S.; Serini, S.; Cassano, R.; Calviello, G. Xanthan gum-based materials for omega-3 PUFA delivery: Preparation, characterization and antineoplastic activity evaluation. Carbohydr. Polym. 2019, 208, 431–440.
- Serini, S.; Cassano, R.; Trombino, S.; Calviello, G. Nanomedicine-based formulations containing ω-3 polyunsaturated fatty acids: Potential application in cardiovascular and neoplastic diseases. Int. J. Nanomed. 2019, 14, 2809–2828.
- Serini, S.; Cassano, R.; Facchinetti, E.; Amendola, G.; Trombino, S.; Calviello, G. Anti-irritant and anti-inflammatory effects of DHA encapsulated in resveratrol-based solid lipid nanoparticles in human keratinocytes. Nutrients 2019, 11, 1400.
- Serini, S.; Cassano, R.; Bruni, M.; Servidio, C.; Calviello, G.; Trombino, S. Characterization of a hyaluronic acid and folic acid-based hydrogel for cisplatin delivery: Antineoplastic effect in human ovarian cancer cells in vitro. Int. J. Pharm. 2021, 606, 120899.
- Cassano, R.; Serini, S.; Curcio, F.; Trombino, S.; Calviello, G. Preparation and Study of Solid Lipid Nanoparticles Based on Curcumin, Resveratrol and Capsaicin Containing Linolenic Acid. Pharmaceutics 2022, 14, 1593.
- Fernandes, R.S.; Silva, J.O.; Mussi, S.V.; Lopes, S.C.A.; Leite, E.A.; Cassali, G.D.; Cardoso, V.N.; Townsend, D.M.; Colletti, P.M.; Ferreira, L.A.M.; et al. Nanostructured lipid carrier co-loaded with doxorubicin and docosahexaenoic acid as a theranostic agent: Evaluation of biodistribution and antitumor activity in experimental model. Mol. Imaging Biol. 2018, 20, 437–447.
- Fang, J. EPR effect-based tumor targeted nanomedicine: A promising approach for controlling cancer. J. Pers. Med. 2022, 12, 95.
- Huang, D.; Sun, L.; Huang, L.; Chen, Y. Nanodrug delivery systems modulate tumor vessels to increase the enhanced permeability and retention effect. J. Pers. Med. 2021, 11, 124.
- Tagde, P.; Najda, A.; Nagpal, K.; Kulkarni, G.T.; Shah, M.; Ullah, O.; Balant, S.; Rahman, M.H. Nanomedicine-based delivery strategies for breast cancer treatment and management. Int. J. Mol. Sci. 2022, 23, 2856.
- WHO. Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 26 March 2021).
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Prim. 2019, 5, 66.
- Augimeri, G.; Montalto, F.I.; Giordano, C.; Barone, I.; Lanzino, M.; Catalano, S.; Andò, S.; De Amicis, F.; Bonofiglio, D. Nutraceuticals in the mediterranean diet: Potential avenues for breast cancer treatment. Nutrients 2021, 13, 2557.
- Masuelli, L.; Benvenuto, M.; Focaccetti, C.; Ciuffa, S.; Fazi, S.; Bei, A.; Miele, M.T.; Piredda, L.; Manzari, V.; Modesti, A.; et al. Targeting the tumor immune microenvironment with “nutraceuticals”: From bench to clinical trials. Pharmacol. Ther. 2021, 219, 107700.
- Sindhu, R.K.; Verma, R.; Salgotra, T.; Rahman, M.H.; Shah, M.; Akter, R.; Murad, W.; Mubin, S.; Bibi, P.; Qusti, S.; et al. Impacting the remedial potential of nano delivery-based flavonoids for breast cancer treatment. Molecules 2021, 26, 5163.
- Mondal, A.; Banerjee, S.; Bose, S.; Mazumder, S.; Haber, R.A.; Farzaei, M.H.; Bishayee, A. Garlic constituents for cancer prevention and therapy: From phytochemistry to novel formulations. Pharmacol. Res. 2022, 175, 105837.
- Kavyani, Z.; Musazadeh, V.; Fathi, S.; Hossein Faghfouri, A.; Dehghan, P.; Sarmadi, B. Efficacy of the omega-3 fatty acids supplementation on inflammatory biomarkers: An umbrella meta-analysis. Int. Immunopharmacol. 2022, 111, 109104.
- Fodil, M.; Blanckaert, V.; Ulmann, L.; Mimouni, V.; Chénais, B. Contribution of n-3 Long-Chain Polyunsaturated Fatty Acids to the Prevention of Breast Cancer Risk Factors. Int. J. Environ. Res. Public Health 2022, 19, 7936.
- Serini, S.; Calviello, G. Modulation of Ras/ERK and phosphoinositide signaling by long-chain n-3 PUFA in breast cancer and their potential complementary role in combination with targeted drugs. Nutrients 2017, 9, 185.
- Asselin, C.Y.; Lam, A.; Cheung, D.Y.C.; Eekhoudt, C.R.; Zhu, A.; Mittal, I.; Mayba, A.; Solati, Z.; Edel, A.; Austria, J.A.; et al. The cardioprotective role of flaxseed in the prevention of doxorubicin- and trastuzumab-mediated cardiotoxicity in C57BL/6 Mice. J. Nutr. 2020, 150, 2353–2363.
- McCarty, M.F.; DiNicolantonio, J.J. Minimizing membrane arachidonic acid content as a strategy for controlling cancer: A review. Nutr. Cancer 2018, 70, 840–850.
- Serini, S.; Fasano, E.; Piccioni, E.; Monego, G.; Cittadini, A.R.; Celleno, L.; Ranelletti, F.O.; Calviello, G. DHA induces apoptosis and differentiation in human melanoma cells in vitro: Involvement of HuR-mediated COX-2 mRNA stabilization and β-catenin nuclear translocation. Carcinogenesis 2012, 33, 164–173.
- Serini, S.; Zinzi, A.; Ottes Vasconcelos, R.; Fasano, E.; Riillo, M.G.; Celleno, L.; Trombino, S.; Cassano, R.; Calviello, G. Role of β-catenin signaling in the anti-invasive effect of the omega-3 fatty acid DHA in human melanoma cells. J. Dermatol. Sci. 2016, 84, 149–159.
- Newell, M.; Baker, K.; Postovit, L.M.; Field, C.J. A Critical review on the effect of docosahexaenoic acid (DHA) on cancer cell cycle progression. Int. J. Mol. Sci. 2017, 18, 1784.
- Newell, M.; Brun, M.; Field, C.J. Treatment with DHA modifies the response of MDA-MB-231 breast cancer cells and tumors from nu/nu mice to doxorubicin through apoptosis and cell cycle arrest. J. Nutr. 2019, 149, 46–56.
- Serini, S.; Ottes Vasconcelos, R.; Fasano, E.; Calviello, G. How plausible is the use of dietary n-3 PUFA in the adjuvant therapy of cancer? Nutr. Res. Rev. 2016, 29, 102–125.
- Newell, M.; Mazurak, V.; Postovit, L.M.; Field, C.J. N-3 Long-chain polyunsaturated fatty acids, eicosapentaenoic and docosahexaenoic acid, and the role of supplementation during cancer treatment: A scoping review of current clinical evidence. Cancers 2021, 13, 1206.
- Kim, W.J.; Yockman, J.W.; Jeong, J.H.; Christensen, L.V.; Lee, M.; Kim, Y.H.; Kim, S.W. Anti-angiogenic inhibition of tumor growth by systemic delivery of PEI-g-PEG-RGD/pCMV-sFlt-1 complexes in tumor-bearing mice. J. Control. Release 2006, 114, 381–388.
- Kim, J.; Kim, S.W.; Kim, W.J. PEI-g-PEG-RGD/small interference RNA polyplex-mediated silencing of vascular endothelial growth factor receptor and its potential as an anti-angiogenic tumor therapeutic strategy. Oligonucleotides 2011, 21, 101–107.
- Aslan, C.; Maralbashi, S.; Kahroba, H.; Asadi, M.; Soltani-Zangbar, M.S.; Javadian, M.; Shanehbandi, D.; Baradaran, B.; Darabi, M.; Kazemi, T. Docosahexaenoic acid (DHA) inhibits pro-angiogenic effects of breast cancer cells via down-regulating cellular and exosomal expression of angiogenic genes and microRNAs. Life Sci. 2020, 258, 118094.
- Gu, J.H.; Gong, T.T.; Wu, Q.J.; Liu, F.H.; Wen, Z.Y.; Gao, C.; Wei, Y.F.; Yang, Z. Association between pre-diagnostic dietary supplements intake and ovarian cancer survival: Findings from a prospective cohort study in chinese women. Front. Nutr. 2021, 8, 758178.
- Wang, Y.; Liu, K.; Long, T.; Long, J.; Li, Y.; Li, J.; Cheng, L. Dietary fish and omega-3 polyunsaturated fatty acids intake and cancer survival: A systematic review and meta-analysis. Crit. Rev. Food. Sci. Nutr. 2022, 1–17.
- West, L.; Yin, Y.; Pierce, S.R.; Fang, Z.; Fan, Y.; Sun, W.; Tucker, K.; Staley, A.; Zhou, C.; Bae-Jump, V. Docosahexaenoic acid (DHA), an omega-3 fatty acid, inhibits tumor growth and metastatic potential of ovarian cancer. Am. J. Cancer Res. 2020, 10, 4450–4463.
- Chandrashekhara, S.H.; Triveni, G.S.; Kumar, R. Imaging of peritoneal deposits in ovarian cancer: A pictorial review. World J. Radiol. 2016, 8, 513–517.
- Chambers, S.K.; Chow, H.-H.S.; Janicek, M.F.; Cragun, J.M.; Hatch, K.D.; Cui, H. Phase I trial of intraperitoneal pemetrexed, cisplatin, and paclitaxel in optimally debulked ovarian cancer. Clin. Cancer Res. 2012, 18, 2668e78.
- Bae, K.H.; Tan, S.; Yamashita, A.; Ang, W.X.; Gao, S.J.; Wang, S. Hyaluronic acid-green tea catechin micellar nanocomplexes: Fail-safe cisplatin nanomedicine for the treatment of ovarian cancer without off-target toxicity. Biomaterials 2017, 148, 41–53.
- Kuznetsova, L.; Chen, J.; Sun, L.; Wu, X.; Pepe, A.; Veith, J.M.; Pera, P.; Bernacki, R.J.; Ojima, I. Syntheses and evaluation of novel fatty acid-second-generation taxoid conjugates as promising anticancer agents. Bioorg. Med. Chem. Lett. 2006, 16, 974–977.
- Udumula, M.P.; Poisson, L.M.; Dutta, I.; Tiwari, N.; Kim, S.; Chinna-Shankar, J.; Allo, G.; Sakr, S.; Hijaz, M.; Munkarah, A.R.; et al. Divergent metabolic effects of metformin merge to enhance eicosapentaenoic acid metabolism and inhibit ovarian cancer in vivo. Cancers 2022, 14, 1504.
- Wang, Y.C.; Wu, Y.N.; Wang, S.L.; Lin, Q.H.; He, M.F.; Liu, Q.L.; Wang, J.H. Docosahexaenoic acid modulates invasion and metastasis of human ovarian cancer via multiple molecular pathways. Int. J. Gynecol. Cancer 2016, 26, 994–1003.
- Astell, K.R.; Sieger, D. Zebrafish in vivo models of cancer and metastasis. Cold Spring Harb. Perspect. Med. 2020, 10, a037077.
- Teng, Y.; Xie, X.; Walker, S.; White, D.T.; Mumm, J.S.; Cowell, J.K. Evaluating human cancer cell metastasis in zebrafish. BMC Cancer 2013, 13, 453.
- Pal, P.; Starkweather, K.N.; Hales, K.H.; Hales, D.B. A review of principal studies on the development and treatment of epithelial ovarian cancer in the laying hen Gallus gallus. Comp. Med. 2021, 71, 271–284.
- Barua, A.; Bahr, J.M. Ovarian Cancer: Applications of chickens to humans. Annu. Rev. Anim. Biosci. 2022, 10, 241–257.
- Pal, P.; Hales, K.; Petrik, J.; Hales, D.B. Pro-apoptotic and anti-angiogenic actions of 2-methoxyestradiol and docosahexaenoic acid, the biologically derived active compounds from flaxseed diet, in preventing ovarian cancer. J. Ovarian Res. 2019, 12, 49.
- Dikshit, A.; Hales, K.; Hales, D.B. Whole flaxseed diet alters estrogen metabolism to promote 2-methoxtestradiol-induced apoptosis in hen ovarian cancer. J. Nutr. Biochem. 2017, 42, 117–125.
- Ziegler, R.G.; Fuhrman, B.J.; Moore, S.C.; Matthews, C.E. Epidemiologic studies of estrogen metabolism and breast cancer. Steroids 2015, 99, 67–75.
- Lokman, N.A.; Elder, A.S.; Ricciardelli, C.; Oehler, M.K. Chick chorioallantoic membrane (CAM) assay as an in vivo model to study the effect of newly identified molecules on ovarian cancer invasion and metastasis. Int. J. Mol. Sci. 2012, 13, 9959–9970.
- Wan, X.H.; Fu, X.; Ababaikeli, G. Docosahexaenoic acid induces growth suppression on epithelial ovarian cancer cells more effectively than eicosapentaenoic acid. Nutr. Cancer 2016, 68, 320–327.
- Goenka, L.; Dubashi, B.; Selvarajan, S.; Ganesan, P. Use of “repurposed” drugs in the treatment of epithelial ovarian cancer: A systematic review. Am. J. Clin. Oncol. 2022, 45, 168–174.
More
Information
Subjects:
Nanoscience & Nanotechnology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
03 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




