Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Fatmi Ahlam | -- | 2533 | 2023-01-17 12:32:23 | | | |
| 2 | Rita Xu | -3 word(s) | 2530 | 2023-01-18 02:31:04 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Fatmi, A.; Saadi, W.; Beltrán-García, J.; García-Giménez, J.L.; Pallardó, F.V. Endothelial Glycocalyx and Neonatal Sepsis. Encyclopedia. Available online: https://encyclopedia.pub/entry/40288 (accessed on 08 February 2026).
Fatmi A, Saadi W, Beltrán-García J, García-Giménez JL, Pallardó FV. Endothelial Glycocalyx and Neonatal Sepsis. Encyclopedia. Available at: https://encyclopedia.pub/entry/40288. Accessed February 08, 2026.
Fatmi, Ahlam, Wiam Saadi, Jesús Beltrán-García, José Luis García-Giménez, Federico V. Pallardó. "Endothelial Glycocalyx and Neonatal Sepsis" Encyclopedia, https://encyclopedia.pub/entry/40288 (accessed February 08, 2026).
Fatmi, A., Saadi, W., Beltrán-García, J., García-Giménez, J.L., & Pallardó, F.V. (2023, January 17). Endothelial Glycocalyx and Neonatal Sepsis. In Encyclopedia. https://encyclopedia.pub/entry/40288
Fatmi, Ahlam, et al. "Endothelial Glycocalyx and Neonatal Sepsis." Encyclopedia. Web. 17 January, 2023.
Copy Citation
Sepsis carries a substantial risk of morbidity and mortality in newborns, especially preterm-born neonates. Endothelial glycocalyx (eGC) is a carbohydrate-rich layer lining the vascular endothelium, with important vascular barrier function and cell adhesion properties, serving also as a mechano-sensor for blood flow. eGC shedding is recognized as a fundamental pathophysiological process generating microvascular dysfunction, which in turn contributes to multiple organ failure and death in sepsis.
biomarkers
endothelium
endothelial glycocalyx
neonatal sepsis
1. Introduction
Neonatal sepsis is a life-threatening condition in newborns. It is associated with a high risk of mortality, serious disability in survivors, and psychological problems, and thus responsible for a severe burden on healthcare services [1][2][3]. Importantly, neonatal sepsis in premature newborns is considered a rare disease (ORPHA:90051). Among the different research areas explored in sepsis, vascular endothelium is considered one of the most important organs affected during the initial onset of sepsis, particularly due to its systemic involvement, and its role in inflammation and in the coagulation process. Endothelial injury increases blood vessel permeability, vessel diameter, and fluid leakage from blood vessels into the interstitial space. Induced hypovolemia affecting tissue perfusion pressure, decrease in oxygen delivery, and hypotension development within a few hours, lead neonates to septic shock [4][5].
Danielli (1940) was the first who described this protein layer on the endothelium [6]. Glycocalyx, from the Greek ‘sugar coat’ (from glykys, sweet and kalyx husk) [7], is a carbohydrate-rich layer (mainly composed of proteoglycans and glycosaminoglycans), which is found surrounding the membrane of many cell types, covering the luminal surface of vascular endothelial cells (EC) [8][9] and comprises membrane-attached proteoglycans, glycosaminoglycan chains, glycoproteins, and adherent plasma proteins, of which syndecans and glypicans are the most prevalent [10]. Endothelial glycocalyx (eGC) thickness differs with location and vascular bed, and can range from 0.1 to 4.5 µm [11][12]. According to a study by Xia et al. using confocal microscopy, the culture of human cerebral microvascular endothelial cells was determined: the thickness of heparan sulfate elements was 1.53 μm and hyaluronic elements was 2.23 μm, comparable to those observed at the microvessels [13]. In rat myocardial capillaries the thickness of eGC ranged from 0.2 to 0.5 µm and its degradation induced myocardial tissue edema [14]. These measures can differ in rat mesenteric capillaries and post-capillary venules (0.9 ± 0.1 and 1.2 ± 0.3 μm, respectively) [15]. In different models, it was found that under inflammatory conditions, the eGC is heavily damaged and takes about 5–7 days to initiate the recovery, after which endothelial and microcirculatory functions can be restored [16]. However, the visualization of the eGC to determine its composition and thickness has proven difficult due to its extremely delicate and rapidly disrupted structure [17].
Glycocalyx in newborns takes special relevance because it is one of the dominant components of the mucosal immune system [18], given that it is one of the earliest sites of injury during inflammation [19]. eGC assessment in pediatric clinical studies is based mainly on two different approaches which provide only indirect information about the eGC. The first approach measures the levels of eGC-detached components in plasma/serum and urine, such as syndecan-1 (SDC-1), hyaluronan (HA), heparan sulphate (HS), and heparan sulphate chondroitin (CS). The second approach uses the video microscopic assessment of the eGC in the microcirculation vessels [20].
2. Structure of Endothelial Glycocalyx
The thickness of the eGC is an essential parameter in measuring the functional performance of this layer in the blood vessels, where this thickness changes according to the organ in which the endothelium is present. eGC is found in continuous and fenestrated capillaries, in which it is thicker than sinusoid capillaries (Figure 1) [21]. eGC appears in continuous-type heart capillaries in moss- or broccoli-like structures covering the entire luminal endothelial cell surface. In the surface of renal podocytes, eCG is located near to occlude the endothelial pores of the fenestrated capillaries. In the sinusoid capillaries, it is in the liver, and in hematopoietic organs, covering both the luminal and the opposite side. Particularly, in the liver, the eGC is located between a hepatocyte and a sinusoid, known as the space of Disse [22].
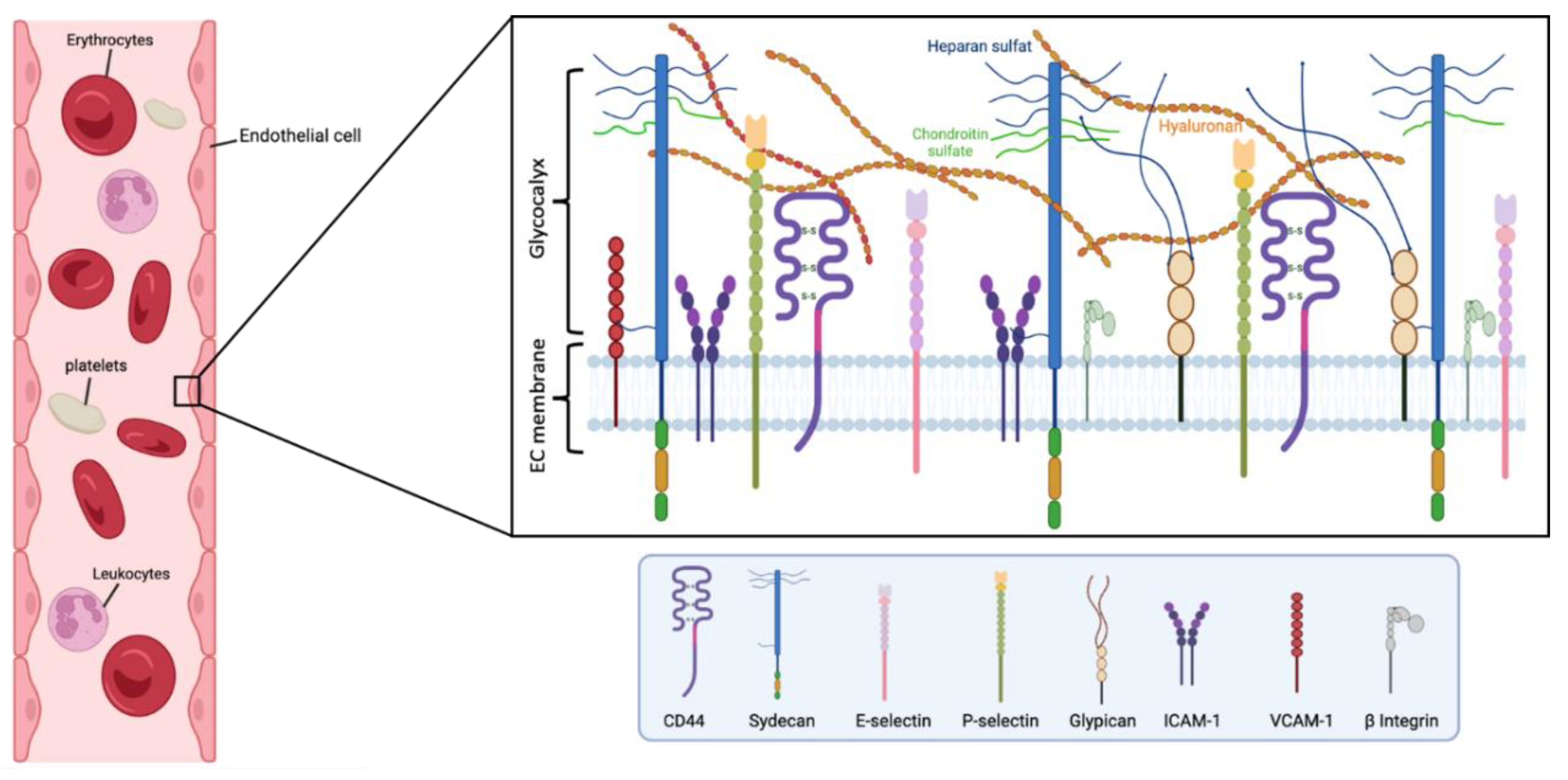
Figure 1. Main components of the human endothelial glycocalyx. The eGC consists of proteoglycans, glycoproteins, and glycosaminoglycans, associated with plasma proteins. Among others, CD44 is a cell-surface receptor for HA, it plays an important role in cell proliferation, and migration, and participates in vascular barrier integrity via the regulation of CD31 expression. β Integrin participates in leukocyte homing and subsequent diapedesis, and platelet interactions via a link to collagen, fibronectin, and laminin in the subendothelial matrix. P-selectin and E-selectin are implicated in the ‘tethering’ and rolling’ of leukocytes in stimulated‘ endothelial cells. Particularly, E-selectin needs direct stimulation by cytokines such as IL-1, TNF-α, or LPS for production and surface expressio. Syndecans and glypicans are proteoglycans that contain GAG chains such as HS and CS. Moreover, syndecans represent the principal effector in cell adhesion or shape changes by their interaction with the cytoskeleton, while the glypicans have a role in flow-induced endothelial NO synthase activation. The intercellular adhesion glycoprotein (ICAM-1) is expressed in the endothelial cell surface participating in cell-to-cell interactions and facilitates leukocyte endothelial transmigration. VCAM-1 is a glycoprotein which contributes to the cell adhesion of molecules to the endothelium. Figure created with BioRender.com.
Generally, eGC appears as a gel-like layer of glycoproteins covering the luminal surface of the capillary endothelium. eGC has an intricate architecture of different components incorporating either plasma- or endothelium-derived soluble molecules. eGC is formed by soluble plasma components linked together either directly or via proteoglycans and/or glycosaminoglycans (GAG) [23]. The components of proteoglycans entail a core protein attached to GAG chains [21], where HS comprises 50–90% of proteoglycans. Small proteogylcans such as syndecans and glypicans bind to the endothelial membrane, and other proteoglycans such as perlecan, versican, decorin, biglycan, and mimecan can be secreted as soluble proteoglycans, which can also bind to multiple GAG side chains (e.g., HS and CS, which bind to syndecans, glypicans, and perlecan; CS and dermatan sulfate, which bind to versican, decorin, and biglycan; and mimecan, which binds to keratan sulfate). These components are responsible for a charge-negative mesh through capillaries, facilitating a frictionless blood flow [24]. Importantly, the parameters of rheology in vessels continuously affect the thickness of the glycocalyx by affecting the composition and structure, through enzymatic or shear-induced shedding processes. Notably, the dynamic balance between biosynthesis and shedding makes it quite complicated to describe properly the geometrical disposition and distribution of the eGC [23].
Several components of the glycocalyx, including syndecans, HS, and HA are altered in cases of ischemia, hypoxia, sepsis, atherosclerosis, renal disease, diabetes, and several viral infections [25][26]. This alteration induces deleterious effects on the eGC and therefore in the endothelium, leading to dysfunction of microcirculatory with subsequent organ ischemia, and finally consequent organ damage.
3. Physiological Function of the Endothelial Glycocalyx
Under physiological conditions, there is a dynamic balance between the biosynthesis of new GAGs and the shear-dependent removal of different components of the eGC. This gives the eGC high structural stability, working as a vasoprotective nanobarrier against vascular leakage and adhesion, and avoiding vessel inflammation [8][27]. Importantly, the eGC can respond to environmental changes by adapting its nanomechanical properties [28]. It is known that the alterations in the hydrostatic pressure, the flow rate, and the influences of the gradient concentration in blood vessels, play an important role in the permeability properties of the eGC [7]. Although the eGC has a mince layer, it has a prominent enzyme regulatory system, which can participate in modulating the expression of functional mediators, that ultimately are involved in the blood vessel barrier integrity (i.e., albumin, antithrombin, HS, and antioxidants) [29]. That means eGC is a physical transducer, which can mediate shear-dependent endothelial responses and act as a selective plasma-filtering system of different macromolecules. In addition, eGC conserves binding sites for endothelial growth factors, fibroblast growth factor, lipoprotein lipase, superoxide dismutase, and antithrombin III [19], which in turn contributes to hydrolyzing triglycerides, balancing oxidative stress, and regulating oxidative stress, respectively. Finally, the eGC also regulates the leukocyte-endothelial adhesion process [5][30], a crucial event in immune responses, and especially important during sepsis [31].
4. Endothelial Glycocalyx and Neonatal Sepsis Therapy
The eGC is altered and shedded in sepsis, affecting the normal endothelial homeostasis. Therefore, eGC components are potential biomarkers for early diagnosis and prognosis of sepsis, and their restoration may set the basis to design potential therapeutic strategies against sepsis (Figure 2) [32].
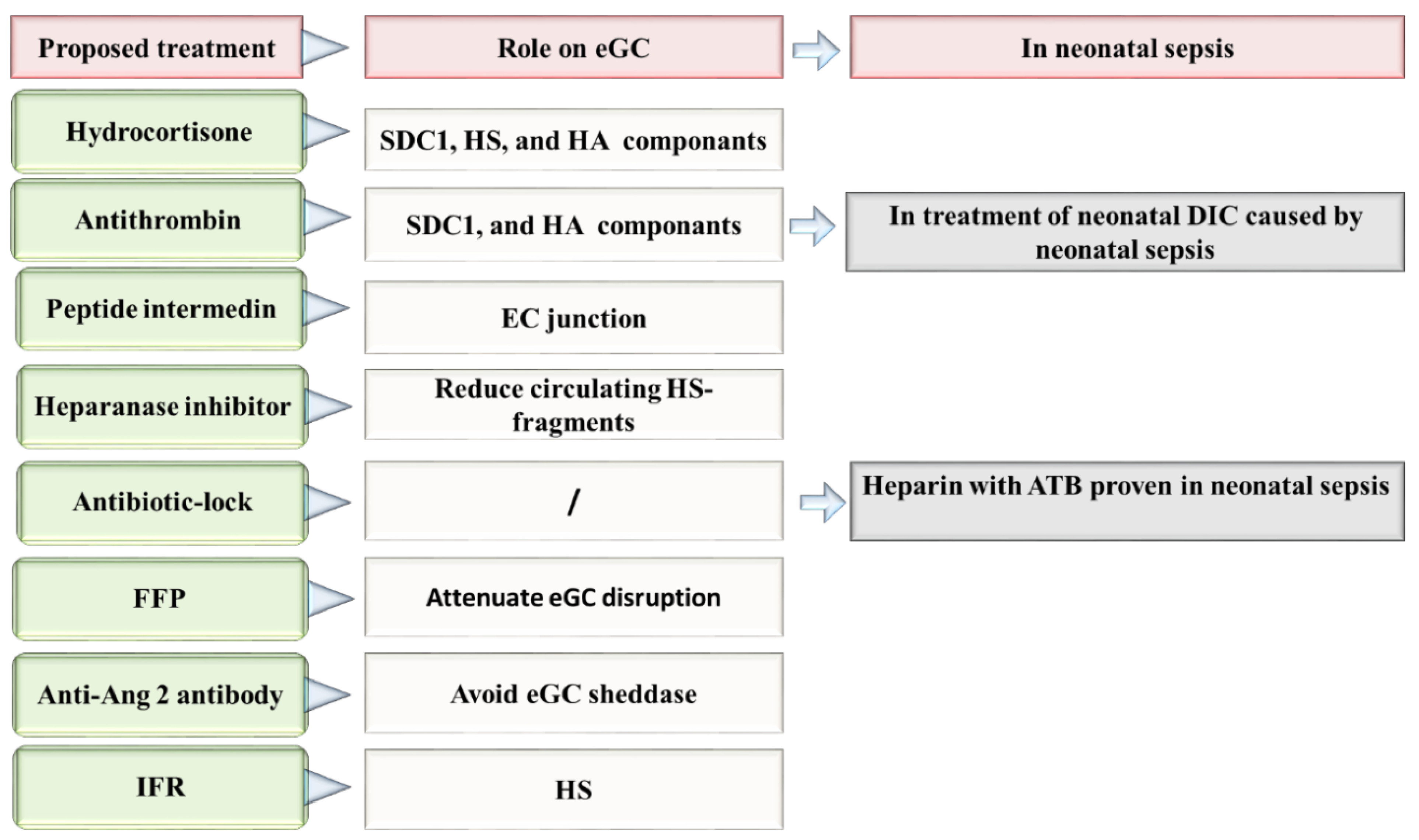
Figure 2. Proposed therapies directed to the repair of eGC in neonatal sepsis. Ang 2: Angiopoietin-2, ATB: antibiotic, DIC: disseminated intravascular coagulation, EC: Endothelial cells, eGC: Endothelial glycocalyx, FFP: fresh frozen plasma, HA: hyaluronan, HS: heparan sulfate, IFR: Intravenous fluid resuscitation, SDC-1: syndecan 1.
It has been demonstrated that hypervolemia and hyperglycemia can be toxic to the glycocalyx. Some studies have explored the therapeutic effects of many molecules to avoid eGC damage. However, to date, all tested glycocalyx-based treatments have failed [33][34]. Among the molecules studied, hydrocortisone, besides its cytokine-suppressing effects, can increase effective circulating blood volume and systemic vascular resistance [35]. In fact, it has been found that hydrocortisone and antithrombin can preserve the eGC during inflammatory-mediated degradation initiated by TNF-α [36]. In experimental models, intravenous hydrocortisone reduced the shedding of glycocalyx components SDC-1, HS, and HA, and decreased the formation of extravascular edema [37]. In a rat sepsis model, the antithrombin-treatment downregulated the circulating levels of SDC-1 and HA, and improved leukocyte adhesion, and blood circulation [38]. In the neonatal period, hydrocortisone represents the third-line response to treat neonatal shock. However, its role as a potential treatment for neonatal septic shock has not been yet evaluated. Hydrocortisone treatment in neonatal period has been found able to increase systemic arterial pressure, reduce the heart rate, and the necessity to use vasoactive drugs in newborns [39][40]. In contrast, the use of antithrombin during neonatal sepsis remains uncertain [41]. Nonetheless, in a study performed by Hayato et al., the authors observed the efficacy of antithrombin to treat neonatal DIC occurring during neonatal sepsis [42].
The peptide intermedin, a calcitonin family member, plays the role of self-protective factor in sepsis. In a septic mice model, intermedin participates in the mechanisms of repairing the endothelial junction disruption. In addition, it decreases the responsiveness of inflammatory and macrophage infiltration, thus preventing organ injury and therefore increasing the survival of infected mice [43]. Nevertheless, as far as researchers are aware, no study has been published so far on intermedin as a therapeutic tool or molecule in neonatal sepsis.
The study of Schmidt et al. is based into the role of endothelial heparanase in the shedding of eGC in mice after LPS-induced sepsis. In this work, the authors noted that the mice pre-treated with heparin or the non-anticoagulant heparanase inhibitor N-desulfated/re-N-acetylated heparin avoid the LPS-induced eGC shedding, thus attenuating sepsis-induced inflammatory lung injury [44]. The use of low molecular weight heparin avoided thrombosis in neonates [45]. In newborns, it was observed a decrease of culture-positive catheter-related sepsis via heparin. Specifically, Birch et al., reported that adding 0.5 IU/mL of heparin to total parenteral nutrition was a very effective manner of reducing sepsis without any adverse complications [46]. Until now, low doses of heparin can reduce the risk of catheter obstruction, and maintenance of percutaneous central venous catheters, thus allowing successful sepsis therapy completion [47]. In addition, the association between heparin and vancomycin (vancomycin-lock) has prevented catheter-related sepsis in VLBW preterm neonates and reduced antibiotic exposure, without causing common complications, including hypoglycemia [48]. In general, all antibiotics combined with heparin “antibiotic-lock solution” appear to decreases the risk of catheter-related bloodstream infection in the neonatal population with a high efficacy [49]. However, despite the fact that heparin seems to improve further complications in septic cases, the existing studies do not elucidate heparin’s true mechanism of action on endothelial vascular cells, or particularly on eGC.
Intravenous fluid resuscitation, generally with crystalloids or some mineral salts, or other soluble molecules, is commonly used nowadays in sepsis treatment [50][51]. However, as a therapeutic strategy may induce iatrogenic endothelial injury. This idea is based on the results found by Hippensteel et al. who mentioned the relation between the volume of intravenous fluids injected and plasma HS during resuscitation. Regardless of the sepsis severity and patient age, every liter of intravenous fluids can increase up to 200 ng/mL of circulating HS. Thus overaggressive fluid therapy can induce glycocalyx degradation [52]. Therefore, there exist undesirable effects produced by the administration of fluid resuscitation in VLBW infants. In fact, there is evidence that after two days of birth, the use of fluid resuscitation can increase the risk of chronic lung disease, patent ductus arteriosus, intraventricular hemorrhage, and the increase of risk of death [53]. There are no research published so far about the role of fluid resuscitation in the treatment regimen of neonatal sepsis. Nonetheless, the results published by Bakshi et al., urge caution regarding the use of fluid resuscitation in newborns with sepsis until new studies provide more data on this issue.
Alternatively, it has been proposed that the use of fresh frozen plasma (FFP) containing albumin to attenuate eGC breakdown [52]. Unfortunately, the benefits or possible side effects of FFP on glycocalyx integrity in sepsis have not been yet studied. Therefore, further efforts and clinical research is needed to demonstrate how these feasible therapies may improve treatment options in neonatal sepsis [54]. Acunas et al., observed that the administration of FFP and gamma-globulin can modulate humoral immunity in neonatal sepsis and induce the increases of immunoglobulins IgA, IgM, and C4 concentrations. Importantly, the authors observed that the likelihood of survival augmented in septic patients after the administration of FFP and gamma-globulin [55]. However, these results should be taken with caution because the use of only FFP did not improve the overall state of neonates diagnosed with neonatal sepsis [56]. In any case, further research evaluating how FFP treatment can mitigate endothelial injury in sepsis, particularly by avoiding the eGC layer, would improve the outcome in neonatal sepsis. This may help to further clarify the potential therapeutic possibilities of this kind of treatment and avoid the transfusion of adverse reactions [57].
In sepsis, HS-fragments released into the bloodstream act as strongly damage-associated molecular patterns, inducing pro-inflammatory phenotypes through TLR4-dependent pathways. Reducing circulating HS fragments represents a new therapeutic strategy against sepsis [58]. Similarly, the administration of heparanase inhibitors for 2 h during early sepsis in mice models attenuated the loss of glomerular filtration rate and attenuated the serum levels of IL-10 [59]. However, despite the prominent role of the endothelial glycocalyx in vascular homeostasis, its importance in some therapies, such as intravenous fluid resuscitation therapies, is still largely unknown.
The pathway Angiopoietin-Tie2 was implicated in bacteremia and mortality in neonatal sepsis [60]. Ang-2 was demonstrated to reduce the expression of receptor Tie2 in the EC, to increase endothelial permeability, and therefore contribute to edema formation in vivo [61]. In mouse models of sepsis, the use of an anti-Ang2 antibody ABTAA (ANG2-binding and Tie2-activating antibody) aids in vascular protection, via reducing cytokine storm, avoiding eGC sheddase, and vascular leakage [62]. These results make the role of the Ang-Tie2 axis feasible in sepsis. Particularly, low Ang-1 and high levels of Ang-2, as well as a high Ang-2/Ang-1 protein ratio in serum have been previously associated with EOS in Surinamese newborns [63]. Therefore, because angiopoietins may play a role in the vascular pathophysiology of EOS, it is feasible that the Tie2 activation may ameliorate sepsis progression. If this hypothesis is demonstrated, the control of the ratio Ang-Tie2 can become a sepsis-specific treatment via restoring the eGC and the microvascular barrier, thus accelerating mechanisms mediating angiogenic repair.
References
- Fatmi, A.; Rebiahi, S.A.; Chabni, N.; Zerrouki, H.; Azzaoui, H.; Elhabiri, Y.; Benmassour, S.; Ibáñez-Cabellos, J.S.; Aribi, M.; García-Giménez, J.L.; et al. miRNA-23b as a biomarker of culture-positive neonatal sepsis. Mol. Med. 2020, 26, 94.
- Bhandari, V. Effective Biomarkers for Diagnosis of Neonatal Sepsis. J. Pediatr. Infect. Dis. Soc. 2014, 3, 234–245.
- Panwar, C.; Kaushik, S.; Kaushik, R.; Sood, A. Correlation of neonatal and maternal clinico-hematological parameters as predictors of early onset neonatal sepsis. Int. J. Contemp. Pediatr. 2017, 4, 36–42.
- Reitsma, S.; Slaaf, D.W.; Vink, H.; van Zandvoort, M.A.; oude Egbrink, M.G. The endothelial glycocalyx: Composition, functions, and visualization. Pflugers Arch. 2007, 454, 345–359.
- Lupu, F.; Kinasewitz, G.; Dormer, K. The role of endothelial shear stress on haemodynamics, inflammation, coagulation and glycocalyx during sepsis. J. Cell. Mol. Med. 2020, 24, 12258–12271.
- Danielli, J.F. Capillary permeability and oedema in the perfused frog. J. Physiol. 1940, 98, 109–129.
- Butler, M.J.; Down, C.J.; Foster, R.R.; Satchell, S.C. The Pathological Relevance of Increased Endothelial Glycocalyx Permeability. Am. J. Pathol. 2020, 190, 742–751.
- Liu, H.; Li, J.; Xuan, C.; Ma, H. A review on the physiological and pathophysiological role of endothelial glycocalyx. J. Biochem. Mol. Toxicol. 2020, 34, e22571.
- Machin, D.R.; Phuong, T.T.; Donato, A.J. The role of the endothelial glycocalyx in advanced age and cardiovascular disease. Curr. Opin. Pharmacol. 2019, 45, 66–71.
- Tarbell, J.M.; Cancel, L.M. The glycocalyx and its significance in human medicine. J. Intern. Med. 2016, 280, 97–113.
- Pries, A.R.; Secomb, T.W.; Gaehtgens, P. The endothelial surface layer. Pflüg. Arch.-Eur. J. Physiol. 2000, 440, 653–666.
- Savery, M.D.; Jiang, J.X.; Park, P.W.; Damiano, E.R. The endothelial glycocalyx in syndecan-1 deficient mice. Microvasc. Res. 2013, 87, 83–91.
- Differential Effects of Vascular Endothelial Growth Factor on Glycocalyx of Endothelial and Tumor Cells and Potential Targets for Tumor Metastasis: APL Bioengineering: Volume 6, No 1. Available online: https://aip.scitation.org/doi/10.1063/5.0064381 (accessed on 7 December 2022).
- van den Berg, B.M.; Vink, H.; Spaan, J.A.E. The endothelial glycocalyx protects against myocardial edema. Circ. Res. 2003, 92, 592–594.
- Yen, W.-Y.; Cai, B.; Zeng, M.; Tarbell, J.M.; Fu, B.M. Quantification of the endothelial surface glycocalyx on rat and mouse blood vessels. Microvasc. Res. 2012, 83, 337–346.
- Fernández-Sarmiento, J.; Salazar-Peláez, L.M.; Carcillo, J.A. The Endothelial Glycocalyx: A Fundamental Determinant of Vascular Permeability in Sepsis. Pediatr. Crit. Care Med. 2020, 21, e291–e300.
- Gaudette, S.; Hughes, D.; Boller, M. The endothelial glycocalyx: Structure and function in health and critical illness. J. Vet. Emerg. Crit. Care 2020, 30, 117–134.
- Pavlova, V.; Paunova-Krasteva, T.; Stoitsova, S.; Nikolova, E. Distribution patterns of carbohydrates in murine glycocalyx. Biotechnol. Biotechnol. Equip. 2015, 29, 357–362.
- Cerny, V.; Astapenko, D.; Brettner, F.; Benes, J.; Hyspler, R.; Lehmann, C.; Zadak, Z. Targeting the endothelial glycocalyx in acute critical illness as a challenge for clinical and laboratory medicine. Crit. Rev. Clin. Lab. Sci. 2017, 54, 343–357.
- Puchwein-Schwepcke, A.; Genzel-Boroviczény, O.; Nussbaum, C. The Endothelial Glycocalyx: Physiology and Pathology in Neonates, Infants and Children. Front. Cell Dev. Biol. 2021, 9, 2432.
- Villalba, N.; Baby, S.; Yuan, S.Y. The Endothelial Glycocalyx as a Double-Edged Sword in Microvascular Homeostasis and Pathogenesis. Front. Cell Dev. Biol. 2021, 9, 711003.
- Okada, H.; Yoshida, S.; Hara, A.; Ogura, S.; Tomita, H. Vascular endothelial injury exacerbates coronavirus disease 2019: The role of endothelial glycocalyx protection. Microcirculation 2021, 28, e12654.
- Lipowsky, H.H. Microvascular rheology and hemodynamics. Microcirculation 2005, 12, 5–15.
- Drost, C.C.; Rovas, A.; Kümpers, P. Protection and rebuilding of the endothelial glycocalyx in sepsis–Science or fiction? Matrix Biol. Plus 2021, 12, 100091.
- Haymet, A.B.; Bartnikowski, N.; Wood, E.S.; Vallely, M.P.; McBride, A.; Yacoub, S.; Biering, S.B.; Harris, E.; Suen, J.Y.; Fraser, J.F. Studying the Endothelial Glycocalyx in vitro: What Is Missing? Front. Cardiovasc. Med. 2021, 8, 280.
- Kalagara, T.; Moutsis, T.; Yang, Y.; Pappelbaum, K.I.; Farken, A.; Cladder-Micus, L.; Vidal-Y-Sy, S.; John, A.; Bauer, A.T.; Moerschbacher, B.M.; et al. The endothelial glycocalyx anchors von Willebrand factor fibers to the vascular endothelium. Blood Adv. 2018, 2, 2347–2357.
- Cosgun, Z.C.; Fels, B.; Kusche-Vihrog, K. Nanomechanics of the Endothelial Glycocalyx. Am. J. Pathol. 2020, 190, 732–741.
- Zou, Z.; Li, L.; Schäfer, N.; Huang, Q.; Maegele, M.; Gu, Z. Endothelial glycocalyx in traumatic brain injury associated coagulopathy: Potential mechanisms and impact. J. Neuroinflamm. 2021, 18, 134.
- Myers, G.J.; Wegner, J. Endothelial Glycocalyx and Cardiopulmonary Bypass. J. Extra. Corpor. Technol. 2017, 49, 174–181.
- LaRivière, W.B.; Schmidt, E.P. The Pulmonary Endothelial Glycocalyx in ARDS: A Critical Role for Heparan Sulfate. Curr. Top. Membr. 2018, 82, 33–52.
- Joyce, D.E.; Nelson, D.R.; Grinnell, B.W. Leukocyte and endothelial cell interactions in sepsis: Relevance of the protein C pathway. Crit. Care Med. 2004, 32, S280–S286.
- Colbert, J.F.; Schmidt, E.P. Endothelial and Microcirculatory Function and Dysfunction in Sepsis. Clin. Chest Med. 2016, 37, 263–275.
- Iba, T.; Levy, J.H. Derangement of the endothelial glycocalyx in sepsis. J. Thromb. Haemost. 2019, 17, 283–294.
- Becker, B.F.; Jacob, M.; Leipert, S.; Salmon, A.H.J.; Chappell, D. Degradation of the endothelial glycocalyx in clinical settings: Searching for the sheddases: Endothelial glycocalyx–emerging clinical impact. Br. J. Clin. Pharmacol. 2015, 80, 389–402.
- Martin, L.; Koczera, P.; Zechendorf, E.; Schuerholz, T. The Endothelial Glycocalyx: New Diagnostic and Therapeutic Approaches in Sepsis. BioMed Res. Int. 2016, 2016, 375827.
- Chappell, D.; Hofmann-Kiefer, K.; Jacob, M.; Rehm, M.; Briegel, J.; Welsch, U.; Conzen, P.; Becker, B.F. TNF-alpha induced shedding of the endothelial glycocalyx is prevented by hydrocortisone and antithrombin. Basic Res. Cardiol. 2009, 104, 78–89.
- Pietrasanta, C.; Pugni, L.; Ronchi, A.; Bottino, I.; Ghirardi, B.; Sanchez-Schmitz, G.; Borriello, F.; Mosca, F.; Levy, O. Vascular Endothelium in Neonatal Sepsis: Basic Mechanisms and Translational Opportunities. Front. Pediatr. 2019, 7, 340.
- Iba, T.; Levy, J.H.; Hirota, T.; Hiki, M.; Sato, K.; Murakami, T.; Nagaoka, I. Protection of the endothelial glycocalyx by antithrombin in an endotoxin-induced rat model of sepsis. Thromb. Res. 2018, 171, 1–6.
- Wynn, J.L.; Wong, H.R. Pathophysiology of Neonatal Sepsis. Fetal Neonatal Physiol. 2017, 2, 1536–1552.e10.
- Wynn, J.L.; Wong, H.R. Pathophysiology and treatment of septic shock in neonates. Clin. Perinatol. 2010, 37, 439–479.
- Bassler, D.; Schmidt, B. Antithrombin replacement in neonates: Is there any indication? Thromb. Res. 2006, 118, 107–111.
- Go, H.; Ohto, H.; Nollet, K.E.; Kashiwabara, N.; Ogasawara, K.; Chishiki, M.; Hiruta, S.; Sakuma, I.; Kawasaki, Y.; Hosoya, M. Risk factors and treatments for disseminated intravascular coagulation in neonates. Ital. J. Pediatr. 2020, 46, 54.
- Xiao, F.; Wang, D.; Kong, L.; Li, M.; Feng, Z.; Shuai, B.; Wang, L.; Wei, Y.; Li, H.; Wu, S.; et al. Intermedin protects against sepsis by concurrently re-establishing the endothelial barrier and alleviating inflammatory responses. Nat. Commun. 2018, 9, 2644.
- Schmidt, E.P.; Yang, Y.; Janssen, W.J.; Gandjeva, A.; Perez, M.J.; Barthel, L.; Zemans, R.L.; Bowman, J.C.; Koyanagi, D.E.; Yunt, Z.X.; et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat. Med. 2012, 18, 1217–1223.
- Romantsik, O.; Bruschettini, M.; Zappettini, S.; Ramenghi, L.A.; Calevo, M.G. Heparin for the treatment of thrombosis in neonates. Cochrane Database Syst. Rev. 2016, 2016, CD012185.
- Birch, P.; Ogden, S.; Hewson, M. A randomised, controlled trial of heparin in total parenteral nutrition to prevent sepsis associated with neonatal long lines: The Heparin in Long Line Total Parenteral Nutrition (HILLTOP) trial. Arch. Dis. Child. Fetal Neonatal Ed. 2010, 95, F252–F257.
- Uslu, S.; Ozdemir, H.; Comert, S.; Bolat, F.; Nuhoglu, A. The effect of low-dose heparin on maintaining peripherally inserted percutaneous central venous catheters in neonates. J. Perinatol. 2010, 30, 794–799.
- Liang, H.; Zhang, L.; Guo, X.; Sun, L. Vancomycin-lock therapy for prevention of catheter-related bloodstream infection in very low body weight infants. BMC Pediatr. 2021, 21, 3.
- Taylor, J.E.; Tan, K.; Lai, N.M.; McDonald, S.J. Antibiotic lock for the prevention of catheter-related infection in neonates. Cochrane Database Syst. Rev. 2015, 6, CD010336.
- Du Pont-Thibodeau, G.; Joyal, J.-S.; Lacroix, J. Management of neonatal sepsis in term newborns. F1000Prime Rep. 2014, 6, 67.
- Tseng, C.-H.; Chen, T.-T.; Wu, M.-Y.; Chan, M.-C.; Shih, M.-C.; Tu, Y.-K. Resuscitation fluid types in sepsis, surgical, and trauma patients: A systematic review and sequential network meta-analyses. Crit. Care 2020, 24, 693.
- Hippensteel, J.A.; Uchimido, R.; Tyler, P.D.; Burke, R.C.; Han, X.; Zhang, F.; McMurtry, S.A.; Colbert, J.F.; Lindsell, C.J.; Angus, D.C.; et al. Intravenous fluid resuscitation is associated with septic endothelial glycocalyx degradation. Crit. Care 2019, 23, 259.
- Bakshi, S.; Singh, R.; Vaidya, R.; Koerner, T.; Knee, A. Impact of Fluid Resuscitation on Clinical Outcomes for Very Low Birth Weight infants in Neonatal Intensive Care Unit. Pediatrics 2019, 144, 647.
- Uchimido, R.; Schmidt, E.P.; Shapiro, N.I. The glycocalyx: A novel diagnostic and therapeutic target in sepsis. Crit. Care 2019, 23, 16.
- Acunas, B.A.; Peakman, M.; Liossis, G.; Davies, E.T.; Bakoleas, B.; Costalos, C.; Gamsu, H.R.; Vergani, D. Effect of fresh frozen plasma and gammaglobulin on humoral immunity in neonatal sepsis. Arch. Dis. Child. Fetal Neonatal Ed. 1994, 70, F182–F187.
- Blood Component Transfusion in Tertiary Care Neonatal Intensive Care Unit and Neonatal Intermediate Care Unit: An Audit–PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7510180/ (accessed on 24 April 2022).
- Qin, X.; Zhang, W.; Zhu, X.; Hu, X.; Zhou, W. Early Fresh Frozen Plasma Transfusion: Is It Associated With Improved Outcomes of Patients With Sepsis? Front. Med. 2021, 8, 754859.
- Martin, L.; De Santis, R.; Koczera, P.; Simons, N.; Haase, H.; Heinbockel, L.; Brandenburg, K.; Marx, G.; Schuerholz, T. The Synthetic Antimicrobial Peptide 19-2.5 Interacts with Heparanase and Heparan Sulfate in Murine and Human Sepsis. PLoS ONE 2015, 10, e0143583.
- Lygizos, M.I.; Yang, Y.; Altmann, C.J.; Okamura, K.; Hernando, A.A.; Perez, M.J.; Smith, L.P.; Koyanagi, D.E.; Gandjeva, A.; Bhargava, R.; et al. Heparanase mediates renal dysfunction during early sepsis in mice. Physiol. Rep. 2013, 1, e00153.
- Wright, J.K.; Hayford, K.; Tran, V.; Al Kibria, G.M.; Baqui, A.; Manajjir, A.; Mahmud, A.; Begum, N.; Siddiquee, M.; Kain, K.C.; et al. Biomarkers of endothelial dysfunction predict sepsis mortality in young infants: A matched case-control study. BMC Pediatr. 2018, 18, 118.
- Cao, R.-N.; Tang, L.; Xia, Z.-Y.; Xia, R. Endothelial glycocalyx as a potential theriapeutic target in organ injuries. Chin. Med. J. 2019, 132, 963–975.
- Leligdowicz, A.; Richard-Greenblatt, M.; Wright, J.; Crowley, V.M.; Kain, K.C. Endothelial Activation: The Ang/Tie Axis in Sepsis. Front. Immunol. 2018, 9, 838.
- Zonneveld, R.; Jongman, R.; Juliana, A.; Zijlmans, W.; Plötz, F.; Molema, G.; van Meurs, M. Low Serum Angiopoietin-1, High Serum Angiopoietin-2, and High Ang-2/Ang-1 Protein Ratio are Associated with Early Onset Sepsis in Surinamese Newborns. Shock 2017, 48, 638–643.
More
Information
Subjects:
Health Care Sciences & Services
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
653
Revisions:
2 times
(View History)
Update Date:
18 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




