
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Miguel | -- | 3954 | 2023-01-19 21:00:46 | | | |
| 2 | Catherine Yang | -1949 word(s) | 2005 | 2023-01-28 03:36:50 | | |
Video Upload Options
Euphorbia officinarum L. is a Moroccan endemic plant known as “Tikiout” and “Daghmus” that can also be found in Mauritania, Western Sahara, and Algeria. This species has been used in folk medicine as anti-diabetic; in the treatment of skin diseases when associated with Opuntia ficus-barbarica, Zea mays and Ziziphus lotus, and honey for eliminating helminths, in the treatment of pyelonephritis and cystitis. Triterpenes, phytosterols and ingol diterpenes have been isolated and identified in the latex of Moroccan E. officinarum, nevertheless the biggest interest has been to obtain derivatives by hemisynthesis from natural triterpenes with insecticidal and antimicrobial activity. In Morocco, the E. officinarum honey is considered the most precious; nevertheless, many times it is mixed with other Euphorbiaceae honeys. To increase the commercial value of a monofloral E. officinarum honey, it would be important to find one or more specific markers for this type of honey to be sure of its authenticity.
1. Introduction
The succulent plants of the genus Euphorbia are the largest in the spurge family, which can be found in Africa, Canary Islands, in Madagascar, India, and the Americas and even Australia [1]. Euphorbia officinarum L., a Moroccan endemic plant, can be found in Mauritania, Western Sahara, and Algeria [1]. This species has milky white latex, which chemical characterization has been done as well as their biological attributes [2]. The term Euphorbia was named in honour of “Euphorbus”, the physician of King Juba II of Mauritania, who paid attention to the medicinal properties of E. officinarum, for the first time [3]. There is one infra-specific taxon of the species Euphorbia officinarum L (E. officinarum subsp. echinus (Hook.f. & Coss.) Vindt) [4].
From olden times that the milky sap of E. officinarum has been used in earache and as emetic in Buxar district, India [5], although this utilization as emetic has already been considered outdated [6]. In different regions of Morocco, E. officinarum subsp. echinus has been used in folk medicine particularly as anti-diabetic [7][8][9][10][11]. E. officinarum L. is also used as anti-diabetic, according to different authors [10][11][12][13]. Other applications of this species as well as its subsp echinus include treatment of pyelonephritis and cystitis, when in association with Opuntia ficus-barbarica, Zea mays and Ziziphus lotus) and honey [14]; treatment of wounds, skin infections and abscesses [15][16][17][18] and by the Sahrawi refugees in Algerian refugee camps [19]; elimination of helminths [18]; treatment of cancer, although not specifying which type [16][20][21]; for respiratory and circulatory systems [22], and as gum-resin for headache, paralysis and apoplexy [23], although an ethnobotanical study made by Blanco and Carrière [24] showed that this species was weakly cited by the informants. E. officinarum subsp. echinus aerial parts may also be chopped and cooked as a vegetable salad [25][26], despite its relative high toxicity [2].
2. Secondary Metabolites Isolated from Euphorbia officinarum L. of Morocco
In the last century, the authors [27][28][29] isolated from the extracts of the latex of E. officinarum, collected in the North Atlantic coast of Agadir (Morocco), the triterpenic compounds lupeol (1) and lupeol acetate (2)), and seven steroidal compounds (lanostenol (3), lanosterol (4), 24-methylene lanostenol (5), 4α,14α-dimethyl-24-methylen-5α-cholest-8-en-3β-ol or obtusifoliol (6), 24(R)-4α,14α,24-trimethyl-5α-cholesta-8,25-dien-3-β-ol (7), 4α,14α-dimethyl-5α-cholest-8,24-dien- 3β-ol (8), and 4α,14α-dimethyl-5 α-cholest-8-en-3-β-ol (9), 3,7-dihydroxy-4,14-dimethyl-5-cholest-8-en-11-one (10) and 3,7-dihydroxy-4,14-dimethyl-5-ergost-8-en-11-one (11) (Figure 1). In the 90s and from the same region, some authors [28][29] isolated more 2 new steroidal compounds:


Figure 1. Triterpenic (1 and 2) and steroid compounds isolated and identified in the Moroccan latex of Euphorbia officinarum.
More recently, Daoubi et al. [2] isolated and identified the highly functionalized ingol diterpenes ingol 7,8,12-triacetate 3-phenylacetate) (1), ingol 7,8,12-triacetate 3-(4-methoxyphenyl) acetate (2) and 8-methoxyingol 7,12-diacetate 3-phenylacetate (3) (Figure 2), along with the novel spirotriterpene 3S,4S,5R,7S,9R,14R-3,7-dihydroxy-4,14-dimethyl-7[8→9]-abeo-cholestan-8-one (4) (Figure 2), in samples also collected in the North Atlantic coast of Agadir (Morocco).
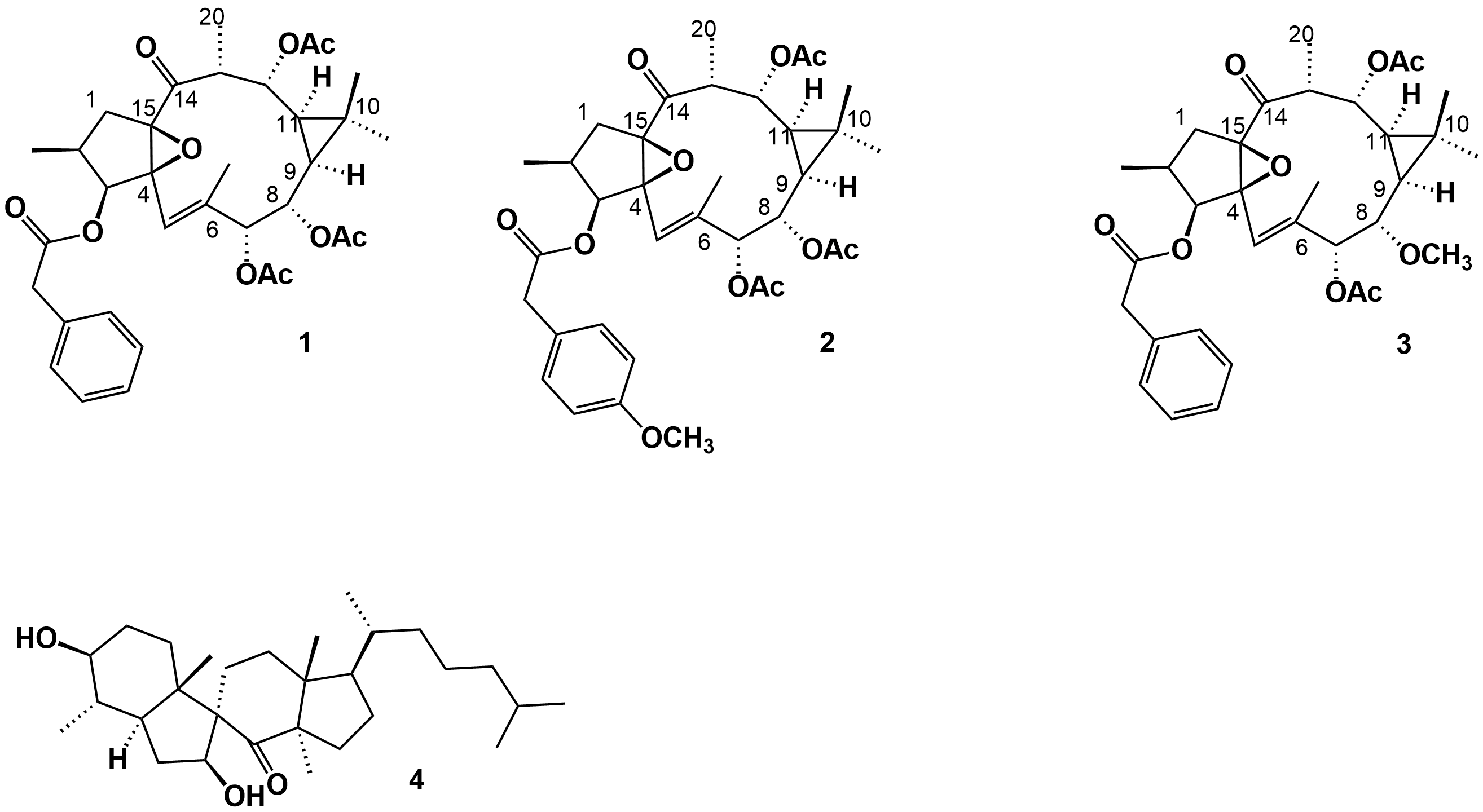
Figure 2. Phenylacetylingol derivatives (1-3) and spirotriterpenoid (4) isolated from the methanolic extract of E. officinarum latex.
3. Hemisynthesis of Triterpene Derivatives Isolated from E. officinarum Latex and Their Biological Properties
The biological properties (cytotoxic, antimicrobial, human immunodeficiency virus type 1 reactivation, among others) found in latex or some of their isolated compounds of Euphorbia species [1][2][30][31][32][33] have led chemical modifications to obtain derivative compounds with enhanced biological properties. For example, Mazoir et al. and other authors (see below) published several works in order to obtain triterpenic or steroidal derivatives that show good biological activities (Table 1) (Figure 3).
Table 1. Triterpene and steroidal derivatives obtained by hemisynthesis and their biological properties.
|
Start compounds |
Reagents |
Final product |
Biological properties |
References |
|
24-Methylene lanostenol |
Chromic anhydride, acetone |
(3S)-Acetyl-24-methyl-elemo-lanosta -8,24-diene-7,11-dione (1) |
ND |
[34] |
|
3(S)-Tosyl-24-methylene lanostenol |
(3S)-Tosyl-24-methyl-elemo-lanosta-8,24-diene-7,11-dione (2) |
ND |
[35] |
|
|
Eupho-lanosta-8,24-dien-3-ol |
Ruthenium(III) chloride trihydrate, esterification, acetylation reactions |
(3S,5S,10S,13S,14S,17S)-Methyl-3-acetyl-25,26,27-trisnorlanost-8-en-24-oate (3) |
ND |
[29] |
|
(6, Fig.1) |
Chromic anhydride, acetone |
4,14-Dimethyl-5-ergost-8,24-dien-3-one (4) |
ND |
[36] |
|
(9, Fig.1) |
Chromic anhydride, acetone |
4,14-Dimethyl-5-cholest-8-en-3-one (5) |
ND |
[36] |
|
(4) |
Ethyl formate, benzene, sodium methoxide |
2-Formyl-4α,14α-dimethyl-5α-ergost-2,8,24-trien-3-ol (6) |
ND |
[36] |
|
(5) |
Ethyl formate, benzene, sodium methoxide |
2-Formyl-4α,14α-dimethyl-5α-cholesta-2,8-dien-3-ol (7) |
ND |
[36] |
|
(6) |
Acetic acid, hydroxylamine hydrochloride |
[1,2]Isoxazolo [4,3-]-4α,14α-dimethyl-5α-ergosta-8,24-diene (8) |
ND |
[36] |
|
(6) |
Pyridine, hydroxylamine hydrochloride |
[1,2]Isoxazolo [4,5-]-4α,14α-dimethyl-5α-ergosta-8,24-diene (9) |
ND |
[36] |
|
(7) |
Acetic acid, hydroxylamine hydrochloride |
[1,2]isoxazolo [4,3-b]-4α,14α-dimethyl-5α-cholesta-8-ene (10) |
ND |
[36] |
|
(7) |
Pyridine, hydroxylamine hydrochloride |
[1,2]isoxazolo [4,5-b]-4α,14α-dimethyl-5α-cholest-8-ene (11) |
ND |
[36] |
|
3-Tosyl-5-ergost-8,24-diene |
Ruthenium trichloride, allylic oxydation with chromic anhydride |
(3S,4S,5S,10S,13R,14R,17R)-4,14-Dimethyl-3-tosyl-5--ergost-8-ene-7,11,24-trione (12) |
ND |
[37] |
|
4,14-Dimethyl-5-cholest-8-en-3-ol (9, Fig.1) |
Chromic anhydride, meta-chloroperbenzoic acid (mCPBA) |
(4S,5S,10S,13R,14R,17R)-8,9-Epoxy-4,14-dimethyl-5-cholestan-3-one (13) |
ND |
[38] |
|
8,9-Epoxy-4,14-Dimethyl-5-cholestan-3-ol (14) |
Chromic anhydride |
(13) |
ND |
[39] |
|
(9, Fig.1) (5) |
Chromic anhydride Thiosemicarbazide in ethanol, concentrated sulphuric acid |
(5) thiosemicarbazone derivative (15) |
ND ND |
[40] |
|
(9, Fig.1) |
Acylation Oxidation |
3-Acetoxy-4,14-dimethyl-5-cholest-8-en-7-one (16) 3-Acetoxy-4,14-dimethyl-5-cholest-8-ene-7,11-dione (17) |
ND |
[39] |
|
(9, Fig.1) |
Tosyl chloride |
3-Tosyloxy-4,14-dimethyl-5-cholest-8-en-7-one (18) 3-tosyloxy-4,14-dimethyl-5-cholest-8-ene-7,11-dione (19) |
ND |
[39] |
|
(9, Fig.1) |
Chromic anhydride in acetone at 273 K, phenylhydrazine, acetic acid |
1-(1,5-Dimethylhexyl)-3a,5b,12a,14a-tetramethyl-2,3,3a,4,5,5a,5b,11,12,13,14, 14a-dodecahydro-1H,12aH-cyclopenta[1,2]-phenanthro[7,8-b]indole (20) |
ND |
[41] |
|
4-14-Dimethyl-5-ergost-8-en-3-ol |
Tosylation |
3-Tosyloxy-4,14-dimethyl-5-ergost-8-en-24-one (21) |
ND |
[39] |
|
(21) |
Oxidation |
3-Tosyloxy-4,14-dimethyl-5-ergost-8-ene-7,24-dione (22) 3-Tosyloxy-4,14-dimethyl-5-ergost-8-ene-7,11,24-trione (23) |
ND |
[38] |
|
Eupho-lanosta-8,24-dien-3-ol |
Sodium periodate-ruthenium (III), chloride trihydrate (NaIO4-(RuCl3,3H2O)), esterification, acetylation |
(3S,5S,10S,13S,14S,17S)3-acetyl-25,26,27-Trisnorlanost-8-en-24-oate (24) |
ND |
[31] |
|
(6 and 9, Fig.1) |
Modifications on positions 3,7, 11 and 24 |
(21), (4), (17), (19), -Tosyloxy-4,14-dimethyl-5-cholest-8-ene (25), 4,14-dimethyl-5-ergost-8-en-3,24-dione (26), 4,14-dimethyl-5-cholest-8-en-3,11-dione-7-thiosemicarbazone (27), 4,14-dimethyl-5-cholest-8-ene-3,11-dione-7-thiadiazoline (28), 4,14-dimethyl-5-cholesta-7,9-diene-3-thiosemicarbazone (29), 4,14-dimethyl-5-cholest-8-ene-7,11-dione-3-thiadiazoline (30) |
- (9, Fig.1), (19), (26), (27) active in relation to Myzus persicae; (9, Fig.1), (17), (4) active in relation to Rhopalosiphum padi, -Insect cells Sf9 were more sensitive to terpene derivatives than mammalian CHO cells. - (28), (17), (26), (4), and (27) showed better activities on Leishmania infantum promastigotes with ED50 (the effective dose to give 50% cell viability) < 10 g/mL, regarding Trypanosoma cruzi only (26), (27) and (28) had ED50 < 10 g/mL |
|
|
(4), (5), ( (13), (19), 3-Acetoxy-30-nor-20-oxolupane (31), 4α,14α-dimethy-l-5α-cholest-8-ene-3,7,11-trione (32) |
Thiosemicarbazide, oxidation by chromic anhydride Thiosemicarbazone, pyridine, acetic anhydride |
(29), 4,14-Dimethyl-5-ergost-8,24-dien-3-one thiosemicarbazone (33), 4,14-dimethyl-5-cholest-8-en-3-one thiosemicarbazone (34), 3β-acetoxy-28-norlup-20-one thiosemicarbazone (35), 3-tosyloxy-4,14-dimethyl-5-cholest-8-ene-7,11-dione-7-thiosemicarbazone (36), 4α,14α-dimethyl-5α-cholest-8-ene-3,7,11-trione-7-thiosemicarbazone (37) 4,14-dimethyl-5-ergost-8,24-dien-3-one thiadiazoline (38), 4,14-dimethyl-5-cholest-8-en-3-one thiadiazoline (39), 4,14-dimethyl-5-cholest-7,9-diene-3-one thiadiazoline (40), 3-acetoxy-28-Norlup-20-one thiadiazoline (41), 3-tosyloxy-4,14-dimethyl-5-cholest-8-ene-7,11-dione-7-thiadiazoline (42), 4,14-dimethyl-5-cholest-8-ene-3,7,11-trione-7-thiadiazoline (43) |
ND ND |
|
|
(9, Fig.1) |
Acetone, chromic anhydride, (mCPBA) Tosylation Lithium aluminium hydride (LiAlH4), tetrahydrofuran (THF) Sodium methoxide (MeONa)/methanol These reagents were used depending on the final product desired |
4α,14α-Dimethyl-5α-cholest-7,9-dien-3β-ol (44), 3-chloro-4α,14α-dimethyl-5α-cholest-8-en-7-one (45), 2-carbomethoxy-4α,14α-dimethyl-5α-cholest-2,8-dien-3-ol (46), 4α,14α-dimethyl-5α-cholest-8-en-3-one (47), 4α,14α-dimethyl-7-oxo-5α-cholest-8-en-3,4-lactone (48), 4α,14α-dimethyl-7,11-dioxo-5α-cholest-8-en-3,4-lactone (49), 8α,9α-epoxy-4α,14α-dimethyl-5α-cholest-3,4-lactone (50), 4α,14α-dimethyl-5α-cholest-7,9-dien-3,4-lactone (51), 4α,14α-dimethyl-3,4-seco-5α-cholest-7,9-dien-3,4-diol (52), 3-carbomethoxy-4-hydroxy-4α,14α-dimethyl-3,4-seco-5α-cholest-7,9-diene (53) |
- (53) was the strongest antiparasitic with activity levels similar to or better than the reference drugs against L. infantum and T. cruzi, respectively - (53) and (14) had the strongest cytotoxic effects on insect-derived Sf9 cells and not cytotoxic to mammalian CHO cells |
[46] |
|
(6, Fig.1) |
Acetone, chromic anhydride, meta-Chloroperoxybenzoic acid (mCPBA |
8α,9α,24,28-Diepoxy-4α,14α-dimethyl-5α-ergosta-3,4-lactone (54), 8α,9α,24,28-diepoxy-4α,14α-dimethyl-5α-ergost-3β-ol (55) |
[46] |
|
|
4α,14-dimethyl-5α,8α-8,9-epoxycholestan-3β-yl acetate (56), (32), 4α,14-dimethyl-5α-cholesta-7,9-dien-3-one (57), 4α,14-dimethyl-5α-cholest-8-en-3β-yl acetate (58) |
Hydrogen peroxide (H2O2), iodosobenzene (PhIO) catalyzed by porphyrin complexes (cytochrome P-450 models) |
25-Hydroxy-4α,14-dimethyl-5α-cholest-7,9-dien-3β-yl acetate (59), 25-hydroxy-4α,14-dimethyl-5α-cholest-8-ene3,7,11-trione (60), 4α,14-dimethyl-5α,7β-7,8-epoxychol-est-9-en-3-one (61), 8-hydroxy-4α,14-dimethyl-5α-cholest-9-ene-3,7-dione (62), 12α-hydroxy-4α,14-dimethyl-5α,7β-7,8-epoxycholest-9-en-3-one (63), 4α,14-dimethyl-5α,8α-8,9-epoxycholestan-3β-yl acetate (64), |
- None of the compounds tested had significant antifeedant effects for the insects M. persicae, R. padi and S. littoralis. - All were more effective post-ingestive toxicants on Spodoptera littoralis larvae than the natural (9, Fig.1), (64) was the most active |
[47] |
|
(2 and 9, Fig.1) |
Sodium periodate, ruthenium trichloride, tosyl chloride, pyridine / 70 ºC |
(31), 3-chloro-4α,14α-dimethyl-5α-cholest-8-ene (64) |
- They act as fungistatic reducing in vitro the conidia formation and germination of Verticillium dahlia, and Fusarium oxysporum fsp. melonis, and Penicillium expansum, - (64) more effective in inhibiting the growth of Penicillium syringae pv. tabaci and P. syringae pv. syringae, even being similar to the positive control (chloramphenicol). - (64) showed antibacterial activity on Erwinia amylovora but at a moderate level and significantly lower than the positive control |
[48] |
|
(9, Fig.1) |
m-CPBA + HCl TsCl + m-CPBA + HCl |
(44), 3-Tosyloxy-4,14-dimethyl-5-cholest-7,9-diene (65) |
- (65) able to protect tomato plants against Verticillium dahliae in a greenhouse, significantly reducing disease severity at 10 g/mL. (65) able to elicit H2O2 accumulation before and after fungal inoculation, and enhance peroxidase and polyphenol oxidase activities |
[49] |
|
(1 and 9, Fig.1) |
Sodium periodate, ruthenium trichloride, tosyl chloride, pyridine/70 ºC |
(31), (64) |
- Seeds of Nicotiana benthamiana treated with (31) and (64) and in the presence of P. syringae pv. tabaci did not harm germination and significantly reduced the diameter of the lesions in inoculated leaves, - (31) and (64) significantly reduced bacterial growth in plants, - Mock-inoculated leaves of plants that germinated in the presence of (31) and (64) showed enhanced ascorbate peroxidase and catalase activities. - Inoculated plants with P. syringae pv. tabaci previously treated with (31) and (64) made increase guaiacol peroxidase and polyphenol oxidase activities. |
[50] |
|
(1 and 9, Fig.1) |
Sodium periodate, ruthenium trichloride, tosyl chloride, pyridine/70 ºC |
(31), (64) |
Direct application of (31) and (64) on tomato seedlings significantly improved growth rate, leaf area, an increased content of chlorophylls, carotenoids, proline, and the activity of nitrate reductase, - (64) reduced leaf alteration indexes and the browning index of the vessels of tomato seedlings, induced by V. dahliae and Agrobacterium tumefaciens - pre-treatment with (31) and (64) reduced the diameter of lesions caused by the oncogenic strain C58 of A. tumefaciens , - (31) and (64) induced H2O2 accumulation and increased the activity of catalase, ascorbate peroxidase, and guaiacol peroxidase |
[51] |
ND: Not determined



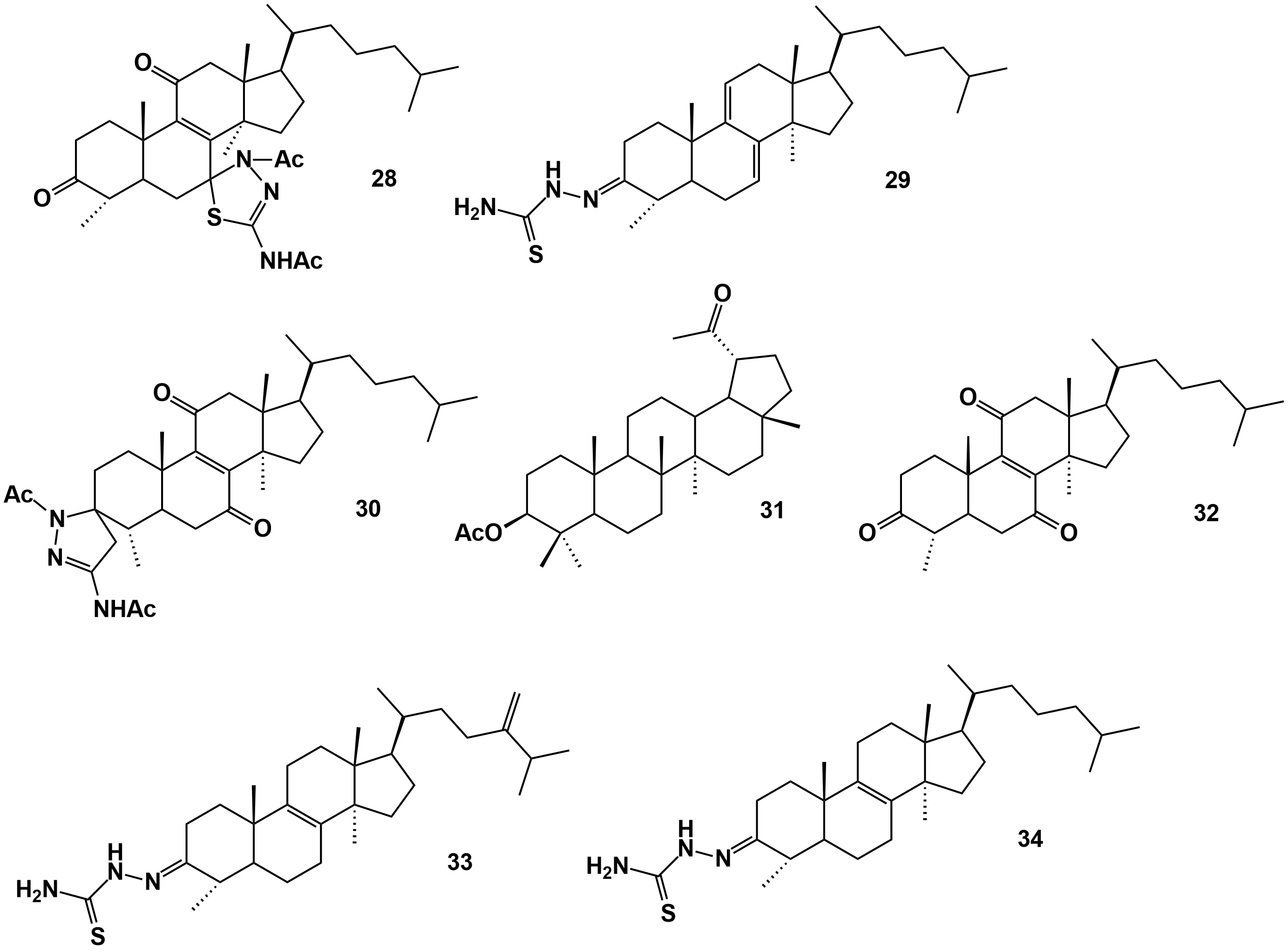
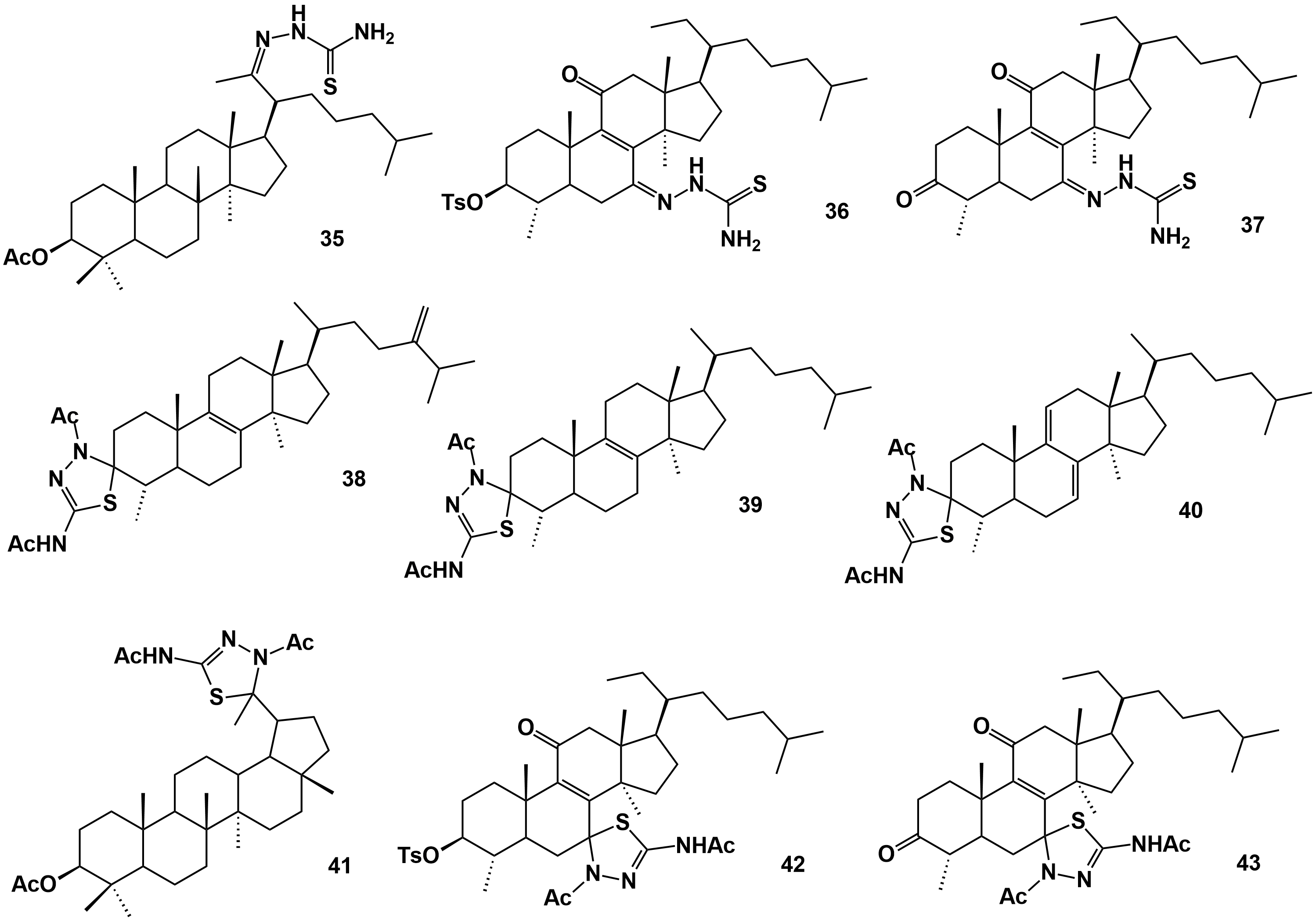


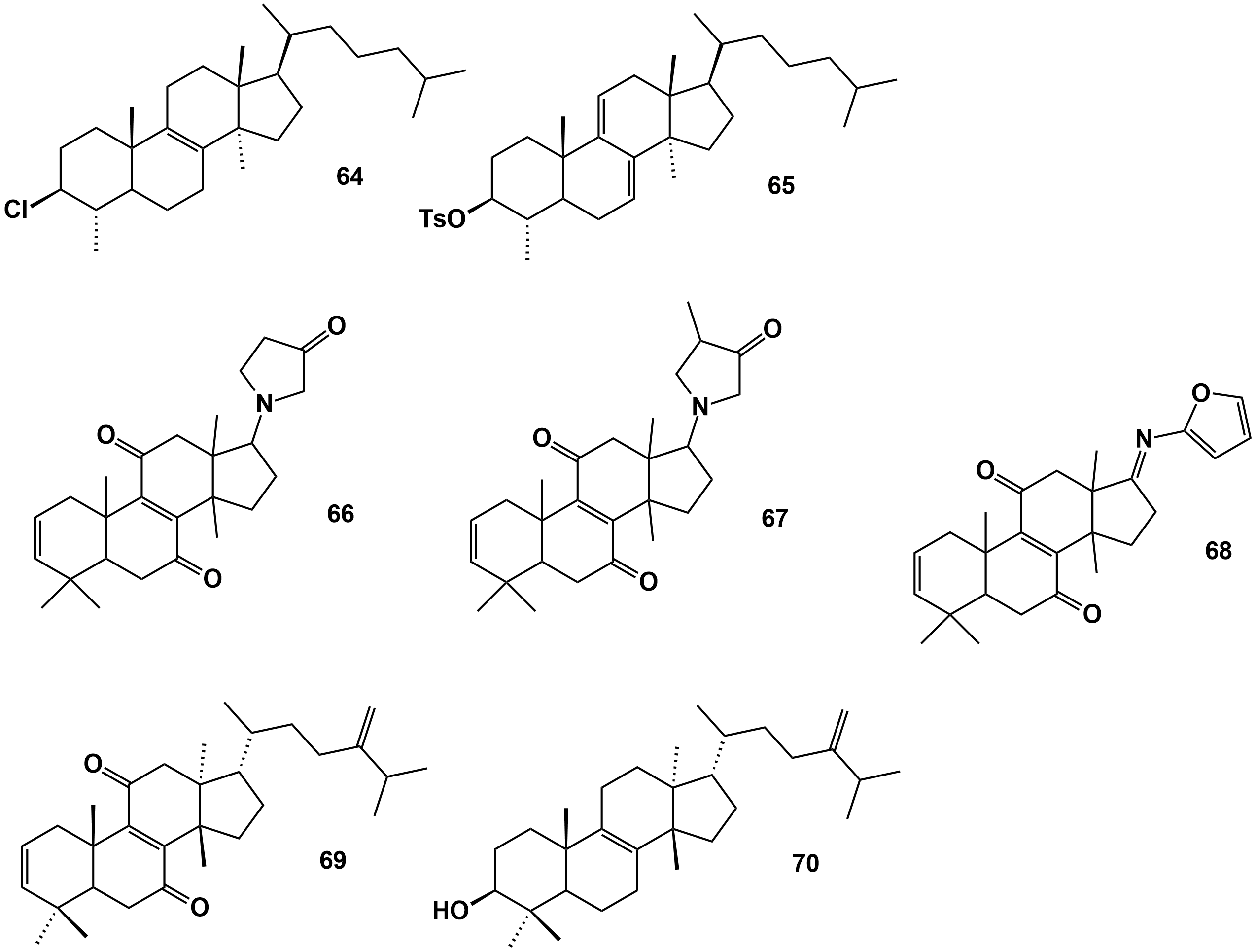
Figure 4. Triterpene derivatives obtained from natural triterpenes isolated from E. officinarum latex. Compounds 66, 67 and 68 according to the structures presented in Figure 9 of Daoui et al. [52].
4. Extracts and Bee Products from Euphorbia Origin
So far, the antimicrobial and insecticidal activities of triterpene derivatives from E. offcinarum latex have been greatly evaluated. Nevertheless, some works start to approach other attributes of E. officinarum extracts [2][33][53][54] and bee products [55][56]. In Morocco the Euphorbia honey is considered valuable but it has been scarcely studied, although the physico-chemical and palynological characteristics have already been reported [55][57][58][59][60]. Generally, in the monofloral honey of Euphorbia origin, there is no specification of which species will be predominant, although three honey types of this genus can be produced (E. officinarum subsp. echinus, E. resinifera, and E. regis-jubae) [52][61]. Abderrahim et al. [62] demonstrated the antioxidant, synergistic antimicrobial and burn wound healing activities of monofloral honey of Euphorbia origin (the specification of the species is not provided) mixed with Allium sativum. Another beeproduct is propolis. The detailed chemical composition and antimicrobial activity of this natural product were evaluated by Chimshirova et al.[63]. Fifteen compounds were isolated and identified, some of them being already reported as constituents of plants in the genus Euphorbia, such as the macrocyclic diterpenes and triterpenoids, and other groups of known compounds (e.g., coumarins, phenolic acids), and new ones (e.g., 29-norlanost-3-hydroxy-8-ene-7,11-dione). The ingol diterpenes found in propolis were those isomers characteristic of the E. resinifera latex. Such results may indicate the utilization of latex of E. resinifera by bees for making propolis, but also from E. officinarum, since obtusifoliol is generally present in the E. officinarum latex [56].
References
- Chamkhi, I.; Hnini, M.; Aurag, J. Conventional medicinal uses, phytoconstituents, and biological activities of Euphorbia officinarum L.: A systematic review. Adv. Pharmacol. Pharm. Sci. 2022, 2022, 1–9. https://doi.org/10.1155/2022/9971085.
- Daoubi, M.; Marquez, N.; Mazoir, N.; Benharref, A.; Hernández-Galán, R.; Muñoz, E.; Collado, I.G. Isolation of new phenylacetylingol derivatives that reactivate HIV-1 latency and a novel spirotriterpenoid from Euphorbia officinarum latex. Bioorg. Med. Chem. 2007, 15, 4577–4584.
- Binoj Kumar, M.S.; Balakrishan, N.P. Ethnobotanical studies of the genus Euphorbia L. (Euphorbiaceae). J. Econ. Taxon. Bot. Addit. Ser. 1996, 12, 46–49.
- The Plant List. Available online: http://www.theplantlist.org/tpl1.1/record/kew-81211 (accessed on 12 September 2022).
- Singh, A.; Singh, M.K.; Singh, R. Traditional medicinal flora of the district Buxar (Bihar, India). J. Pharmacogn. Phytochem. 2013, 2, 41–49.
- Mackonochie, M.; Heinrich, M. Materia medica chests: Investigating the 19th century use of botanicals by different medical professions. J. Herb. Med. 2019, 16, 100255. https://doi.org/10.1016/j.hermed.2019.100255.
- Ouhaddou, H.; Alaoui, A.; Laaribya, S.; Ayan, S. Ethnobotanical survey of medicinal plants used for treating diabetes in Agadir Ida Outaname region, Southwestern Morocco. Arab. J. Med. Aromat. Plants 2020, 6, 72–86.
- Abouri, M.; El Mousadik, A.; Msanda, F.M.; Boubaker, H.; Saadi, B.; Cherifi, K. An ethnobotanical survey of medicinal plants used in the Tata Province, Morocco. J. Med. Plants Res. 2012, 1, 99–123.
- Barkaoui, M.; Katiri, A.; Boubaker, H.; Msanda, F. Ethnobotanical survey of medicinal plants used in the traditional treatment of diabetes in Chtouka Ait Baha and Tiznit (Western Anti-Atlas), Morocco. J. Ethnopharmacol. 2017, 198, 338–350, https://doi.org/10.1016/j.jep.2017.01.023.
- Boufous, H.; Marhoume, F.; Chait, A.; Bagri, A. Ethnopharmacological survey of medicinal plants with hallucinogenic effect and used against pain, inflammatory diseases, diabetes and urinary lithiasis in Zagora “Morocco”. J. Intercult. Ethnopharmacol. 2017, 6, 342–350.
- Idm’hand, E.; Msanda, F.; Cherifi, K. Ethnopharmacological review of medicinal plants used to manage diabetes in Morocco. Clin. Phytosci. 2020, 6, 18. https://doi.org/10.1186/s40816-020-00166-z.
- Bouyahya, A.; El Omari, N.; Elmenyiy, N.; Guaouguaou, F.-E.; Balahbib, A.; Belmehdi, O.; Salhi, N.; Imtara, H.; Mrabti, H.N.; El-Shzly, M.; et al. Moroccan antidiabetic medicinal plants: Ethnobotanical studies, phytochemical bioactive compounds, preclinical investigations, toxicological validations and clinical evidences; challenges, guidance and perspectives for future management of diabetes worlwide. Trends Food Sci. Technol. 2021, 115, 147–254.
- Chetoui, A.; Kaoutar, K.; Boutahar, K.; El Kardoudi, A.; BenChaoucha-Chekir, R.; Chigr, F.; Najimi, M. Herbal medicine use among Moroccan type 2 diabetes patients in the Beni Mellal-Khenifra region. J. Herb. Med. 2021, 29, 100480. https://doi.org/10.1016/j.hermed.2021.100480.
- Ghourri, M.; Zidane, L.; Douira, A. La phytothérapie et les inféctions urinaires (la pyélonéphrite et la cystite) au Sahara marocain (Tan-Tan). J. Anim. Plant Sci. 2014, 20, 3171–3193.
- Noureddine, B.; Mostafa, E.; Mandal, S.C. Ethnobotanical, pharmacological, phytochemical, and clinical investigations on Moroccan medicinal plants traditionally used for the management of renal dysfunctions. J. Ethnopharmacol. 2022, 292, https://doi.org/10.1016/j.jep.2022.115178.
- Idm’Hand, E.; Msanda, F.; Cherifi, K. Ethnobotanical study and biodiversity of medicinal plants used in the Tarfaya Province, Morocco. Acta Ecol. Sin. 2020, 40, 134–144. https://doi.org/10.1016/j.chnaes.2020.01.002.
- Ajjoun, M.; Kharchoufa, L.; Merrouni, I.A.; Elachouri, M. Moroccan medicinal plants traditionally used for the treatment of skin diseases: From ethnobotany to clinical trials. J. Ethnopharmacol. 2022, 297. https://doi.org/10.1016/j.jep.2022.115532.
- Mekkaoui, M.; Assaggaf, H.; Qasem, A.; El-Shemi, A.; Abdallah, E.M.; Bouidida, E.H.; Mrabti, H.N.; Cherrah, Y.; Alaoui, K. Ethnopharmacological Survey and Comparative Study of the Healing Activity of Moroccan Thyme Honey and Its Mixture with Selected Essential Oils on Two Types of Wounds on Albino Rabbits. Foods 2021, 11, 28. https://doi.org/10.3390/foods11010028.
- Volpato, G.; Kourková, P.; Zelený, V. Healing war wounds and perfuming exile: The use of vegetal, animal, and mineral products for perfumes, cosmetics, and skin healing among Sahrawi refugees of Western Sahara. J. Ethnobiol. Ethnomed. 2012, 8, 49. Available online: http://www.ethnobiomed.com/content/8/1/49 (accessed on 1 July 2022).
- El Hafian, M.; Benlamdini, N.; Elyacoubi, H.; Zidane, L.; Rochdi, A. Étude floristique et ethobotanique des plantes médicinales utilisées au niveau de la préfecture d’Agadir-Ida-Outanane (Maroc). J. Appl. Biosci. 2014, 81, 7198–7213.
- Merrouni, I.A.; Elachouri, M. Anticancer medicinal plants used by Moroccan people: Ethnobotanical, preclinical, phytochemical and clinical evidence. J. Ethnopharmacol. 2021, 266, 113435. https://doi.org/10.1016/j.jep.2020.113435.
- Blanco, J.; Carrière, S.M. Sharing local ecological knowledge as a human adaptation strategy to arid environments: Evidence from na ethnobotany survey in Morocco. J. Arid Environ. 2016, 127, 30–43.
- Ouhaddou, H.; Boubaker, H.; Msanda, F.; El Mousadik, A. An ethnobotanical stydy of medicinal plants of the Agadir Ida Ou Tanane province (Southwest Morocco). J. Appl. Biosci. 2014, 84, 7707–7722.
- Abolhasanzadeh, Z.; Ashrafi, H.; Badr, P.; Azadi, A. Traditional neurotherapeutics approach intended for direct nose to brain delivery. J. Ethnopharmacol. 2017, 209, 116–123, https://doi.org/10.1016/j.jep.2017.07.026.
- Nassif, F.; Tanji, A. Gathered food plants in Morocco: The long forgotten species in ethnobotanical research. Life Sci. Leafl. 2013, 3, 17–54.
- Geraci, A.; Amato, F.; di Noto, G.; Bazan, G.; Schcchi, R. The wild taxa utilized as vegetables in Sicily (Italy): A traditional componente of the Mediterranean diet. J. Ethnobiol. Ethnomed. 2018, 14, 14. https://doi.org/10.1186/s13002-018-0215-x.
- Benharref, A.; Lavergne, J.P. Triterpenes issus des latex des euphorbes cactóides marocaines E. resinífera, E.echinus et E. officinarum: Isolement, étude comparative par RMN 13C des quatres classes tetracycliques eupho-lanostane, elemolanostane, lanostane et nor-31-lanostane. Bull. Soc. Chim. Fr. 1985, 5, 965–972.
- Daoubi, M.; Benharref, A.; Hernandez-Galán, R.; Macías-Sánchez, A.J.; Collado, I.G. Two novel steroids from Euphorbia officinarum latex. Nat. Prod. Res. 2004, 18, 177–181.
- Mazoir, N.; Giorgi, M.; Auhmani, A. (3 S,5 S,10 S,13 S,14 S,17 S )-Methyl 3?-acetyl-25,26,27-trisnorlanost-8-en-24-oate. Acta Crystallogr. Sect. E Struct. Rep. Online 2005, 61. https://doi.org/10.1107/s1600536805020726.
- Avila, L.; Perez, M.; Sánchez-Duffhues, G.; Hernández-Galán, R.; Muñoz, E.; Cabezas, F.; Quiñones, W.; Torres, F.; Echeverri, F. Effects of diterpenes from latex of Euphorbia lactea and Euphorbia laurifolia on human immunodeficiency vírus type 1 reactivation. Phytochemistry 2010, 71, 243–248.
- Singh, I.P.; Bodiwala, H.S. Recent advances in anti-HIV natural products. Nat. Prod. Rep. 2010, 27, 1781–1800.
- Sanchez-Duffhues, G.; Vo, M.Q.; Perez, M.; Calzado, M.A.; Moreno, S.; Appendino, G.B.; Munoz, E. Activation of Latent HIV-1 Expression by Protein Kinase C Agonists. A Novel Therapeutic Approach to Eradicate HIV-1 Reservoirs. Curr. Drug Targets 2011, 12, 348–356.
- El-Hawary, S.S.; Mohammed, R.; Tawfike, A.F.; Lithy, N.M.; Abouzid, S.F.; Amin, M.N.; Abdelmohsen, U.R.; Amin, E. Cytotoxic Activity and Metabolic Profiling of Fifteen Euphorbia Species. Metabolites 2020, 11, 15, https://doi.org/10.3390/metabo11010015.
- Mazoir, N.; Auhmani, A.; Itto, M.Y.A.; Benharref, A. (3S)-acetyl-24-methyl-elemo-lanosta-8,24-diene-7,11-dione. Molbank 2004, 2004, M365. https://doi.org/10.3390/M365.
- Mazoir, N.; Auhmani, A.; Dakir, M.; Itto, M.Y.A.; Benharref, A. (3S)-Tosyl-24-methyl-elemo-lanosta-8,24(31)-diene-7,11-dione. Molbank 2004, 2004, M366. https://doi.org/10.3390/m366.
- Mazoir, N.; Liazid, A.; Auhmani, A.; Daoubi, M.; Dakir, M.; Benharref, A.; Kenz, A.; Pierrot, M. 1H and 13C-NMR structural study of products obtained by hemisynthesis on the triterpenes isolated from latex of Euphorbia officinarum. Phys. Chem. News 2005, 21, 123–128.
- Mazoir, N.; Berraho, M.; Fraisse, B.; Benharref, A.; Bouhmaida, N. (3S,4S,5S,10S,13R,14R,17R)-4,14-Dimethyl-3-tosyl-5-ergost-8-ene-7,11,24-trione at 100 K. Acta Crystallogr. Sect. E Struct. Rep. Online 2006, E62, o2153–o2155.
- Mazoir, N.; Giorgi, M.; Benharref, A. (4S,5S,10S,13R,14R,17R)-8α,9α-epoxy-4α,14α-dimethyl-5α-cholestan-3-one. Acta Crystallogr. Sect. E Struct. Rep. Online 2005, E61, o3709–o3711.
- Mazoir, N.; Auhmani, A.; Daoubi, M.; Collado, I.G.; Benharref, A. Hemisynthesis of New Triterpene Derivatives using Oxidation by CrO3 and NaIO4-(RuCl3, 3H2O). Synth. Commun. 2007, 37, 1289–1299.
- Mazoir, N.; Itto, M.Y.A.; Artiles, M.R.; Benharref, A. 4α,14α-Dimethyl-5α-cholest-8-en-3-one. Molbank 2006, M504. https://www.mdpi.com/1422-8599/2006/6/M504.
- López-Rodríguez, M.; Mazoir, N.; Daoubi, M.; Reina, M.; Benharref, A. 1-(1,5-dimethylhexyl)-3a,5b,12a,14atetramethyl-2,3,3a,4,5,5a,5b,11,12,13,-14,14a-dodecahydro-1H,12aH-cyclopenta [1,2]-phenanthro [7,8-b]índole. Acta Crystallogr. Sect. E Struct. Rep. Online 2007, E63, 04911.
- Mazoir, N.; Benharref, A.; Bailén, M.; Reina, M.; González-Coloma, A. Bioactive triterpene derivatives from latex of two Euphorbia species. Phytochemistry 2008, 69, 1328–1338.
- Mazoir, N.; Benharref, A.; Bailén, M.; Reina, M.; González-Coloma, A., Martínez-Díaz, R.A. Antileishmanial and antitrypanosomal activity of triterpene derivatives from latex of two Euphorbia species. Z. Naturforsch. 2011, 66, 360–366.
- Mazoir, N.; Benharref, A. Hemisynthesis of new thiosemicarbazone derivatives resulting from latex of Moroccan endemic plant: Euphorbia officinarum. J. Mater. Environ. Sci. 2015, 6, 592–597.
- Mazoir, N.; Belahyan, A.; Benharref, A. New 1,3,4—Thiadiazolines hemisynthesized from Moroccan endemic plants: Euphorbia Officinarum latex. J. Mater. Environ. Sci. 2015, 6, 3604–3608.
- Bailen, M.; Khamlichi, M.D.; Benharref, A.; Martinez-Diaz, R.A.; Gonzalez-Coloma, A. New bioactive semisynthetic derivatives of 31-norlanosterol and obtusifoliol from Euphorbia officinarum. Nat. Prod. Commun. 2016, 11, 733–738.
- Mazoir, N.; Benharref, A.; Vaca, L.; Reina, M.; González-Coloma, A. Optimization of insecticidal triterpene derivatives by biomimetic oxidations with hydrogen peroxide and iodosobenzene catalyzed by MnIII and FeIII porphyrin complexes. Chem. Biochem. 2020, 17, e2000287. https://doi.org/10.1002/cbdv.202000287.
- Smaili, A.; Mazoir, N.; Rifai, A.L.; Koussa, T.; Makroum, K.; Benharref, A.; Faize, L.; Albuquerque, N.; Burgos, L.; Belfaiza, M.; et al. Antimicrobial activity of two semisynthetic triterpene derivatives from Euphorbia officinarum latex against fungal and bacterial phytopathogens. Nat. Prod. Commun. 2017, 12, 331–336.
- Smaili, A.; Mazoir, N.; Rifai, A.L.; Koussa, T.; Makroum, K.; Kabil, E.M.; Benharref, A.; Faize, M. Triterpene derivatives from Euphorbia enhance resistance against Verticillium wilt of tomato. Phytochemistry 2017, 135, 169–180.
- Smaili, A.; Mazoir, N.; Rifai, A.L.; Koussa, T.; Makroum, K.; Belfaiza, M.; Benharref, A.; Faize, M. Induced resistance to wild fire disease of Nicotiana benthamiana using seed treated with triterpene derivatives from Euphorbia. J. Plant Pathol. 2018, 100, 75–83.
- Smaili, A.; Rifai, L.A.; Mazoir, N.; Koussa, T.; Faize, L.; Albuquerque, N.; Burgos, L.; Makroum, K.; Malika, B.; Benharref, A.; et al. Semisynthetic triterpenes derived from Euphorbia officinarum as plant growth promoters and inducers of disease resistance. J. Plant Growth Regul. 2019, 38, 262–272.
- Daoui, O.; Mazoir, N.; Bakhouch, M.; Salah, M.; Benharref, A.; Gonzalez-Coloma, A.; Elkhattabi, S.; El Yazidi, M.; Chtita, S. 3D-QSAR, ADME-Tox, and molecular docking of semisynthetic triterpene derivatives as antibacterial and insecticide agents. Struct. Chem. 2022, 33, 1063–1084.
- Battistini, A.; Sgarbanti, M. HIV-latency: An update of molecular mechanisms and therapeutic strategies. Viruses 2014, 6, 1715–1758.
- Boutoub, O.; Aazza, S.; El‑Guendouz; S.; El Ghadraoui, L.; Miguel, M.G. Response surface methodology (RSM) for optimization of Euphorbia resinifera and Euphorbia officinarum extracts with antioxidant and antidiabetic activities. Pharmacogn. Mag. 2022,, 18(80), 151-163.
- Boutoub, O.; El-Guendouz, S.; Estevinho, L.M.; Paula, V.B.; Aazza, S.; El Ghadraoui, L.; Rodrigues, B.; Raposo, S.; Carlier, J.; Costa, M.C.; et al. Antioxidant activity and enzyme inhibitory potential of Euphorbia resinifera and E. officinarum honeys from Morocco and plant aquous extracts. Environ. Sci. Pollut. Res. 2020, 28, 503–517.
- Boutoub, O.; El-Guendouz, S.; Manhita, A.; Dias, C.B.; Estevinho, L.M.; Paula, V.B.; Carlier, J.; Costa, M.C.; Rodrigues, B.; et al. Comparative study of the antioxidante and enzyme inhibitory activities of two types of Moroccan Euphorbia entire honey and their phenolic extracts. Foods 2021, 10, 1909. https://doi.org/10.3390/foods10081909.
- Bettar, I.; González-Miret, M.; Hernanz, D.; Marconi, A.; Heredia, F.; Terrab, A. Characterisation of Moroccan spurge (Euphorbia) honeys by their physicochemical characteristics, mineral contents and colour. Arab. J. Chem. 2019, 12, 2052–2060.
- Terrab, A.; Marconi, A.; Bettar, I.; Msanda, F.; Díez, M.J. Palynological characterisation of Euphorbia honeys from Morocco. Palynology 2014, 38, 138–146.
- Terrab, A.; Moujanni, A.; Essamadi, A.K.; Hernanz, D.; Díez, M.J.; Berjano, R. A palynological and geographical characterization of labeled resin spurge honey: Euphorbia resinifera. Palynology 2021, 46, 1–10, https://doi.org/10.1080/01916122.2021.1933639.
- Chakir, A.; Romane, A.; Marcazzan, G.L.; Ferrazzi, P. Physicochemical properties of some honeys produced from different plants in Morocco. Arab. J. Chem. 2016, 9, S946–S954.
- Moujanni, A.; Partida, L.; Essamadi, A.K.; Hernanz, D.; Heredia, F.J.; Terrab, A. Physicochemical characterisation of unique unifloral honey: Euphorbia resinifera. Cyta. J. Food 2018, 16, 27–35.
- Abderrahim, L.A.; Taïbi, K.; Abderrahim, N.A.; Boussaid, M.; Rios-Navarro, C.; Ruiz-Saurí, A. Euphorbia honey and garlic: Biological activity and burn wound recovery. Burns 2019, 45, 1695–1706.
- Chimshirova, R.; Popova, M.; Chakir, A.; Valcheva, V.; Dimitrov, S.; Trusheva, B.; Romane, A.; Bankova, V. Antimicrobial Triterpenoids and Ingol Diterpenes from Propolis of Semi-Arid Region of Morocco. Molecules 2022, 27, 2206, https://doi.org/10.3390/molecules27072206.




