
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marina López-Pozo | -- | 1987 | 2023-01-23 09:50:17 | | | |
| 2 | William Walter Adams III | Meta information modification | 1987 | 2023-01-23 19:16:37 | | | | |
| 3 | William Walter Adams III | Meta information modification | 1987 | 2023-01-23 19:21:50 | | | | |
| 4 | Dean Liu | + 1 word(s) | 1988 | 2023-01-29 03:22:49 | | | | |
| 5 | Dean Liu | Meta information modification | 1988 | 2023-01-29 03:25:29 | | | | |
| 6 | Dean Liu | Meta information modification | 1988 | 2023-01-29 03:26:24 | | | | |
| 7 | Dean Liu | Meta information modification | 1988 | 2023-01-29 03:26:58 | | |
Video Upload Options
Rising atmospheric carbon dioxide (CO2) levels can have negative impacts on food security through effects on plant photosynthesis, productivity, and nutritional quality, especially in the presence of additional environmental stressors. This entry presents a multi-factorial analysis of how differing levels of atmospheric CO2 and mineral nutrient supply affect productivity and nutritional quality of the aquatic floating plant Lemna minor (common duckweed) in the presence or absence of its microbiome. Elevated CO2 in combination with low nutrient supply decreased plant area-expansion rate and increased biomass accumulation, with minimal negative impacts on protein-to-biomass ratio but stronger declines in the content of essential human micronutrients. Inoculation with plant-associated microorganisms restored area-expansion rate and further stimulated accumulation of biomass with an unaltered protein-to-biomass ratio compared to uninoculated plants under a combination of elevated CO2 and low nutrient supply. Under ample nutrient supply, inoculation ameliorated the declines in micronutrient content induced by elevated CO2. These findings add additional insight into possible roles of duckweed in sustainable systems and support a role for the plant microbiome in protecting plant productivity and nutritional quality in a manner that varies with specific growth conditions and plant traits. This understanding is relevant in both agricultural and natural contexts during a time of rapid environmental change.
1 .Introduction
A changing climate has intensified both abiotic (e.g., droughts, floods, extreme temperatures) and biotic stresses (pests and pathogens) threatening food security [1]. While CO2 is required for photosynthesis and the growth of plants and algae, too much CO2 can have negative effects, especially in combination with low nutrient supply [2]. Such adverse effects include diminished plant nutritional quality for human and non-human consumers [3]. Future agriculture will require climate-resilient crops [4], capable of maintaining both productivity and nutritional quality under changing environmental conditions, as well as identification of mitigating factors that can support crop cultivation (as well as the primary productivity of natural systems). Elevated CO2 can alter not only protein but also micronutrient content because feedback downregulation of photosynthesis impacts proteins that bind carotenoids that are essential human micronutrients, such as provitamin A (β-carotene), lutein, and zeaxanthin (needed to support human vision, immune health, and cognitive performance [5][6][7][8]). Such effects vary by plant species and growth conditions [9][10].
It has been proposed that plant-microbiome interaction may counteract photosynthetic downregulation and the loss of plant nutritional quality [11][12][13]. Such effects depend on environmental factors [14][15], including CO2 level [11] and nitrogen availability [16]. Specifically, the plant microbiome has the potential to (i) increase plant nitrogen content in support of new growth [17], (ii) lessen build-up of excess carbohydrates by serving as an additional carbohydrate-consuming sink, (iii) produce growth-stimulating plant hormones [18][19][20], and/or (iv) induce routing of electrons into alternative pathways in various compartments [21] (for a recent general overview, see [12]). Duckweeds grow exceptionally fast and can double both frond number and area in 1–3 days [22]. They also accumulate high-quality edible protein at levels up to 20x that produced by soybean per unit of cultivation area [23]. Moreover, duckweed accumulates high concentrations of essential human micronutrients [24], including carotenoids [25]. Duckweeds also support environmental sustainability by being able to remove excess nitrogen and phosphorus [23][26][27][28] as well as heavy metals and other toxins [29][30] from freshwater bodies [31][32].
2. Effects of Growth Environment and Inoculation on Plant Growth Rate
The most notable result for area expansion (via new growth; Figure 1A,B) over the course of the experimental phase was a strong inhibition of its relative growth rate (RGR) by a combination of elevated CO2 and low nutrient supply in uninoculated fronds (Figure 1A, light-red solid column) and the complete prevention of this inhibition by inoculation (Figure 1A, light-red dotted column).
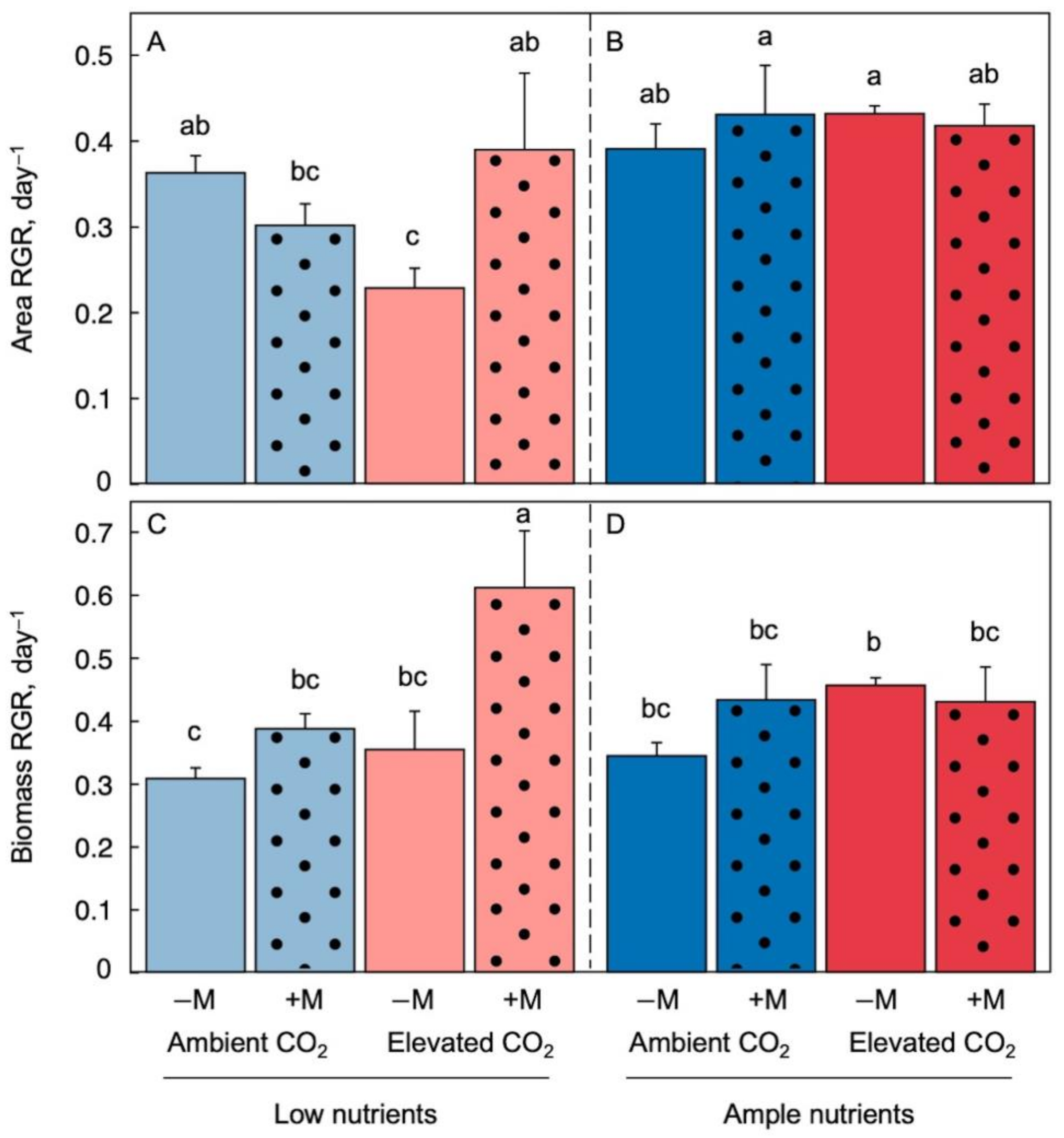
Figure 1. Relative growth rate (RGR) of frond area expansion under low (A) and ample (B) nutrients as well as RGR of dry biomass production under low (C) and ample (D) nutrients for Lemna minor grown in 1/20 (light blue or light red) or 1/2 (dark blue or red) strength Schenk & Hildebrandt medium under either ambient (blue) or elevated (red) CO2 levels. Blue and red-color columns indicate ambient and elevated CO2, respectively. Solid-fill columns represent plants that were not inoculated (−M) and dotted columns plants that were inoculated (+M) with microorganisms from a pond with L. minor. Mean values ± standard deviations; n = 3. Different lower-case letters represent significant differences at p < 0.05.
The RGR of dry biomass accumulation (Figure 1C,D) exhibited both similar and different effects compared to RGR of area expansion. On the one hand, under low nutrient supply (Figure 1C) biomass accumulation was not significantly different under elevated CO2 (light-red solid column) versus ambient CO2 (light-blue solid column), which was a different response from that of RGR of area expansion (Figure 1A). On the other hand, inoculation caused a significant stimulation of the RGR of biomass production (Figure 1C, light-red dotted compared to light-red solid column), as was also seen for the RGR of area expansion (Figure 1A).
All these findings are consistent with altered source-sink and carbon to nitrogen relationships in duckweed grown under elevated CO2, as has been reported for other plants [33][34]. Conversely, the plant microbiome may have restored balance in these systems, and allowed new area growth, via (i) consumption of excess carbohydrate produced by the fronds and (ii) possible improvement in nitrogen availability by increased uptake from the medium and/or from microbial N2 fixation [12]. Moreover, Figure 1B,D show that neither elevated (versus ambient) CO2 nor inoculation of fronds (versus uninoculated fronds) resulted in significantly different RGRs of area expansion or biomass accumulation under ample nutrient supply. This result further emphasizes that the combination of elevated CO2 with low nutrient supply caused the apparent imbalances [2].
3.Effect of Growth Environment and Inoculation on Dry Biomass per Area and Protein per Dry Biomass
More dry biomass accumulated per unit frond area under elevated CO2 especially in combination with low nutrient supply in the medium (Figure 2A,B, light-red compared to light-blue solid columns). This dry biomass had a ratio of protein to biomass that was a little – albeit not significantly – lower in elevated versus ambient CO2 (Figure 2C,D, light-red compared to light-blue solid columns). Protein-to-biomass ratio can be used as a proxy for the relative proportion of nitrogen to carbon in the biomass and thus biomass quality.
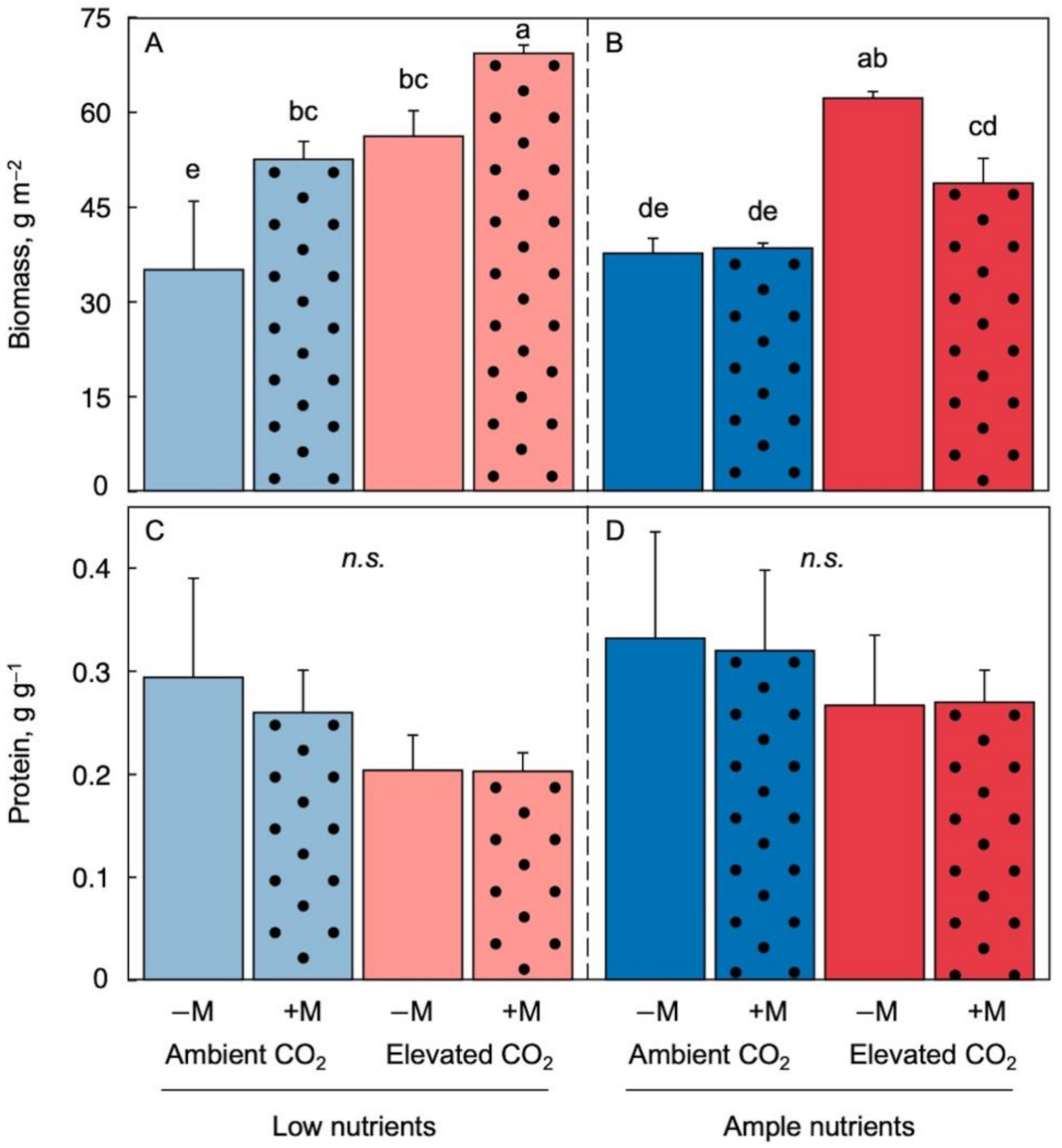
Figure 2. Dry biomass production per frond area under low (A) and ample (B) nutrients as well as protein to dry biomass ratio under low (C) and ample (D) nutrients for Lemna minor grown in 1/20 (light blue or light red) or 1/2 strength (dark blue or red) Schenk & Hildebrandt medium and either ambient (blue) or elevated (red) CO2 levels. Solid-fill columns represent fronds that were not inoculated (−M) and dotted columns plants that were inoculated (+M) with microorganisms from a pond with L. minor. Mean values ± standard deviations; n = 3. Different lower-case letters represent significant differences at p < 0.05.
The finding that there were only minor, non-significant differences in protein to biomass ratio among treatments with respect to nutrient supply, CO2 level, or inoculation (Figure 2C,D) is consistent with duckweed’s ability to store large quantities of protein that is apparently rather insensitive to feedback downregulation and may also provide nitrogen for new growth for some time upon transfer to low nutrient supply [26]. In contrast, terrestrial plants often exhibit pronounced effects of elevated CO2 on foliar protein, especially under limiting nutrient supply, associated with feedback downregulation of photosynthetic proteins [35][36]. Moreover, elevated CO2 under low nitrogen supply can also lead to a lowering of grain protein content in barley, wheat, and rice [37][38]. In such cases, any enhanced biomass production is accompanied by a significantly diminished nutritional quality of this biomass under elevated CO2 and low nutrient supply [39][40]. These findings indicate a benefit of plant-microbe interaction on the nutritional quality of plant biomass (protein-to-biomass ratio) in duckweed under low nutrient supply irrespective of CO2 level.
Trends were somewhat different under ample nutrient supply, where inoculation did not enhance biomass per frond area under either ambient or elevated CO2 (Figure 2B) and also did not affect protein-to-biomass ratio (Figure 2D). This outcome is consistent with the absence of any inhibition of area expansion by elevated CO2 under ample nutrient supply (Figure 1B).
Taken together, these findings suggest that association of a microbiome with the duckweed L. minor is beneficial for production of large amounts of high-quality biomass with respect to protein-to-biomass ratio. This benefit manifested especially under the combination of elevated CO2 and low nutrient supply where inoculation not only prevented inhibition of area expansion by elevated CO2 but allowed accumulation of more biomass per area with a high protein content.
4. Effect of Growth Environment and Inoculation on Chlorophyll and Carotenoid Micronutrients
Figures 3 and 4 show plant pigment levels on a dry biomass basis. There was a rather consistent trend for a decline (relative to dry biomass) in pigments associated with chlorophyll-binding proteins upon transfer to low-nutrient medium, resulting in lower chlorophyll-to-biomass and carotenoid-to-biomass ratios under low (Figure 3A,C and Figure 4A,C) compared to ample (Figure 3B,D and Figure 4B,D) nutrient supply and irrespective of CO2 level and inoculation status. The more pronounced declines in pigments compared to total protein in L. minor is consistent with chlorophyll/carotenoid-binding proteins, but not vegetative storage protein (Rubisco), being under the control of feedback downregulation.
In addition, inoculation counteracted the declines in the ratios of chlorophyll-to-biomass and carotenoid-to-biomass seen in elevated CO2 (dark-red dotted versus solid columns) but did so only under ample (Figure 3B,D and Figure 4B,D) but not low (Figure 3A,C and Figure 4A,C) nutrient supply. In fact, inoculation showed a trend for exacerbating the decline in carotenoid-to-biomass ratio under low nutrient supply (Figure 3A,C and Figure 4A,C, dotted compared to solid columns). This response may be associated with the potential for both competition for, and provision of, plant mineral nutrients by microorganisms [18][41][42].
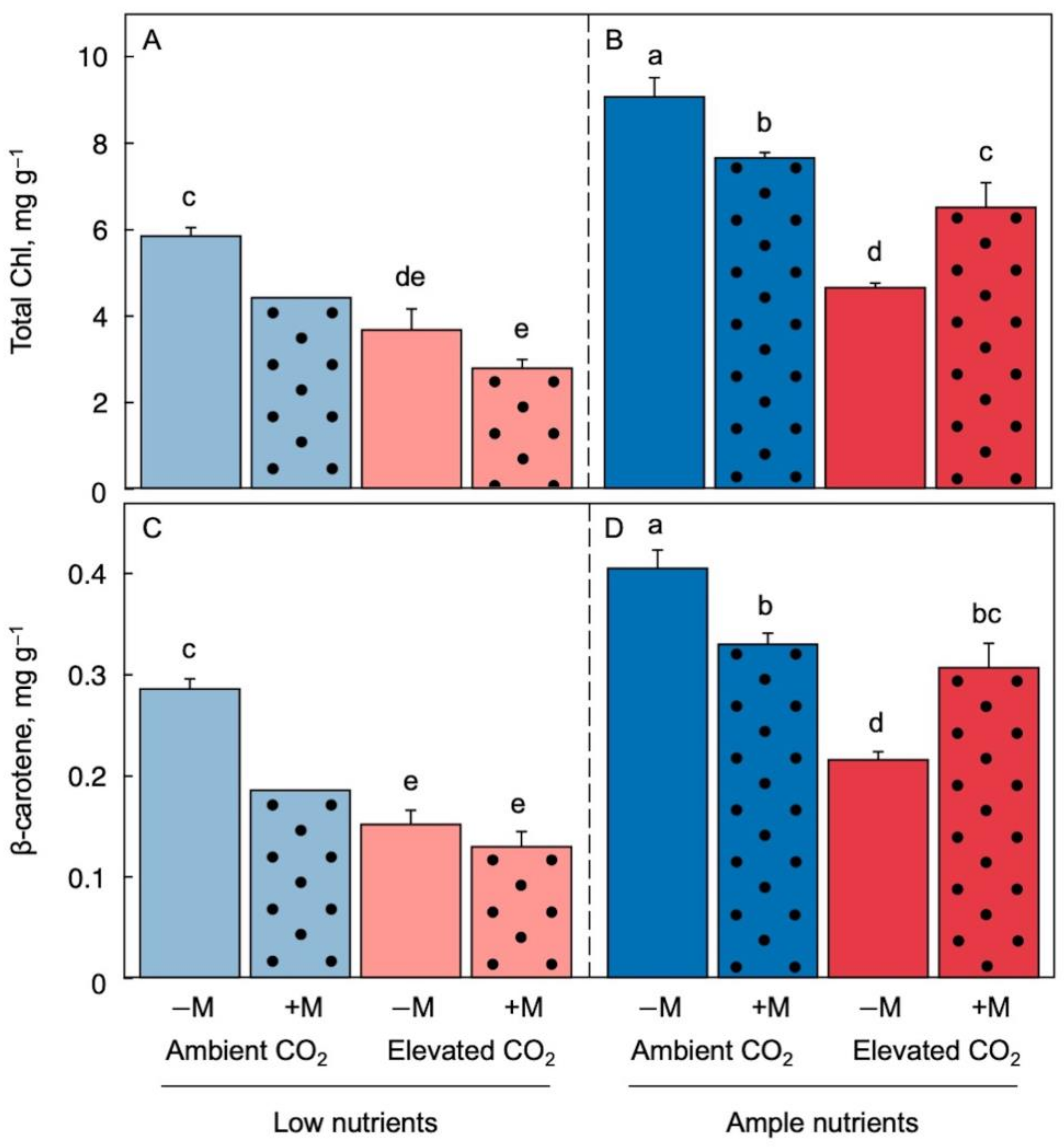
Figure 3. Chlorophyll (a + b) content (A,B) and β-carotene content (C,D) as fractions of dry biomass under low (A,C) and ample (B,D) nutrient supply for Lemna minor grown in 1/20 (light blue or light red) or 1/2 strength (dark blue or dark red) Schenk & Hildebrandt medium and either ambient (blue) or elevated (red) CO2 levels. Solid columns represent plants that were not inoculated (−M) and dotted columns represent plants that were inoculated (+M) with microorganisms from a pond with L. minor. Mean values ± standard deviations; n = 3 under all conditions except for n = 2 in the treatment of inoculated fronds in ambient CO2 and low nutrients. Different lower-case letters represent significant differences at p < 0.05.
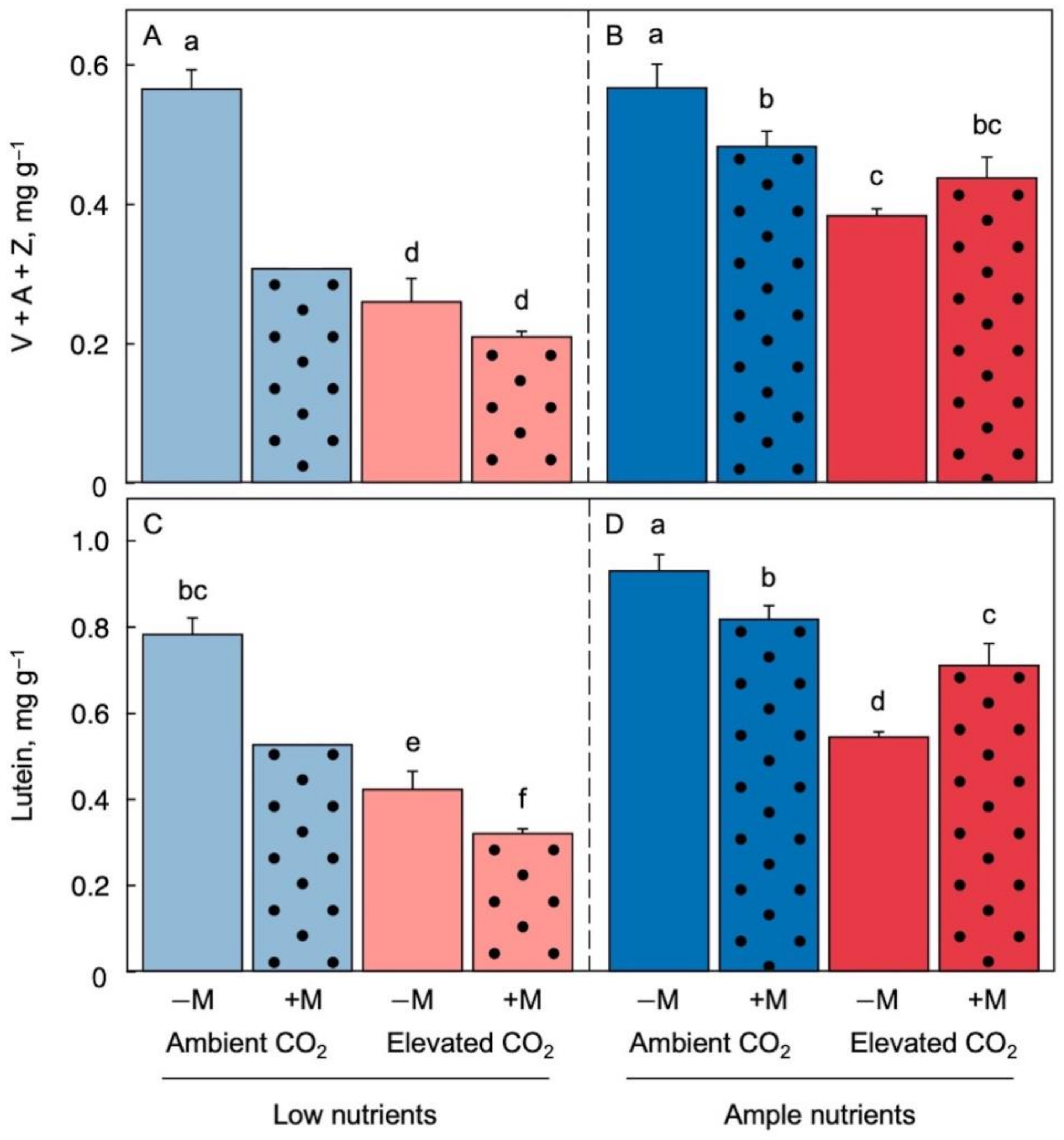
Figure 4. Ratios of the three carotenoids of the xanthophyll cycle to biomass under low (A) and ample (B) nutrients as well as lutein to biomass under low (C) and ample (D) nutrients for Lemna minor grown in 1/20 (light blue or light red) or 1/2 strength (dark blue or red) Schenk & Hildebrandt medium and either ambient (blue) or elevated (red) CO2 levels. Solid columns represent plants that were not inoculated (−M) and dotted columns represent plants that were inoculated (+M) with microorganisms from a pond with L. minor. A, antheraxanthin; V, violaxanthin; Z, zeaxanthin. Mean values ± standard deviations; n = 3 under all conditions except for n = 2 in the treatment of inoculated fronds in ambient CO2 and low nutrients. Different lower-case letters represent significant differences at p < 0.05.
5. Take-Home Messages and Benefits of Plant-Microbiome Interaction
Inoculation treatment in the duckweed L. minor showed clear benefits under conditions of elevated CO2 and low nutrient supply in preventing area-growth inhibition and allowing greater accumulation of biomass with an unaltered protein-to-biomass ratio. Presence of the plant microbiome ameliorated decreases in carotenoid/biomass ratios for several carotenoids that are essential human micronutrients under ample but not limiting nutrient supply. These findings are consistent with a view that the impact of plant-microbiome interaction varies with plant species, growth conditions, and specific aspects of plant productivity and plant nutritional quality considered. Duckweed exhibited a notable insensitivity to declines in plant protein content under elevated CO2, which was further aided by plant-microbiome interaction especially under limiting nutrient supply.
References
- Albert KR, Ro-Poulsen H, Mikkelsen TN, Michelsen A, Van Der Linden L, Beier C. Interactive effects of elevated CO2, warming, and drought on photosynthesis of Deschampsia flexuosa in a temperate heath ecosystem. J Exp Bot. 2011;62(12):4253-4266. doi:10.1093/jxb/err133
- Agüera E, De la Haba P. Leaf senescence in response to elevated atmospheric CO2 concentration and low nitrogen supply. Biol Plant. 2018;62(3):401-408. doi:10.1007/s10535-018-0798-z
- Dong J, Gruda N, Lam SK, Li X, Duan Z. Effects of elevated CO2 on nutritional quality of vegetables: A review. Front Plant Sci. 2018;9(August):1-11. doi:10.3389/fpls.2018.00924
- Dhankher OP, Foyer CH. Climate resilient crops for improving global food security and safety. Plant Cell Environ. 2018;41(5):877-884. doi:10.1111/pce.13207
- Demmig-Adams B, Polutchko SK, Adams WW. Structure-function-environment relationship of the isomers zeaxanthin and lutein. Photochem. 2022;2(2):308-325. doi:10.3390/photochem2020022
- Demmig-Adams B, López-Pozo M, Stewart JJ, Adams WW. Zeaxanthin and lutein: Photoprotectors, anti-inflammatories, and brain food. Molecules. 2020;25(16). doi:10.3390/molecules25163607
- Polutchko SK, Adams WW, Escobar CM, Demmig-Adams B. Conquering space with crops that produce ample oxygen and antioxidants. Oxygen. 2022;2(2):211-226. doi:10.3390/oxygen2020016
- Polutchko SK, Glime GNE, Demmig-Adams B. Synergistic action of membrane-bound and water-soluble antioxidants in neuroprotection. Molecules. 2021;26(17). doi:10.3390/molecules26175385
- Dhami N, Cazzonelli CI. Environmental impacts on carotenoid metabolism in leaves. Plant Growth Regul. 2020;92(3):455-477. doi:10.1007/s10725-020-00661-w
- Demmig-Adams B, Stewart JJ, Adams WW, López-Pozo M, Polutchko SK. Zeaxanthin, a molecule for photoprotection in many different environments. Molecules. 2020;25(24). doi:10.3390/MOLECULES25245825
- Nie M, Bell C, Wallenstein MD, Pendall E. Increased plant productivity and decreased microbial respiratory C loss by plant growth-promoting rhizobacteria under elevated CO2. Sci Rep. 2015;5:1-6. doi:10.1038/srep09212
- Demmig-Adams B, Polutchko SK, Zenir MC, et al. Intersections: photosynthesis, abiotic stress, and the plant microbiome. Photosynthetica. 2022;60(1):59-69. doi:10.32615/ps.2021.065
- Irigoyen JJ, Goicoechea N, Antolín MC, et al. Growth, photosynthetic acclimation and yield quality in legumes under climate change simulations: An updated survey. Plant Sci. 2014;226:22-29. doi:10.1016/j.plantsci.2014.05.008
- O’Brien AM, Yu ZH, Luo D ya, Laurich J, Passeport E, Frederickson ME. Resilience to multiple stressors in an aquatic plant and its microbiome. Am J Bot. 2020;107(2):273-285. doi:10.1002/ajb2.1404
- O’Brien AM, Laurich J, Lash E, Frederickson ME. Mutualistic outcomes across plant populations, microbes, and environments in the duckweed lemna minor. Microb Ecol. 2020;80(2):384-397. doi:10.1007/s00248-019-01452-1
- Moreau D, Pivato B, Bru D, et al. Plant traits related to nitrogen uptake influence plant-microbe competition. Ecology. 2018;96(8):2300-2310.
- Richardson AE, Barea JM, McNeill AM, Prigent-Combaret C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil. 2009;321(1-2):305-339. doi:10.1007/s11104-009-9895-2
- Ishizawa H, Kuroda M, Morikawa M, Ike M. Evaluation of environmental bacterial communities as a factor affecting the growth of duckweed Lemna minor. Biotechnol Biofuels. 2017;10(1):1-10. doi:10.1186/s13068-017-0746-8
- Stefan M, Munteanu N, Stoleru V, Mihasan M. Effects of inoculation with plant growth promoting rhizobacteria on photosynthesis, antioxidant status and yield of runner bean. Rom Biotechnol Lett. 2013;18(2):8132-8143.
- Vacheron J, Desbrosses G, Bouffaud ML, et al. Plant growth-promoting rhizobacteria and root system functioning. Front Plant Sci. 2013;4:1-19. doi:10.3389/fpls.2013.00356
- Asha AD, Nivetha N, Krishna GK, et al. Amelioration of short-term drought stress during different growth stages in Brassica juncea by rhizobacteria mediated maintenance of ROS homeostasis. Physiol Plant. 2021;172(4):1880-1893. doi:10.1111/ppl.13399
- Ziegler P, Adelmann K, Zimmer S, Schmidt C, Appenroth KJ. Relative in vitro growth rates of duckweeds (Lemnaceae) - the most rapidly growing higher plants. Plant Biol. 2015;17(s1):33-41. doi:10.1111/plb.12184
- Mohedano RA, Costa RHR, Tavares FA, Belli Filho P. High nutrient removal rate from swine wastes and protein biomass production by full-scale duckweed ponds. Bioresour Technol. 2012;112:98-104. doi:10.1016/j.biortech.2012.02.083
- Appenroth KJ, Sree KS, Böhm V, et al. Nutritional value of duckweeds (Lemnaceae) as human food. Food Chem. 2017;217:266-273. doi:10.1016/j.foodchem.2016.08.116
- Stewart JJ, Adams WW, Escobar CM, López-Pozo M, Demmig-Adams B. Growth and essential carotenoid micronutrients in lemna gibba as a function of growth light intensity. Front Plant Sci. 2020;11:1-14. doi:10.3389/fpls.2020.00480
- Cheng JJ, Stomp AM. Growing Duckweed to recover nutrients from wastewaters and for production of fuel ethanol and animal feed. Clean - Soil, Air, Water. 2009;37(1):17-26. doi:10.1002/clen.200800210
- Zhao Z, Shi H, Liu Y, et al. The influence of duckweed species diversity on biomass productivity and nutrient removal efficiency in swine wastewater. Bioresour Technol. 2014;167:383-389. doi:10.1016/j.biortech.2014.06.031
- Sońta M, Rekiel A, Batorska M. Use of Duckweed (Lemna L.) in sustainable livestock production and aquaculture - a review. Ann Anim Sci. 2019;19(2):257-271. doi:10.2478/aoas-2018-0048
- Al-Khafaji MS, Al-Ani FH, Ibrahim AF. Removal of some heavy metals from industrial wastewater by Lemmna minor. KSCE J Civ Eng. 2018;22(4):1077-1082. doi:10.1007/s12205-017-1112-x
- Torbati S. Toxicological risks of Acid Bordeaux B on duckweed and the plant potential for effective remediation of dye-polluted waters. Environ Sci Pollut Res. 2019;26(27):27699-27711. doi:10.1007/s11356-019-05898-1
- Saha P, Banerjee A, Sarkar S. Phytoremediation potential of duckweed (Lemna minor L.) on steel wastewater. Int J Phytoremediation. 2015;17(6):589-596. doi:10.1080/15226514.2014.950410
- Verma R, Suthar S. Synchronized urban wastewater treatment and biomass production using duckweed Lemna gibba L. Ecol Eng. 2014;64:337-343. doi:10.1016/j.ecoleng.2013.12.055
- Rho H, Doty SL, Kim SH. Endophytes alleviate the elevated CO2-dependent decrease in photosynthesis in rice, particularly under nitrogen limitation. J Exp Bot. 2020;71(2):707-718. doi:10.1093/jxb/erz440
- Zhu X, Song F, Liu S, Liu F. Arbuscular mycorrhiza improve growth, nitrogen uptake, and nitrogen use efficiency in wheat grown under elevated CO2. Mycorrhiza. 2016;26(2):133-140. doi:10.1007/s00572-015-0654-3
- Moore BD, Cheng SH, Sims D, Seemann JR. The biochemical and molecular basis for photosynthetic acclimation to elevated atmospheric CO2. Plant, Cell Environ. 1999;22(6):567-582. doi:10.1046/j.1365-3040.1999.00432.x
- Ainsworth EA, Long SP. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005;165(2):351-372. doi:10.1111/j.1469-8137.2004.01224.x
- Kimball BA, Morris CF, Pinter PJ, et al. Elevated CO2, drought and soil nitrogen effects on wheat grain quality. New Phytol. 2001;150(2):295-303. doi:10.1046/j.1469-8137.2001.00107.x
- Taub DR, Miller B, Allen H. Effects of elevated CO2 on the protein concentration of food crops: A meta-analysis. Glob Chang Biol. 2008;14(3):565-575. doi:10.1111/j.1365-2486.2007.01511.x
- Fangmeier A, Grüters U, Högy P, Vermehren B, Jäger HJ. Effects of elevated CO2, nitrogen supply and tropospheric ozone on spring wheat - II. Nutrients (N, P, K, S, Ca, Mg, Fe, Mn, Zn). Environ Pollut. 1997;96(1):43-59. doi:10.1016/S0269-7491(97)00013-4
- Myers SS, Zanobetti A, Kloog I, et al. Increasing CO2 threatens human nutrition. Nature. 2014;510(7503):139-142. doi:10.1038/nature13179
- Cheng YT, Zhang L, He SY. Plant-microbe interactions facing environmental challenge. Cell Host Microbe. 2019;26(2):183-192. doi:10.1016/j.chom.2019.07.009
- Kuzyakov Y, Xu X. Competition between roots and microorganisms for nitrogen: Mechanisms and ecological relevance. New Phytol. 2013;198(3):656-669. doi:10.1111/nph.12235




