Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Humira Assad | -- | 7107 | 2023-01-22 04:24:03 | | | |
| 2 | Conner Chen | + 2 word(s) | 7109 | 2023-01-28 10:08:03 | | | | |
| 3 | Conner Chen | + 11 word(s) | 7120 | 2023-01-29 09:01:26 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Assad, H.; Assad, A.; Kumar, A. Materials for Three-Dimensional Bio-Printing. Encyclopedia. Available online: https://encyclopedia.pub/entry/40471 (accessed on 07 February 2026).
Assad H, Assad A, Kumar A. Materials for Three-Dimensional Bio-Printing. Encyclopedia. Available at: https://encyclopedia.pub/entry/40471. Accessed February 07, 2026.
Assad, Humira, Arvina Assad, Ashish Kumar. "Materials for Three-Dimensional Bio-Printing" Encyclopedia, https://encyclopedia.pub/entry/40471 (accessed February 07, 2026).
Assad, H., Assad, A., & Kumar, A. (2023, January 22). Materials for Three-Dimensional Bio-Printing. In Encyclopedia. https://encyclopedia.pub/entry/40471
Assad, Humira, et al. "Materials for Three-Dimensional Bio-Printing." Encyclopedia. Web. 22 January, 2023.
Copy Citation
The fast-developing field of three-dimensional (3D) bio-printing has been extensively used to improve the usability and performance of scaffolds filled with cells. To further 3D-printing uses in tissue engineering, research on novel, suitable biomaterials with quick cross-linking capabilities is a prerequisite. A wider variety of acceptable 3D-printed materials are still needed, as well as better printing resolution (particularly at the nanoscale range), speed, and biomaterial compatibility.
3D bio-printing
biomaterials
bio-ink
1. Materials for 3D Bio-Printing
The initial, non-biological uses of three-dimensional (3D) printing were for the deposition of metals, ceramics, and thermoplastics. Living cells and biomaterials cannot be processed using increased processing temperatures, organic solvents, or cross-linking agents [1]. Therefore, finding biological materials that are companionable with printing procedures that can also encounter the mechanical and functional requirements for tissue constructs remains the focus [2]. The most crucial aspects for the biomaterials used in 3D bio-printing are biocompatibility, uniformity in disintegration, and printability. Biomaterials are often defined as organic or synthetic materials utilized in biological equipment to mend or even transplant any organ of the body [3]. They are separated into four divisions based on their chemical makeup: metals, polymers, ceramics, and composites. While ceramics and composites have a stronger corrosion resistance [4][5][6] than other classes of materials, metals and composites also have a high mechanical strength. In contrast to other materials, polymers are notable for being biocompatible (BC) and biodegradable [7]. The most practical ingredients for 3D bio-printing are thermoplastic polymers. Nevertheless, they are divided into two primary classes for 3D bio-printing: synthetic polymers and natural polymers (often isolated from animal or human tissues) [8]. Additionally, in the tissue and organ bio-printing industries, the term “bio-ink” is crucial. Biomaterials and living cells are both included in bio-inks, which are created in a cellular matrix. Bio-inks must contain nontoxic, bioactive constituents and have a print temperature lower than physiological temperatures [9] when compared to conventional 3D-printing substances. Both natural and artificial polymers are used in bio-inks because of the substances that are appropriate for them. Bio-inks are being used to create 3D-printed constructions using a broad range of materials (ceramics, hydrogels, elastomers, and polymers) [10]. The highlights and drawbacks of these materials for 3D bio-printing are discussed in the following sections.
2. Synthetic Polymers
Synthetic polymers play a noteworthy role as highly applicabile constituents in 3D bio-printing owing to the characteristics they offer, including high robustness, a dominant microstructure, and measured degradability [11]. Chemical synthesis is used to create synthetic polymers, which may be precisely tailored with certain mechanical and chemical properties to match various bio-printing applications. In 3D scaffold construction, poly-glycolic acid (PGA) is regarded as a primary synthetic polymer due to its chemical adaptability, processing simplicity, biocompatibility, and biological characteristics. The glycolic acid monomer created by the biological degradation of PGA is effortlessly eliminated from the physique through certain catabolic routes including carbon dioxide and water [12]. The copolymers of PGA can also retain the mechanical and physical characteristics of PGA. PGA is employed when creating restorable grafts and internal bone-fixation devices. However, PGA’s breakdown products are not hazardous. When PGA’s surface is functionalized via the breakdown of ester bonds, the seeding density and cell spreading can be increased. Additionally, the primary polymer utilized as an antecedent in the FDM process is PLA, a popular, polymeric bio-ink that is a hydrolytically compostable aliphatic polyester with characteristics comprising biocompatibility, process ability, and printing capability [13]. Moreover, one non-toxic polymer with considerable durability is polycaprolactone (PCL) [14]. PCL is a less costly polymer that has admirable BI properties such as rigidity, biocompatibility, and degradability [15]. Its stability typically lasts for six months, with a biological half-life of three years. SLS-printed PCL scaffoldings have properties such as a porous architecture that promotes connectivity, a rough surface, and a bone-like tightness that promotes bone regeneration and cell ingrowth. Nevertheless, the prolonged biological half-life of the scaffold creates an additional barrier in scaffolds designed for uses other than osseous tissue engineering (TE). Additionally, the greater hydrophobicity of the substance results in reduced bioactivity, which slows tissue adhesion and cell development [16]. Additionally, a BC, thermoplastic polyester utilized in FDM printing methods is polybutylene terephthalate (PBT) [17]. PBT exhibits high flexibility, simple processing, and allowable strength and resilience. PBT is one of the primary polymers utilized in the biomedical area for in vivo and in vitro biocompatibility. It continues to be useful for printing canine trabecular bone scaffolds and for tissue regeneration. It is also used as a filler in orthopedic surgery. It does not have any distinct benefits and shares the same physical and chemical properties as PCL or PLA. Like most polymers, PBTs will break down in an aqueous medium via oxidation or hydrolysis. Its high melting point (225 °C) and non-biodegradable essence, which results in the production of crystalline residues during in vivo application, restrict its utilization [18]. In musculoskeletal tissue regeneration, filaments manufactured from PLA can be employed to substitute ligaments and non-biodegradable fibers. Poly-D, L-lactic acid (PDLLA) is an amorphous polymer having lactic acid as its primary component. Its inherent hydrophobicity, accessible biocompatibility, and durable mechanical properties make it suitable for biomedical implementations, predominantly in SLA methods. It is one of the polymers that is frequently used to create porous, BC scaffolds. As a result, it is used in tissue engineering and in restorable implants for orthopedic rehabilitation. Another prospective biodegradable elastomer with great biocompatibility and mechanical strength is polyurethane (PU), which has a thermosetting tendency. Water-based PU regular PU can be distinguished by the type of solvent used. The latter uses water as a solvent, whereas the former uses volatile organics [19]. SLA and digital light processing (DLP) printing methods use PU, and PLA can be used to assess how well it degrades. The strengths of 3D-bio-printing properties are enhanced by a high printing resolution and good cyto-compatibility. Chondrocyte manufacturing is preferred in cartilage tissue engineering and the creation of bone, muscle, and nerve scaffolds and. It exhibits an optimum elastomeric quality that stands up to repeated contraction and relaxation and is a suitable option for muscle generation [19]. By contrast, an H2O-dissolvable polymer—PVA—is utilized in the SLS printing procedure. PVA has a tensile strength comparable to human articular cartilage. With the right adhesive, PVA can build complex structures and offers a suitable matrix for bone-cell ingrowth. Its preferred hydrophilicity and chemical stability enable it to withstand extreme pH and temperature changes, and its semi-crystalline structure ensures that oxygen and other nutrients are efficiently transported to the cell. PVA is frequently utilized in a variety of load-bearing therapies, such as bone-tissue engineering or the repair of craniofacial defects. Due to its water solubility, PVA willfully expands in the presence of water and can be difficult to control [20].
Despite the variety of materials mentioned above, each material has a specific application. The inadequacy of conventional treatments still poses a serious threat to organ regeneration caused by severe losses and/or damages. Nowadays, 3D bio-printing is a noteworthy factor in the quick production of organs as an alternative to conventional techniques. Organ printing begins by employing specific polymers (such as PCL) as a scarified layer that provides strength during printing. The scarified coating is then eliminated by soaking the generated construct in a solution without inflicting suffering to the framework [21]. Recently, researchers attempted to use PU and PCL to apply polymers in the 3D printing of muscle tissue. Due to their adequate firmness and flexible characteristics, many studies have concentrated on developing these materials for printing muscle tissue. These experiments have included a variety of conventional methods, including solvent casting and phase separation, etc. In essence, scaffolds function as essential structural elements in tissue engineering, enabling their incorporation into organ architecture and supplying the required forms. Generalized scaffold fabrication is a requirement for bone-tissue engineering, which includes the steps listed below [22]:
- Selecting the materials for the scaffold and the bone tissue;
- Selecting the cell structure to be used;
- Bio-printing the cells into the scaffoldings;
- Determining the viability of the cells;
- Conducting experiments on animals.
3. Natural Polymers
Natural polymers, also known as bio-derived resources, are formed from living things and can be removed physically or chemically from their natural environments. Silk, wool, cellulose, and other materials are examples of naturally occurring polymers. These polymers have are used extensively in a variety of commercial sectors, including the food, paper, pharmaceutical, and other industries. Natural polymers that are H2O-soluable can dissolve in inorganic solvents that are pleasant to cells, such as cell culture media and phosphate-buffered saline, to produce solutions or hydrogels. Examples of these polymers include gelatin, alginate, fibrinogen, and hyaluronic acid. Natural polymers can be 3D-printed layer-by-layer, employing discrete-stacking rapid prototyping (RP), additive manufacturing (AM), or solid, free-form manufacturing (SFM) principles as their solution or hydrogel states exhibit a certain fluidity [23]. Theoretically, any natural polymer that, under particular circumstances, contains a sol–gel phase transition (i.e., a gelation point) can be printed utilizing a spontaneous, layer-by-layer (LBL) deposition process. In reality, only a very limited number of natural polymers can be printed in films at cell-friendly temperatures (such as room temperature) without the help of the physical, chemical, or biological cross-linking of the included polymer chains. This is due to the fact that only a few naturally occurring polymers can completely satisfy all requirements for the 3D bio-printing of cells, tissues, and organs [24]. Natural polymers have been crucial both during and after the 3D-bio-printing process in a number of ways, including by providing adequate spaces for extracellular matrix (ECM) configurations, biophysical/chemical cues for tissue/organ morphologies, and hierarchical network settings (vascular, neural, and lymphatic). It is possible to successfully and effectively avoid unexpected processing conditions, such as high temperatures, organic solvents, and H2O shortages, which have a negative impact on the bioactivities of the compressed cells and/or biomolecules [25]. Numerous natural polymers (such as alginate, chitosan, and decellularized extracellular matrix (dECM)) have been used as the primary ingredient in bio-inks over the past ten years. According to F. Pati et al., bio-inks containing dECM were successfully printed from three tissues [26]. The bio-ink’s dECM compositions, which include traits and biological functions from many tissues, have the ability to closely imitate genuine tissues. In numerous biomedical sectors, these natural polymers have enormous worth for scientific research and extravagant financial profit. These natural polymers for 3D bio-printing have three standout properties:
- Strong biocompatibility;
- Low mechanical strength;
- Quick biodegradability.
Consequently, natural polymers can provide a pleasant and secure situation for cells—especially stem cells—to sprout, relocate, propagate, and/or develop, in contrast to synthetic polymers. The details of a few exemplary natural polymers for 3D bio-printing are discussed in the section that follows.
3.1. Gelatin
Gelatin is a naturally occurring protein that is generated by the hydrolysis of collagen [27] and exhibits amphoteric activity with relation to alkaline and acidic amino acid functional moieties.
Mammalian-derived gelatin has been utilized as a biomaterial for regenerative goals. Due to their good biocompatibility, low immunogenicity, non-cytotoxicity, water solubility, and ability to promote cell adhesion, gelatin and its byproducts have been extensively used in 3D bio-printing [28][29][30]. Gelatin mixture exhibits a peculiar sol–gel transition at 28 °C, which corresponds to the melting point of G hydrogel. Thus, the unique thermal properties of gelati -based solutions enable the injection or extrusion of cells and/or bioactive substances via the plungers of 3D bio-printing, followed by the layering of those substances at relatively tolerable environmental temperatures between 1 and 28 °C. Moreover, gelatin-based solutions and hydrogels are used during and after 3D-printing procedures to sustain the structural performance of the 3D fabricates and to provide spaces for cells and bioactive substances inside the pre-defined 3D constructions. Due to these characteristics, a gelatin hydrogel in the form of gelatin methacryloyl (GelMA) is popular and substantially utilized for DIW printing. Two different types of GelMA for cell-laden bio-printing were evaluated by Lee et al. [31]. They claimed that cell viability had a value of up to 75% in the printed architectures of A and B GelMA. These components’ porous structures and reduced rigidity could help cells survive and proliferate more adequately.
However, naturally gelatin-based hydrogels have two distinct drawbacks in the field of 3D organ-printing [32]:
- Poor mechanical potency;
- Organizational unsteadiness at physiological temperatures (such as 37 °C).
When the printed, unit-filled 3D assemblies are placed in culture media at around 37 °C, it can be observed that the physiological, cross-linked gel phases (or structures) break down quickly. This occurs as a result of the gelatin molecules’ intrinsic cross-linking links disorganizing above the melting point of 28 °C, which compromises the structural performance of the three-dimensional (3D) formations [32]. To put it another way, reversible, physical cross-linking links cause gelled gelati -based constructions to disband instantly in a culture medium. To produce a stable structure [33][34], structures made of gelatin via 3D printing need to be further strengthened.
3.2. Alginate
Brown algae is the source of alginate (commonly known as algin), an anionic polysaccharide. In general, the term “alginate” refers to the salts of alginic acid, which is made up of the building blocks “β-D-mannuronic acid” (M block) and “σ-L-glucuronic acid” (G block) and can signify both the acid itself and all of its derivatives [35]. Divalent cations such as Ca2+ (calcium), Sr2+ (strontium), and Ba2+ (barium) ions can structurally cross-link a material called alginate, which dissolves in water. This property has made alginate exceptionally appealing in the sectors of regenerative medicine (RM), drug delivery, and wound healing [36]. Alginate and composite alginate hydrogels have been enormously utilized as cell-laden bio-inks in numerous 3D-bio-printing methods owing to their low toxicity, non-immunogenicity (excellent biocompatibility), fast degradability, and cross-linkable characteristic (chemical gelling propensity) [37]. Alginate sulfate–nanocellulose bio-inks for cartilage bio-printing implementations were investigated by Michael et al. [38] Alginate Sulfate was mixed with nanocellulose, which has been shown to have excellent printability, to transform it into a printable bio-ink. The results reveal that A-sulfate/nanocellulose ink had worthy printing qualities and that the encapsulated cells’ C II production was stirred by the non-printed BI material. The natural functioning of the cells was greatly influenced by the plunger shape during the printing of the bio-ink [38]. The viscosity of the cell-filled alginate hydrogel, however, is heavily influenced by the density, phenotypic, and molecular weight of the cells throughout the 3D-bio-printing procedures. After chemical crosslinking, cells placed in an alginate hydrogel with an extreme polymer intensity usually have significantly reduced bioactivities. A lower alginate–hydrogel content, meanwhile, allows for greater cell survival and proliferative capacity. Even after chemical cross-linking, however, the mechanical characteristics of the 3D structure rapidly decline when the alginate–hydrogel content is lowered. Therefore, for a standard 3D-bio-printing procedure, an optimal alginate concentration is required to guarantee beneficial cell survival and printing precision [39][40].
3.3. Collagen
Collagen is one of the BC polymers that has been thoroughly investigated in bioprinting [41]. Throughout the last few years, natural collagen has been enormously exploited as scaffold matter for TE. It is the chief component of musculoskeletal tissue and comprises the ECM of the majority of tissues. In a broad sense, V is a triple-helical, BC protein of biological origin. Therefore, immunological reactions to collagen scaffolding occur infrequently. On porous scaffolds, it can considerably increase osteoblasts’, chondroblasts’, and mesenchymal stem cells’ adherence, multiplication, and development capabilities [42]. Additionally, collagen can improve cellular attachment, adhesion, and proliferation. Nevertheless, it is challenging to 3D-print a collagen solution under ambient circumstances because the characteristics of the low-pH-soluble collagen solution are rapidly affected by hydrogel potential (pH) and temperature. This is because when a solution is neutralized at 37 °C, collagenases and metallo-proteinases can quickly break down collagen strands into amino acids, preventing them from assembling to form a hydrogel. The impact of pH and riboflavin photo-cross-linking on the rheological characteristics and printability of collagen was explored by Diamantides et al. [43]. The findings of their pH analysis demonstrated that the appearance stability of printed fragments throughout the gelation of collagen bio-inks was strongly influenced by pH; however, printability was unaffected by the pace of gelation of collagen bio-inks. In particular, collagen type I and type II have been utilized regularly for 3D-printed scaffoldings for chondro and osseous restoration. Ren et al. concentrated on the bio-printed collagen II hydrogel structures with a gradient chondrocyte compactness for manufactured zonal cartilage. In this investigation, type II collagen played a crucial role in promoting chondrogenic development and the ability to maintain the chondrocyte phenotype. The gradient ECM distribution in the 3D-printed zonal cartilage was favorably linked to chondrocyte density. For better biological effects, the bio-printing technique has modified both cell density and cell dispersion patterns in several zonal areas [44]. The utilization of 3D-printed scaffolds for tissue restoration has three clear benefits:
- In contrast to the conventional tissue-engineering of porous scaffolds, most 3D-printed scaffolds characteristically scale up via networks that are useful for transporting nutrients, oxygen, and metabolites;
- The gradient structural morphology and material composition are advantageous for realizing diverse functions in 3D-printed scaffolds;
- For hard or soft TE, living cells can be directly inserted into biocompatible material.
3.4. Hyaluronic Acid
A polymer found in living entities, hyaluronic acid (HA) is made up of d-glucuronic acid and N-acetyl-d-glucosamine [45]. HA has good biocompatibility and biodegradability, which is crucial for cell growth, angiogenesis, and interactions with receptors. Hyaluronidase, β-glucuronidase, and β-N-acetyl-glucosaminidase are enzymes that may quickly break down HA into low-molecular-weight hyaluronic acid and oligosaccharides (i.e., they glycolytically degrade it through a glycolytic pathway) [46]. It is a lubricating hydrophilic polymer that, when added to the previously stated gelatin-based bio-inks, can change viscosity by forming very viscous hydrogels at low concentrations. The limited mechanical properties of HA, as with the majority of natural polymers, lead to a low shape consistency during 3D bio-printing. Although HA has fascinating bioactive qualities and exhibits great biocompatibility for cartilage tissue creation, it lacks the physical characteristics necessary for its use in 3D, extrusion-based bio-printing (EBB). One of these drawbacks is that the material’s solutions lack sufficient viscosity to maintain stability in the reservoir throughout the printing process and, as a result, a uniform, three-dimensional distribution of the cells. Additionally, HA is incapable of gelating, which is necessary to preserve 3D structure after printing [47]. The production of HA formulations appropriate for use as a bio-ink has been made possible by a number of methods based on HA modification [48][49]. However, the majority of these methods have some shortcomings that may restrict their usefulness. Natural gelling agents have drawn a lot of attention in this regard because they do not require any harmful or complicated preparations or gel-forming processes. In this situation, Antich et al. produced highly capable and sustainable bio-printed, 3D hybrid scaffolds for AC restoration using an HA-based bio-ink. Analyzing the mechanical characteristics of the HA-based bio-ink and 3D hybrid construct, it was discovered that HA-based bio-ink increases the production of chondrogenic gene markers—specifically matrix deposition—and tissue development, which enhances cell functionality. These findings point to the bio-printed hybrid scaffold made of PLA and HA-based bio-ink as a suitable candidate for bio-ink that may assist AC formation in vitro [50].
Additionally, many changes have been made to the HA-based 3D-printing techniques to enhance their mechanical characteristics and shape fidelity. The cross-linking of HA with other polymers can be performed physically or chemically. For instance, by employing a UA-light source to photo-chemically cross-link HA and methacrylate, hyaluronic acid methacrylate (HAM) can be formed as a natural/synthetic hybrid polymer [51]. It should be emphasized that although HAMA has improved the poor physical characteristics of HA, the bio-inert characteristics of HAMA, such as the non-biodegradability of polymethacrylate (i.e., PMA), high hardness (or stiffness), and low shape fidelity, have significantly restricted its use in three-dimensional organ-bio-printing areas. As of the present moment, hybrid HAMA–GelMA bio-inks have been used in certain tissue-engineering applications, including the engineering of neuronal, cardiovascular, cartilage, and bone tissues. For instance, Skardal et al. showed that although a low ratio of HAMA/GelMA results in poor mechanical strength but higher cell adhesions, a high ratio of HAMA/GelMA would result in a stiffer structure but a poor cell-adhesive ability. When all factors were considered, the 80/20 HAMA/GelMA ratio was the best option [52].
Furthermore, Hauptstein et al. conducted research on hyaluronic acid (HA)-based bio-ink compositions to support 3D bio-printing and improve the quality of deposited, cartilaginous extracellular matrices by the UV-cross-linking of an allyl-modified poly(glycidol) in a range of concentrations [53]. The gels were additionally enhanced with an unmodified, 1 wt.% high-molecular-weight HA (hmHA), and chondrogenic differentiation of the included human mesenchymal stromal cells was evaluated in order to adapt bio-inks to poly(-caprolactone) (PCL)-supported 3D bio-printing. Surprisingly, the addition of hmHA to gels with a modest polymer content (3 wt.%) led to a noticeable improvement in construct quality with uniform ECM distribution across the constructs, independent of the printing procedure. When compared to higher-concentrated constructs (10 wt.%), which exclusively exhibited peri-cellular matrix deposition, the improved ECM dispersion in those constructs was related to a greater construct stiffness during chondrogenic development.
3.5. Chitosan
The well-known natural polymer chitosan, which is obtained from shrimp shells, is created when chitin is de-acetylated. It is an advantageous choice for tissue engineering due to its biocompatibility, antimicrobial qualities, biodegradability, and inexpensive cost. Lysozymes can biodegrade chitosan to produce amino-sugars [54]. Similar to alginate and HA, the poor mechanical strengths and sluggish gelation characteristics of chitosan solutions have undoubtedly limited their use in 3D organ-printing applications. Chitosan can be physically combined and chemically cross-linked with other supporting polymers, including alginate, gelatin, and collagen, to increase its mechanical qualities as expected [55]. A high chitosan viscosity is typically advised for extrusion-based, hybrid-polymeric hydrogel 3D-bio-printing technologies. Collagen/chitosan, alginate/chitosan, and gelatin/alginate/chitosan have all been widely and recently used as bio-inks in various 3D organ-bio-printing fields. A chitosan hydrogel was employed by Wu et al. [56] to guide cell development. Unusually, chitosan itself can be chemically altered to enhance printability in an acceptable pH range (7–7.4) without compromising its biocompatibility or biodegradability. Gu and colleagues’ research focuses on the proliferation and differentiation of brain cells, and they extruded a mixture of alginate, agarose, and carboxymethyl-chitosan in their experiments [57][58][59]. The mixture of 1.5% alginate and 5% carboxymethyl chitosan (a water-soluble derivative) was first identified as being ideal for print fidelity and the development of human-brain stem cells. The constructs had a depth evaluation of differentiated cell development that reached 169 μm, making them rather thin. In a subsequent work, Gu et al. demonstrated how the scaffold supported the formation of the embryoid body and subsequently controlled differentiation along the neural lineage using induced pluripotent stem cells.
Cheng et al. [60] simultaneously created a composite of chitosan and poly (caprolactone)-diacrylate/poly (ethylene glycol)-diacrylate for 3D printing via photopolymerization. Kingsley et al. (2016) [61] complexed the microspheres with PLL or chitosan using the laser direct-writing technique, in which cells in alginate were expelled by a laser pulse into gelatin/CaCl2. By liquefying the alginate core through incubation in sodium citrate, encapsulated cell spheroids that could be shaped into micro-strands were produced. Using chitosan coating as opposed to PLL coating, a breast-cancer cell line (M231) had higher cell viability. Fibroblasts and M231 cells were printed as a single construct to demonstrate their distinct localization and interplay. Despite being a 3D culture, the spheroids or strands in this study were not stacked, and the z-axis was about 80 μm. For bone tissue engineering, Lee et al. [62] created scaffolds using chitosan, gelatin, and hydroxyapatite. Demirtaş et al. [63] created a chitosan that exhibited temperature-sensitive gelation and remained in solution up to a pH range between 6.9 and 7 using a mixture of chitosan and glycerol phosphate. Alginate and chitosan–glycerol phosphate were compared with and without hydroxyapatite in printing pre-osteoblasts (MC3T3-E1). A good cell vitality (>90%) was observed across all conditions. Chitosan–hydroxyapatite > chitosan > alginate promotes osteogenesis in MC3T3 cells in accordance with osteogenic gene expression. Chitosan is a feasible substrate for iPSCs, MSCs (Mesenchymal Stem Cells), neurons, and other cell lines, according to these bio-printing research. They serve as the foundation for investigations that will improve in vitro models and add controlled complexity. Chitosan and polyethylene glycol diacrylate were utilized as bio-inks by Morris et al. and Elviri et al., respectively, to print scaffolds using the stereolithography technique [64][65].
3.6. Decellularized Extracellular Matrix
Cell and extracellular matrix (ECM) elements such as collagen, fibronectin, laminin, and glycosaminoglycans are found in tissues and organs [66]. Each tissue and organ has a unique composition that is influenced by interactions between its cells and the ECM. The ECM interacts with and controls the behavior of cells while also being produced by cells [67]. Cell receptors such asintegrins are used by cells to interact with the ECM. Several signaling pathways that are crucial for cellular functioning are activated by cell–ECM interactions. The decellularized extracellular matrix (dECM), on the other hand, is a blend of organic polymers made from various animal tissues including the skin, small intestinal submucosa, and liver [68]. The elimination of cellular components while preserving the natural shape and makeup of the tissue or organ is the true aim of a decellularization (dC) phase. Thus, the final qualities of the created dECM bio-ink are greatly influenced by the chosen dC process. Following dC, the composition and structure of the original tissues may still be largely preserved, which may allow for the creation of tissue-specific microenvironments for the preservation of cell-specific functions. The final components of the dECM can be influenced by the dC processes, which can be either physical, chemical, biological (such as enzymatic), or a mixture of these processes. Chemicals such as acids, bases, detergents, and alcohols have all been utilized in the past, but the only biological techniques that are currently available are enzymes, such as trypsin, and nucleases [69]. Other techniques, such as sonication, heating, exerting pressure, and electroporation, can also be used to decellularize an object [70]. Beyond 15 °C, the resulting dECM-based solutions gel instantly and produce physically cross-linked hydrogels. It was reported that dECM produced from porcine liver could be employed as a useful substrate for hepatocyte cultivation. Through albumin secretion, bile-salt export-pump (BSEP) mRNA expression, and sodium taurocholate co-transporting polypeptide (NTCP)—which was considered a potential scaffold material in3D tissue-bio-printing—the liver-specific dECM could maintain hepatocyte activities [26]. Additionally, dECM can offer cells specialized milieus for the 3D bio-printing of organs. Due to their low viscosity, dECM bio-inks frequently require the assistance of additional supporting polymers in order to achieve basic 3D printability and shape fidelity. For instance, a multi-head tissue-building system has printed an adipose-derived dECM/polycaprolactone (PCL) hydrogel with encapsulated ADSCs, which produced a high cell viability (>90%) [71].
The use of dECM-derived bio-inks for various tailored, bio-artificial 3D organ-bio-printing applications is currently gaining popularity in both academic and professional contexts. Some bio-inks made from dECM have been investigated as potential substitutes for clinical uses. However, the degree of mechanical property preservation has a significant impact on clinical outcomes. The ECM of the native tissue or organ is often maintained by dECMs, which provides tissue- or organ-specific microenvironments for cellular growth and function. Growth factors, cytokines, and microRNAs have all been found to be present in a variety of dECM-based scaffolds that have been produced [72][73]. For a transplant to be effective, dECM must meet a number of conditions. After dC, there cannot be more than 50 ng of dsDNA/mg of weight of remaining DNA. Decellularization must be performed gently to prevent harming the ECM and changing its composition. Additionally, it has been widely shown that the decellularized extracellular matrix (dECM) is a bio-instructive scaffold that may drive and modulate cellular responses, such as proliferation and differentiation, as well as aid in vivo tissue repair and regeneration [74]. Additionally, it has been established that cells have a better expansion and differentiation potential when grown on a dECM from their original tissue [74]. Although the precise mechanism is unclear, it is hypothesized that the various tissues’ distinct compositional makeups of the ECM create an environment that is favorable for cell types that are compatible with their respective tissues. This concept gave rise to the idea of using a tissue-specific approach in tissue engineering. For extrusion-based 3D-bio-printing systems, the use of a dECM as an innovative bio-ink has been popular up until recently. The efficient use of dECM bio-inks to create cell-filled, porous adipose, cartilage, and heart tissue, mimicking using a nozzle-based bioprinter, was experimentally verified by Pati et al. [26]. In this instance, when cultured in their corresponding, tissue-matched dECM, bio-inks—particularly in comparison to collagen-I-regulated bio-printed tissues—assisted with high viability and augmented the tissue-specific gene expression of human-adipose-derived stem cells (ADSCs), human turbinate mesenchymal stem cells (hTMSCs), and rat myoblast cells [26]. This further emphasizes the benefits of tissue-specific ECM. Although the use of a dECM provides outstanding benefits for maintaining tissue- or organ-specific functions, it still has numerous other difficulties regarding the 3D bio-printing of complicated organs. First, it is challenging to successfully remove antigenic epitopes that have been produced by the allogeneic or xenogeneic receivers of dECMs. Second, residual DNA or nuclear materials are somewhat preserved in dECMs, which likely influences the behaviors of encapsulated cells. Finally, there are still many issues that need to be resolved in the future, including incredibly bad mechanical qualities, low construction resolution, surprising form-shrinking, and quick degradation rate [75].
3.7. Other Materials
Several actions are necessary to obtain bio-inks (e.g., increasing the printability). These constraints improve the qualities of the bio-printed materials. Numerous studies have been performed in this area to accomplish this milestone. Making multi-material bio-inks possible is one of the most crucial tactics. A collagen–alginate bio-ink was employed in tissue engineering by Yanga et al. [76]. An alginate–methylcellulose hydrogel was employed by Li et al. [77] as the bio-ink for bio-printing. Additionally, there have been various attempts to use 3D bio-printing to produce silk-based constructions due to the growth in the biological and therapeutic uses of silk. For the creation of soft tissue implants, the inkjet bio-printing of silk–alginate as a bio-ink cross-linked with horseradish peroxidase has been documented [78]. In order to bio-fabricate cardiac tissue constructs, Wang et al. created a fibrin-based composite bio-ink made of fibrinogen, gelatin, HA, and rat ventricular cardiomyocytes at a concentration of 10 ×× 106 cells/mL. The fibrin was cross-linked by soaking the bio-printed construct in Dulbecco’s modified minimum essential medium (DMEM) containing thrombin for 20 min. Immunostaining was used to confirm the creation of cardiac tissue constructions and showed that the bio-printed constructs responded to medications for the heart [79]. These findings illustrate the use of fibrin bio-inks in regeneration. The study discussed above can be used to produce many materials for 3D bio-printing. In addition to single- and multi-material bio-inks, a novel idea known as the “self-assembling of bio-inks” has emerged for the manufacture of enormous anatomical structures. Numerous studies have shown that self-assembling tissue strands have been used to create nanofibrous hydrogels for bio-printing [80][81].
The total number of publications retrieved from Science Direct for the past five years is shown in Figure 1, in which the gradual increase of the use of dECMs is clearly discernible.
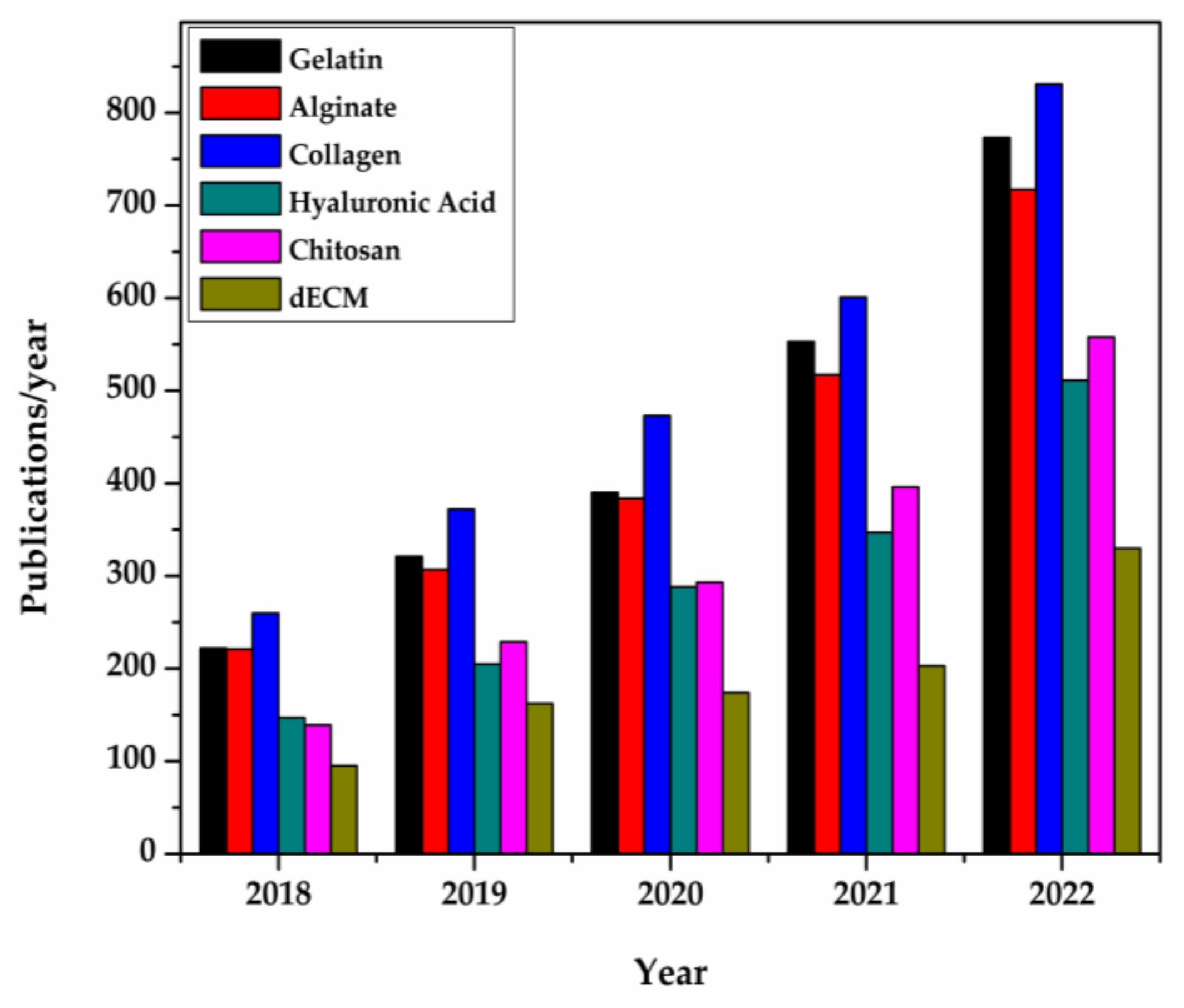
Figure 1. Natural polymer publication/year in 3D bio-printing for tissue engineering [82]. The bars represent the number of publications retrieved from Science Direct. The examined time interval was 2018–2022.
4. Material Characteristics of Bio-Inks
A complicated manufacturing technique is used to create bio-ink formulations with pre-defined rheological properties in order to 3D-print a rigid or elastic 3D scaffold with evenly spaced components and a controlled shape. As a result, the following qualities are preferred for the bio-inks.
4.1. Printability
One of the most crucial characteristics for a component to be appropriate for 3D bio-printing is its capacity to be effectively used by the printer; in particular, how well the element could be precisely coated with the requisite controllability. This differs amongst printing techniques; therefore, it is challenging to define what printability is. Smooth nozzle dispensing, continuous filament formation with high shape retention, and high structural integrity are all characteristics of bio-inks with good printability. Printability can be impacted by a variety of parameters, including ink composition, viscosity, gelation kinetics, and surface tension. Printing characteristics that can affect the results include extrusion pressure, printing velocity, nozzle diameter, nozzle/printed/ambient temperature, and the printing path. Whatever parameter configurations are chosen, a method of evaluation known as printability is required to describe the structural outcome. Bio-ink printability can be assessed using known techniques such as qualitative description, quantitative analysis, and computer simulation [83]. Ouyang et al. [84] described the structural creation of the pore squared by four filaments in a usual lattice arrangement using a semi-quantitative method. The printability factor (Pr) was defined as the ratio of the ideal square circularity (i.e., p = π/4) to the actual pore circularity. An appropriate gelation situation is represented by a lattice structure with a Pr = 1; over-gelation is indicated by a Pr value > 1, and under-gelation is demonstrated by a Pr value < 1. The gelation situation needs a formulation with an acceptable viscosity range and quick transition kinetics from the sol to gel states [85]. For instance, extrusion-based printing can print materials with very high viscosities, whereas inkjet printing has a restriction on material viscosity. However, extrusion-based printing needs the material to have certain inter-layer cross-linking mechanisms or shear-thinning qualities. Bio-inks are anticipated to be shear-thinning liquids with a regulated geometry and porosity that can be extruded in a laminar flow while having adequate mechanical strength to self-support over multiple layers for nozzle-based extrusion bio-printing [86]. Additionally, the viscosity and gel rate of the hydrogel can be changed to increase 3D printability by simply treating the sodium alginate/poly(acrylamide-co-acrylic acid) bio-ink formulation in a solution of pH 14 [87]. It is crucial for the printing material or procedure to shield the cells from this high temperature because some processes demand intense, localized heating of the material for cell deposition. The ability of materials to cushion cells during the deposition process and materials with low thermal conductivity have both been proven to boost cell survivability and biological function after printing. The surface tension between the printing medium and the receiving substrate is another element that significantly affects cell adhesion and development. It is anticipated that the printed material will maintain a vertical tension with the substrate. Before printing, this can be accomplished by covering the substrate with a small layer of substance to increase its hydrophobicity. Rheological characteristics including storage and loss moduli, degree of shear-thinning, and yield stress can also be used to assess bio-ink printability [88][89].
4.2. Biocompatibility
The capacity of a substance to function with a suitable host reaction in a particular situation is referred to as biocompatibility. The primary objective of attaining biocompatibility has evolved over time, shifting from requiring the implantation material to coexist with the host without having any negative local or global consequences to allowing or actively producing positive benefits passively in the host. Additionally, it is anticipated that the material will, in an ideal scenario, allow the cells to replace it with their own ECM proteins that are created at a speed which matches the material’s rate of deterioration after being transplanted into the host and degrading. In a recent study [90], dental implants from mining were subjected to acidic etching and grit-blasting to create surfaces with nanostructures. After four and twelve weeks of implantation, only a very small number of inflammatory cells were observed, demonstrating the biocompatibility of the implants. Additionally, within a few weeks of implantation, the microenvironment within the scaffold was anticipated to promote the growth of blood vessels in or around the implant, creating a favorable environment for the movement of nutrients, oxygen, and waste. Since all byproducts should be harmless, easily digested, and quickly eliminated from the body, the creation of byproducts during the degradation process also defines the biocompatibility of the material.
4.3. Gelation
The kinetics of a bio-ink’s gelation or cross-linking are yet another important factor for structural printability. Kinetics control how quickly the deposited bio-ink can cross-link, which has an impact on the shape fidelity of printed constructions. Bio-inks that gel slowly prevent cross-linking from occurring, and the deposited bio-inks spread adversely. Additionally, because of the excessive exposure to gelation stimuli, prolonged gelation time may have an adverse effect on the viability of cells in the constructions (e.g., light, temperature, pH, or other harsh conditions) [91]. However, it is also crucial to manage gelation kinetics because a bio-ink nozzle’s quick cross-linking can result in a sharp shift in the rheological properties and clog the dispensing nozzle. Biomaterials that have been physically cross-linked produce transient networks that are stabilized by weak interactions such as ionic cross-linking, hydrophobic contact, hydrogen bonding, host–guest interactions, and stereo-complex formation [92]. Physical gelation is unsuitable as the only cross-linking method for the solidification of bio-printed constructions due to its dynamic and mechanically fragile nature. As a result, secondary chemical cross-linking is frequently used in conjunction with physical gelation [9]. In contrast to physically cross-linked gels, chemical cross-linking introduces covalent connections into the network, which are believed to increase mechanical characteristics and structural fidelity. Covalently cross-linked inks are often produced using enzyme-mediated cross-linking, photo-polymerization, and click chemistry (e.g., Michael addition and Schiff base formation).
4.4. Mechanical Properties
For the material to maintain a 3D structure following solidification, it must possess enough structural and mechanical qualities. Additionally, a study framework is necessary for cells to adhere to, multiply, and differentiate in an appropriate environment. Additionally, there are considerable impacts on cell adhesion from interactions between cells and the printing medium. From hard, implanted bone to soft tissues such as skin and cartilage, there are varying mechanical needs for materials for different types of tissue engineering. The mechanical qualities are particularly important because the functions of soft tissues primarily depend on such features. To avoid material failure and to reduce fracture under significant strain, the scaffold’s components should establish an effective network-enhancement mechanism [93]. To enable an appropriate load transfer, the mechanical properties of the constructed scaffold should be compatible with those of the host bone. For instance, Ratheesh and colleagues attempted to improve the biomechanical properties of the scaffold by adding 15% w/v of inorganic bone particles with diameters of 150–500 and 0–500 μm to 10 and 12.5% w/v methacrylated, gelatin-based bio-inks, respectively [94].
4.5. Viscosity
The ability of bio-inks to be printed is greatly influenced by their viscosity; appropriately viscous inks can frequently improve the printing resolution, form retention, and stability. However, high viscosity frequently leads to more shear stress being generated inside the dispensing nozzle, which could harm implanted cells. Therefore, for optimal ink printability, an adjustable bio-ink viscosity is essential. The viscosity of a particular bio-ink formulation is affected by its temperature, shear-thinning characteristics, polymer content, molecular-weight encapsulated cell density, and the addition of rheology-modifying components [95]. Through the use of temperature-controlled nozzles, thermo-responsive biomaterials with temperature-dependent viscosities can be used for 3D bio-printing. A simple method to increase viscosity is to use a high concentration of biomaterials; however, a high polymer density may be hazardous to encapsulated cells and may restrict their access to nutrients and oxygen in a 3D culture system. Depending on the polymer selected, the type of cross-linker used, and the method used to partially cross-link the bio-ink precursor, the polymer’s molecular weight has an impact on the bio-ink viscosity. Recently, the impact of encapsulated cells on the viscosity of bio-ink and the printing quality has been brought to light [96] According to a paper, the viscosity of bio-inks can change after encasing cells. This is likely dependent on the type of cell and the biomaterial. To change the viscosity of ink, rheology-modifying components are frequently used [97]. For instance, Ouyang et al. [98] achieved standard printability for all the examined bio-inks by using gelatin as a universal ingredient to bring thermo-responsive rheology to a variety of photo-cross-linkable hydrogels. In extrusion-based bioprinting, shear-thinning tendencies are advantageous rheological characteristics. The addition of shear-thinning capabilities to bio-inks can be accomplished in a number of ways, including reversible supramolecular and dynamic covalent-bond modifications [99]. Another significant rheological element linked to ink printability is the yield stress. If the applied shear stress is greater than the yield stress, bio-inks behave as a complicated fluid and begin to flow through the printer nozzle [100]. The applied shear stress is removed as the bio-ink exits the nozzle, and the pre-existing yield stress aids in preserving the filament’s form. A bio-ink’s appropriate yield stress also promotes uniform cell distribution in the printed tissue and precludes cell sedimentation in the hydrogel precursor. However, the majority of hydrogels are shear-thinning, which means that as their shear strain increases, so do their viscosities [101]. Some hydrogels also have thixotropic behavior. This means that, when subjected to shear, their viscosity also gradually lowers [102]. Rheological studies of a bio-ink can determine its viscosity, shear-thinning, and thixotropic properties, and numerous mathematical models linking these measures to extrudability and cell injury have been well-established [103]. Rheological characterizations can therefore be utilized to estimate extrudability indirectly. Most significantly, frequency sweeps at varying shear speeds have been used to assess the viscosity of bio-inks. These values can be shown as-is or fitted to the power law equation to determine the consistency index (K) and flow index (n) for comparison between different bio inks. The consistency index relates to the bio-ink’s initial (or zero-shear) viscosity, with lower values indicating increased extrudability. On the other hand, the flow index is related to the shear-thinning properties of the bio-ink, with a flow index of one indicating Newtonian behavior and values closer to zero indicating a higher degree of shear-thinning and, consequently, extrudability [104].
4.6. Biodegradability and Surface Characteristics
The printed scaffold is anticipated to deteriorate over time in vivo. In an ideal situation, the pace at which scaffolds degrade and the rate at which the ECM is produced are equal, and the degradation products have no negative consequences on the host. For instance, a waterborne polyurethane scaffold lost 35.62% of its mass after 90 days of deterioration, yet a fluorescence scan revealed that all of the scaffolds had been coated with rabbit chondrocytes after just seven days of incubation [105]. A scaffold transplantation’s effectiveness also depends on the interface between the scaffold and the tissue that needs to be healed, where a variety of responses and interactions occur. As a result, the surface properties of the scaffold, such as its porosity, wettability, and shape, need to be carefully considered. For instance, the first-linked holes should retain a minimum diameter of 100 μm to permit the diffusion of nutrients and oxygen, which are necessary for cell viability, as well as the transfer of waste created by cells. The suggested pore-size range for bone tissue development is between 200 and 350 μm. The scaffold’s surface should be designed to limit cell migration, proliferation, and differentiation. Additionally, the scaffold’s shape is crucial for securing proteins and cells to the surface [106].
The above-mentioned characteristics of bio-inks and consequent scaffolding do not exist independently at any one stage. These qualities are related to one another and may have an impact on one another during the entire process, which includes both physical and chemical changes beginning with the bio-ink in its liquid condition and ending with the formed scaffold and functional implant.
References
- Ramadan, Q.; Zourob, M. 3D bioprinting at the frontier of regenerative medicine, pharmaceutical, and food industries. Front. Med. Technol. 2021, 2, 607648.
- Tammaro, D.; Detry AL, H.; Landonfi, L.; Napolitano, F.; Villone, M.M.; Maffettone, P.L.; Squillace, A. Bio-lightweight structures by 3D foam printing. In Proceedings of the 2021 IEEE 6th International Forum on Research and Technology for Society and Industry (RTSI), Naples, Italy, 6–9 September 2021; IEEE: New York, NY, USA, 2021; pp. 47–51.
- Arslan-Yildiz, A.; El Assal, R.; Chen, P.; Guven, S.; Inci, F.; Demirci, U. Towards artificial tissue models: Past, present, and future of 3D bioprinting. Biofabrication 2016, 8, 014103.
- Assad, H.; Kumar, A. Understanding functional group effect on corrosion inhibition efficiency of selected organic compounds. J. Mol. Liq. 2021, 344, 117755.
- Assad, H.; Ganjoo, R.; Sharma, S. A theoretical insight to understand the structures and dynamics of thiazole derivatives. J. Phys. Conf. Ser. 2022, 2267, 012063.
- Assad, H.; Fatma, I.; Kumar, A. An overview of the application of graphene-based materials in anticorrosive coatings. Mater. Lett. 2022, 330, 133287.
- Shim, J.H.; Won, J.Y.; Park, J.H.; Bae, J.H.; Ahn, G.; Kim, C.H.; Lim, D.-H.; Cho, D.-W.; Yun, W.-S.; Bae, E.-B.; et al. Effects of 3D-printed polycaprolactone/β-tricalcium phosphate membranes on guided bone regeneration. Int. J. Mol. Sci. 2017, 18, 899.
- Atala, A.; Yoo, J.J. Essentials of 3D Biofabrication and Translation; Academic Press: Cambridge, MA, USA, 2015.
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946.
- O’Grady, B.J.; Balikov, D.A.; Lippmann, E.S.; Bellan, L.M. Spatiotemporal control of morphogen delivery to pattern stem cell differentiation in three-dimensional hydrogels. Curr. Protoc. Stem Cell Biol. 2019, 51, e97.
- Griffin, M.D.; Pereira, S.R.; DeBari, M.K.; Abbott, R.D. Mechanisms of action, chemical characteristics, and model systems of obesogens. BMC Biomed. Eng. 2020, 2, 6.
- Asti, A.; Gioglio, L. Natural and synthetic biodegradable polymers: Different scaffolds for cell expansion and tissue formation. Int. J. Artif. Organs 2014, 37, 187–205.
- Serra, T.; Mateos-Timoneda, M.A.; Planell, J.A.; Navarro, M. 3D printed PLA-based scaffolds: A versatile tool in regenerative medicine. Organogenesis 2013, 9, 239–244.
- Pan, Q.; Gao, C.; Wang, Y.; Wang, Y.; Mao, C.; Wang, Q.; Economidou, S.N.; Douroumis, D.; Wen, F.; Tan, L.P.; et al. Investigation of bone reconstruction using an attenuated immunogenicity xenogenic composite scaffold fabricated by 3D printing. Bio Des. Manuf. 2020, 3, 396–409.
- Murphy, C.; Kolan, K.; Li, W.; Semon, J.; Day, D.; Leu, M. 3D bioprinting of stem cells and polymer/bioactive glass composite scaffolds for bone tissue engineering. Int. J. Bioprinting 2017, 3, 005.
- Gonçalves, E.M.; Oliveira, F.J.; Silva, R.F.; Neto, M.A.; Fernandes, M.H.; Amaral, M.; Vallet-Regí, M.; Vila, M. Three-dimensional printed PCL-hydroxyapatite scaffolds filled with CNT s for bone cell growth stimulation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1210–1219.
- Guvendiren, M.; Molde, J.; Soares, R.M.; Kohn, J. Designing biomaterials for 3D printing. ACS Biomater. Sci. Eng. 2016, 2, 1679–1693.
- Kutikov, A.B.; Gurijala, A.; Song, J. Rapid prototyping amphiphilic polymer/hydroxyapatite composite scaffolds with hydration-induced self-fixation behavior. Tissue Eng. Part C Methods 2015, 21, 229–241.
- Hung, K.C.; Tseng, C.S.; Hsu, S.H. Synthesis and 3D printing of biodegradable polyurethane elastomer by a water-based process for cartilage tissue engineering applications. Adv. Healthc. Mater. 2014, 3, 1578–1587.
- Shuai, C.; Mao, Z.; Lu, H.; Nie, Y.; Hu, H.; Peng, S. Fabrication of porous polyvinyl alcohol scaffold for bone tissue engineering via selective laser sintering. Biofabrication 2013, 5, 015014.
- Ho, C.C.; Fang, H.Y.; Wang, B.; Huang, T.H.; Shie, M.Y. The effects of Biodentine/polycaprolactone three-dimensional-scaffold with odontogenesis properties on human dental pulp cells. Int. Endod. J. 2018, 51, e291–e300.
- Pati, F.; Song, T.H.; Rijal, G.; Jang, J.; Kim, S.W.; Cho, D.W. Ornamenting 3D printed scaffolds with cell-laid extracellular matrix for bone tissue regeneration. Biomaterials 2015, 37, 230–241.
- Liu, F.; Liu, C.; Chen, Q.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Progress in organ 3D bioprinting. Int. J. Bioprinting 2018, 4, 182.
- Wang, X.; Yan, Y.; Zhang, R. Gelatin-based hydrogels for controlled cell assembly. In Biomedical Applications of Hydrogels Handbook; Springer: New York, NY, USA, 2010; pp. 269–284.
- Hou, R.; Nie, L.; Du, G.; Xiong, X.; Fu, J. Natural polysaccharides promote chondrocyte adhesion and proliferation on magnetic nanoparticle/PVA composite hydrogels. Colloids Surf. B Biointerfaces 2015, 132, 146–154.
- Pati, F.; Jang, J.; Ha, D.-H.; Kim, S.W.; Rhie, J.-W.; Shim, J.-H.; Kim, D.-H.; Cho, D.-W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935.
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-based hydrogels for organ 3D bioprinting. Polymers 2017, 9, 401.
- Park, J.; Lee, S.J.; Chung, S.; Lee, J.H.; Kim, W.D.; Lee, J.Y.; Park, S.A. Cell-laden 3D bioprinting hydrogel matrix depending on different compositions for soft tissue engineering: Characterization and evaluation. Mater. Sci. Eng. C 2017, 71, 678–684.
- Yu, Y.; Zhang, Y.; Martin, J.A.; Ozbolat, I.T. Evaluation of cell viability and functionality in vessel-like bioprintable cell-laden tubular channels. J. Biomech. Eng. 2013, 135, 091011–0910119.
- Wang, X.; He, K.; Zhang, W. Optimizing the fabrication processes for manufacturing a hybrid hierarchical polyurethane–cell/hydrogel construct. J. Bioact. Compat. Polym. 2013, 28, 303–319.
- Lee, B.H.; Lum, N.; Seow, L.Y.; Lim, P.Q.; Tan, L.P. Synthesis and characterization of types A and B gelatin methacryloyl for bioink applications. Materials 2016, 9, 797.
- Liu, F.; Chen, Q.; Liu, C.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Natural polymers for organ 3D bioprinting. Polymers 2018, 10, 1278.
- Rutz, A.L.; Hyland, K.E.; Jakus, A.E.; Burghardt, W.R.; Shah, R.N. A multimaterial bioink method for 3D printing tunable, cell-compatible hydrogels. Adv. Mater. 2015, 27, 1607–1614.
- Xavier, J.R.; Thakur, T.; Desai, P.; Jaiswal, M.K.; Sears, N.; Cosgriff-Hernandez, E.; Kaunas, R.; Gaharwar, A.K. Bioactive nanoengineered hydrogels for bone tissue engineering: A growth-factor-free approach. ACS Nano 2015, 9, 3109–3118.
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305.
- Choi, Y.J.; Yi, H.G.; Kim, S.W.; Cho, D.W. 3D cell printed tissue analogues: A new platform for theranostics. Theranostics 2017, 7, 3118.
- Axpe, E.; Oyen, M.L. Applications of alginate-based bioinks in 3D bioprinting. Int. J. Mol. Sci. 2016, 17, 1976.
- Müller, M.; Öztürk, E.; Arlov, Ø.; Gatenholm, P.; Zenobi-Wong, M. Alginate sulfate–nanocellulose bioinks for cartilage bioprinting applications. Ann. Biomed. Eng. 2017, 45, 210–223.
- Izadifar, Z.; Chang, T.; Kulyk, W.; Chen, X.; Eames, B.F. Analyzing biological performance of 3D-printed, cell-impregnated hybrid constructs for cartilage tissue engineering. Tissue Eng. Part C Methods 2016, 22, 173–188.
- Luo, Y.; Luo, G.; Gelinsky, M.; Huang, P.; Ruan, C. 3D bioprinting scaffold using alginate/polyvinyl alcohol bioinks. Mater. Lett. 2017, 189, 295–298.
- Vanaei, S.; Parizi, M.S.; Salemizadehparizi, F.; Vanaei, H.R. An overview on materials and techniques in 3D bioprinting toward biomedical application. Eng. Regen. 2021, 2, 1–18.
- Nagel, T.; Kelly, D.J. The composition of engineered cartilage at the time of implantation determines the likelihood of regenerating tissue with a normal collagen architecture. Tissue Eng. Part A 2013, 19, 824–833.
- Diamantides, N.; Wang, L.; Pruiksma, T.; Siemiatkoski, J.; Dugopolski, C.; Shortkroff, S.; Kennedy, S.; Bonassar, L.J. Correlating rheological properties and printability of collagen bioinks: The effects of riboflavin photocrosslinking and pH. Biofabrication 2017, 9, 034102.
- Ren, X.; Wang, F.; Chen, C.; Gong, X.; Yin, L.; Yang, L. Engineering zonal cartilage through bioprinting collagen type II hydrogel constructs with biomimetic chondrocyte density gradient. BMC Musculoskelet. Disord. 2016, 17, 301.
- Skardal, A.; Devarasetty, M.; Kang, H.-W.; Seol, Y.-J.; Forsythe, S.D.; Bishop, C.; Shupe, T.; Soker, S.; Atala, A. Bioprinting cellularized constructs using a tissue-specific hydrogel bioink. J. Vis. Exp. 2016, 110, e53606.
- Xiang, H.; Yang, X.; Ke, L.; Hu, Y. The properties, biotechnologies, and applications of antifreeze proteins. Int. J. Biol. Macromol. 2020, 153, 661–675.
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343.
- Kiyotake, E.A.; Douglas, A.W.; Thomas, E.E.; Nimmo, S.L.; Detamore, M.S. Development and quantitative characterization of the precursor rheology of hyaluronic acid hydrogels for bioprinting. Acta Biomater. 2019, 95, 176–187.
- Petta, D.; Armiento, A.R.; Grijpma, D.; Alini, M.; Eglin, D.; D’este, M. 3D bioprinting of a hyaluronan bioink through enzymatic-and visible light-crosslinking. Biofabrication 2018, 10, 044104.
- Antich, C.; de Vicente, J.; Jiménez, G.; Chocarro, C.; Carrillo, E.; Montañez, E.; Gálvez-Martín, P.; Marchal, J.A. Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs. Acta Biomater. 2020, 106, 114–123.
- Abbadessa, A.; Mouser, V.H.; Blokzijl, M.M.; Gawlitta, D.; Dhert, W.J.; Hennink, W.E.; Malda, J.; Vermonden, T. A synthetic thermosensitive hydrogel for cartilage bioprinting and its biofunctionalization with polysaccharides. Biomacromolecules 2016, 17, 2137–2147.
- Xu, W.; Wang, X.; Yan, Y.; Zhang, R. A polyurethane-gelatin hybrid construct for manufacturing implantable bioartificial livers. J. Bioact. Compat. Polym. 2008, 23, 409–422.
- Hauptstein, J.; Böck, T.; Bartolf-Kopp, M.; Forster, L.; Stahlhut, P.; Nadernezhad, A.; Blahetek, G.; Zernecke-Madsen, A.; Detsch, R.; Jüngst, T.; et al. Hyaluronic acid-based bioink composition enabling 3D bioprinting and improving quality of deposited cartilaginous extracellular matrix. Adv. Healthc. Mater. 2020, 9, 2000737.
- Cui, T.; Wang, X.; Tan, Y.; Zhang, R. Rapid prototyping a double-layer polyurethane—Collagen conduit and its Schwann cell compatibility. J. Bioact. Compat. Polym. 2009, 24, 5–17.
- Huang, Y.; Onyeri, S.; Siewe, M.; Moshfeghian, A.; Madihally, S.V. In vitro characterization of chitosan–gelatin scaffolds for tissue engineering. Biomaterials 2005, 26, 7616–7627.
- Wu, Q.; Maire, M.; Lerouge, S.; Therriault, D.; Heuzey, M.C. 3D printing of microstructured and stretchable chitosan hydrogel for guided cell growth. Adv. Biosyst. 2017, 1, 1700058.
- Gu, Q.; Tomaskovic-Crook, E.; Lozano, R.; Chen, Y.; Kapsa, R.M.; Zhou, Q.; Wallace, G.G.; Crook, J.M. Functional 3D neural mini-tissues from printed gel-based bioink and human neural stem cells. Adv. Healthc. Mat. 2016, 5, 1429–1438.
- Gu, Q.; Tomaskovic-Crook, E.; Wallace, G.G.; Crook, J.M. 3D bioprinting human induced pluripotent stem cell constructs for in situ cell proliferation and successive multilineage differentiation. Adv. Healthc. Mater. 2017, 6, 1700175.
- Gu, Q.; Tomaskovic-Crook, E.; Wallace, G.G.; Crook, J.M. Engineering human neural tissue by 3D bioprinting. In Biomaterials for Tissue Engineering; Humana Press: New York, NY, USA, 2018; pp. 129–138.
- Cheng, Y.L.; Chen, F. Preparation and characterization of photocured poly (ε-caprolactone) diacrylate/poly (ethylene glycol) diacrylate/chitosan for photopolymerization-type 3D printing tissue engineering scaffold application. Mater. Sci. Eng. C 2017, 81, 66–73.
- Kingsley, D.M.; Dias, A.D.; Corr, D.T. Microcapsules and 3D customizable shelled microenvironments from laser direct-written microbeads. Biotechnol. Bioeng. 2016, 113, 2264–2274.
- Lee, C.M.; Yang, S.W.; Jung, S.C.; Kim, B.H. Oxygen plasma treatment on 3D-printed chitosan/gelatin/hydroxyapatite scaffolds for bone tissue engineering. J. Nanosci. Nanotechnol. 2017, 17, 2747–2750.
- Demirtaş, T.T.; Irmak, G.; Gümüşderelioğlu, M. A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication 2017, 9, 035003.
- Mosesson, M.W. Fibrinogen and fibrin structure and functions. J. Thromb. Haemost. 2005, 3, 1894–1904.
- Elviri, L.; Foresti, R.; Bergonzi, C.; Zimetti, F.; Marchi, C.; Bianchera, A.; Bernini, F.; Silvestri, M.; Bettini, R. Highly defined 3D printed chitosan scaffolds featuring improved cell growth. Biomed. Mater. 2017, 12, 045009.
- Choudhury, D.; Tun, H.W.; Wang, T.; Naing, M.W. Organ-derived decellularized extracellular matrix: A game changer for bioink manufacturing? Trends Biotechnol. 2018, 36, 787–805.
- Ma, X.; Yu, C.; Wang, P.; Xu, W.; Wan, X.; Lai, C.S.E.; Liu, J.; Koroleva-Maharajh, A.; Chen, S. Rapid 3D bioprinting of decellularized extracellular matrix with regionally varied mechanical properties and biomimetic microarchitecture. Biomaterials 2018, 185, 310–321.
- Aamodt, J.M.; Grainger, D.W. Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials 2016, 86, 68–82.
- Keane, T.J.; Swinehart, I.T.; Badylak, S.F. Methods of Tissue Decellularization Used for Preparation of Biologic Scaffolds and in vivo relevance. Methods 2015, 84, 25–34.
- Badylak, S.F.; Taylor, D.; Uygun, K. Whole-organ tissue engineering: Decellularization and recellularization of three-dimensional matrix scaffolds. Annu. Rev. Biomed. Eng. 2011, 13, 27–53.
- Sellaro, T.L.; Ranade, A.; Faulk, D.M.; McCabe, G.P.; Dorko, K.; Badylak, S.F.; Strom, S.C. Maintenance of human hepatocyte function in vitro by liver-derived extracellular matrix gels. Tissue Eng. Part A 2010, 16, 1075–1082.
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239.
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009, 5, 1–13.
- Yu, C.; Kornmuller, A.; Brown, C.; Hoare, T.; Flynn, L.E. Decellularized adipose tissue microcarriers as a dynamic culture platform for human adipose-derived stem/stromal cell expansion. Biomaterials 2017, 120, 66–80.
- Wong, Y.S.; Tay, C.Y.; Wen, F.; Venkatraman, S.S.; Tan, L.P. Engineered polymeric biomaterials for tissue engineering. Curr. Tissue Eng. 2012, 1, 41–53.
- Yang, X.; Lu, Z.; Wu, H.; Li, W.; Zheng, L.; Zhao, J. Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater. Sci. Eng. C 2018, 83, 195–201.
- Li, H.; Tan, Y.J.; Leong, K.F.; Li, L. 3D bioprinting of highly thixotropic alginate/methylcellulose hydrogel with strong interface bonding. ACS Appl. Mater. Interfaces 2017, 9, 20086–20097.
- Agarwal, K.; Srinivasan, V.; Lather, V.; Pandita, D.; Vasanthan, K.S. Insights of 3D bioprinting and focusing the paradigm shift towards 4D printing for biomedical applications. J. Mater. Res. 2022, 1–30.
- Wang, Z.; Lee, S.; Cheng, H.; Yoo, J.; Atala, A. 3D bioprinted functional and contractile cardiac tissue constructs. Acta Biomater. 2018, 70, 48–56.
- Hedegaard, C.L.; Collin, E.; Redondo-Gomez, C.; Castrejón-Pita, J.R.; Mata, A.; Ng, K.W.; Castrejón-Pita, A.A. A self-assembly based supramolecular bioink with hierarchical control as a new bioprinting tool. In Proceedings of the Biofabrication for Hierarchical in Vitro Tissue Models, Hernstein, Austria, 5–9 June 2017.
- Loo, Y.; Hauser, C.A. Bioprinting synthetic self-assembling peptide hydrogels for biomedical applications. Biomed. Mater. 2015, 11, 014103.
- Manohar, P. Re: How Can We Find the Number of Papers per Year Dedicated to a Certain Topic? 2022. Available online: https://www.researchgate.net/post/How-can-we-find-the-number-of-papers-per-year-dedicated-to-a-certain-topic/61e42848f7181f153356f673/citation/download (accessed on 8 January 2023).
- Göhl, J.; Markstedt, K.; Mark, A.; Håkansson, K.; Gatenholm, P.; Edelvik, F. Simulations of 3D bioprinting: Predicting bioprintability of nanofibrillar inks. Biofabrication 2018, 10, 034105.
- Ouyang, L.; Yao, R.; Zhao, Y.; Sun, W. Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication 2016, 8, 035020.
- Kim, S.H.; Seo, Y.B.; Yeon, Y.K.; Lee, Y.J.; Park, H.S.; Sultan, T.; Lee, J.M.; Lee, J.S.; Lee, O.J.; Hong, H.; et al. 4D-bioprinted silk hydrogels for tissue engineering. Biomaterials 2020, 260, 120281.
- Jakus, A.E.; Rutz, A.L.; Shah, R.N. Advancing the field of 3D biomaterial printing. Biomed. Mater. 2016, 11, 014102.
- Li, X.; Wang, H.; Li, D.; Long, S.; Zhang, G.; Wu, Z. Dual ionically cross-linked double-network hydrogels with high strength, toughness, swelling resistance, and improved 3D printing processability. ACS Appl. Mater. Interfaces 2018, 10, 31198–31207.
- Gao, T.; Gillispie, G.J.; Copus, J.S.; Pr, A.K.; Seol, Y.-J.; Atala, A.; Yoo, J.-J.; Lee, S.-J. Optimization of gelatin–alginate composite bioink printability using rheological parameters: A systematic approach. Biofabrication 2018, 10, 034106.
- Paxton, N.; Smolan, W.; Böck, T.; Melchels, F.; Groll, J.; Jungst, T. Proposal to assess printability of bioinks for extrusion-based bioprinting and evaluation of rheological properties governing bioprintability. Biofabrication 2017, 9, 044107.
- Hoornaert, A.; Vidal, L.; Besnier, R.; Morlock, J.F.; Louarn, G.; Layrolle, P. Biocompatibility and osseointegration of nanostructured titanium dental implants in minipigs. Clin. Oral Implant. Res. 2020, 31, 526–535.
- Jana, S.; Lerman, A. Bioprinting a cardiac valve. Biotechnol. Adv. 2015, 33, 1503–1521.
- Zhang, L.G.; Leong, K.; Fisher, J.P. (Eds.) 3D Bioprinting and Nanotechnology in Tissue Engineering and Regenerative Medicine; Academic Press: Cambridge, MA, USA, 2022.
- Zhang, X.Y.; Fang, G.; Zhou, J. Additively manufactured scaffolds for bone tissue engineering and the prediction of their mechanical behavior: A review. Materials 2017, 10, 50.
- Ratheesh, G.; Vaquette, C.; Xiao, Y. Patient-specific bone particles bioprinting for bone tissue engineering. Adv. Healthc. Mater. 2020, 9, 2001323.
- Dubbin, K.; Hori, Y.; Lewis, K.K.; Heilshorn, S.C. Dual-stage crosslinking of a gel-phase bioink improves cell viability and homogeneity for 3D bioprinting. Adv. Healthc. Mater. 2016, 5, 2488–2492.
- Gillispie, G.; Han, A.; Uzun-Per, M.; Fisher, J.; Mikos, A.G.; Niazi, M.K.K.; Yoo, J.J.; Lee, S.J.; Atala, A. The influence of printing parameters and cell density on bioink printing outcomes. Tissue Eng. Part A 2020, 26, 1349–1358.
- Chen, Z.; Zhao, D.; Liu, B.; Nian, G.; Li, X.; Yin, J.; Qu, S.; Yang, W. 3D printing of multifunctional hydrogels. Adv. Funct. Mater. 2019, 29, 1900971.
- OOuyang, L.; Armstrong, J.P.K.; Lin, Y.; Wojciechowski, J.P.; Lee-Reeves, C.; Hachim, D.; Zhou, K.; Burdick, J.A.; Stevens, M.M. Expanding and optimizing 3D bioprinting capabilities using complementary network bioinks. Sci. Adv. 2020, 6, eabc5529.
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855.
- Varchanis, S.; Haward, S.J.; Hopkins, C.C.; Syrakos, A.; Shen, A.Q.; Dimakopoulos, Y.; Tsamopoulos, J. Transition between solid and liquid state of yield-stress fluids under purely extensional deformations. Proc. Natl. Acad. Sci. USA 2020, 117, 12611–12617.
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434.
- Jungst, T.; Smolan, W.; Schacht, K.; Scheibel, T.; Groll, J. Strategies and molecular design criteria for 3D printable hydrogels. Chem. Rev. 2016, 116, 1496–1539.
- Sarker, M.; Chen, X.B. Modeling the flow behavior and flow rate of medium viscosity alginate for scaffold fabrication with a three-dimensional bioplotter. J. Manuf. Sci. Eng. 2017, 139, 081002.
- Sweeney, M.; Campbell, L.L.; Hanson, J.; Pantoya, M.L.; Christopher, G.F. Characterizing the feasibility of processing wet granular materials to improve rheology for 3D printing. J. Mater. Sci. 2017, 52, 13040–13053.
- Feng, Z.; Wang, D.; Zheng, Y.; Zhao, L.; Xu, T.; Guo, Z.; Hussain, M.I.; Zeng, J.; Lou, L.; Sun, Y.; et al. A novel waterborne polyurethane with biodegradability and high flexibility for 3D printing. Biofabrication 2020, 12, 035015.
- Haider, A.; Haider, S.; Kummara, M.R.; Kamal, T.; Alghyamah AA, A.; Iftikhar, F.J.; Bano, B.; Khana, N.; Afridi, M.A.; Han, S.S.; et al. Advances in the scaffolds fabrication techniques using biocompatible polymers and their biomedical application: A technical and statistical review. J. Saudi Chem. Soc. 2020, 24, 186–215.
More
Information
Subjects:
Chemistry, Physical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
960
Revisions:
3 times
(View History)
Update Date:
29 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




