
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nuno Graça | -- | 1790 | 2023-01-13 11:17:18 | | | |
| 2 | Jessie Wu | Meta information modification | 1790 | 2023-01-16 02:12:20 | | | | |
| 3 | Jessie Wu | + 2 word(s) | 1792 | 2023-01-16 02:15:49 | | |
Video Upload Options
The electrocoagulation (EC) process is a possible alternative to conventional wastewater treatment methods. Characteristics of the process, such as its flexibility, easy operation, no need for additional chemicals, and its ability to deal with different contaminants, have been increasing the interest in its implementation. The EC process found application in the treatment of different contaminated waters, and several studies have shown the potential of this technology. Adsorption (AD) is another attractive way of treating wastewaters due to the potential of using low-cost and environmentally friendly adsorbents. Due to their high surface area and well-developed pore structure, activated carbons are the most used adsorbents in wastewater treatment systems. The high price of activated carbon limits its application. The combination of the EC and AD processes can be used to amplify the advantages that each process presents in treating wastewaters. As a first step, the EC process reduces the pollutant loading and the suspended solids concentration, which can benefit the AD process by delaying the adsorbent saturation and preventing clogging. Additionally, each adsorption/regeneration cycle could result in the adsorbent losing some of its capacity; as a result, delaying adsorbent saturation helps to increase its useful life.
1. Applications of Combined Electrocoagulation and Adsorption Processes
| Wastewater | Adsorbent | Electrodes | Removal Efficiency | Reference |
|---|---|---|---|---|
| Industrial wastewater | Ectodermis of Opuntia | Al | 84% (COD) 78% (BOD) 97% (color) 98% (turbidity) 99% (fecal coliforms) |
[1] |
| Aqueous solution | Granular activated carbon | Zn | 99.88% (Pb(II)) | [2] |
| Textile wastewater | Crude Tunisian clay | Fe | 96.87% (color) 89.77% (COD) 84.46% (TSS) |
[3] |
| Beverage industry wastewater | Activated carbon | Al | 98.66% (COD) 92.15% (TSS) 90.12% (color) |
[4] |
| Semiconductor wastewater | Activated carbon | Al | 67.25% (fluoride) | [5] |
| Produced water | Coconut shell activated carbon | Al/Fe | 98.39% (COD) 93.54% (TDS) 75.16% (ammonia) 97.56% (oil content) 92.5% (phenol) |
[6] |
| Nitrate-contaminated ground water | Zeolite | Al | 96% (nitrates) | [7] |
| Automobile wastewater | Activated carbon | Al | 71.58% (COD) 77.91% (surfactant) |
[8] |
| Tanning wastewater | Eggshell | Al | 99% (Cr(VI)) | [9] |
| Anaerobic wastewater | Granular activated carbon | Al | 100% (COD) 100% (BOD) 96.5% (turbidity) 97.5% (phosphorus) |
[10] |
| Paper mill effluent | Granular activated carbon | Al/Fe | 98.97% (COD) | [11] |
| Model solution | Red onion skin | Al | 97% (Cr(VI)) | [12] |
| Cellulose and paper industry wastewater | Granular activated carbon | Al | 93% (humic acid) | [13] |
| Dye solution | Banana peel | Al | 99% (methylene blue) | [14] |
| Mine waters | Rice straw activated carbon | Al | 95.2% (sulphate) | [15] |
| Dairy wastewater | Granular activated carbon | Al | 99.39% (turbidity) 87.12 (COD) |
[16] |
2. The Combined Process from the Perspective of Circular Economy
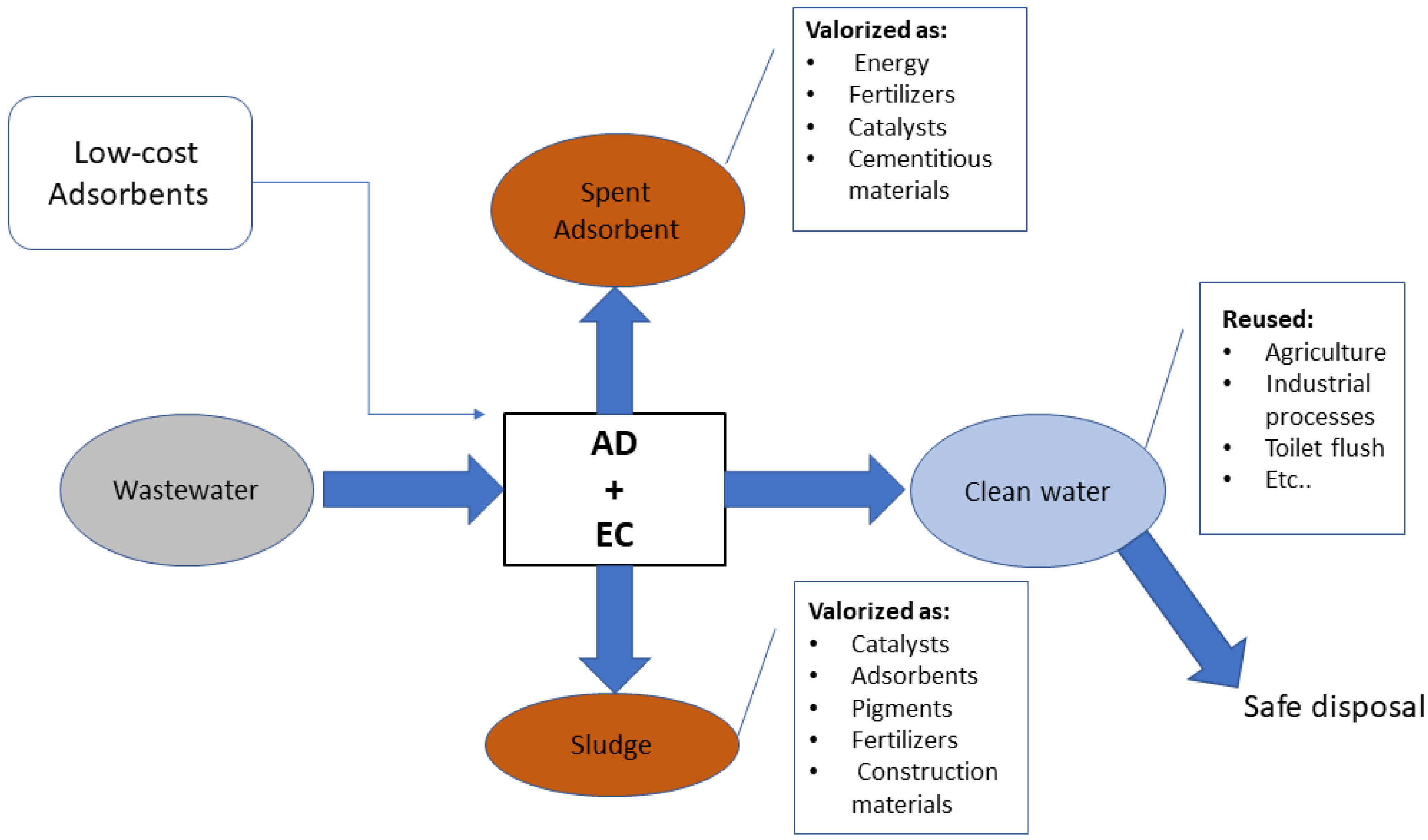
References
- Hernández, I.L.; Barrera-Díaz, C.; Roa, G.; Bilyeu, B.; Ureña-Núñez, F. A combined electrocoagulation–sorption process applied to mixed industrial wastewater. J. Hazard. Mater. 2007, 144, 240–248.
- Hussin, F.; Aroua, M.K.; Szlachta, M. Combined solar electrocoagulation and adsorption processes for Pb(II) removal from aqueous solution. Chem. Eng. Process. Process Intensif. 2019, 143, 107619.
- Hendaoui, K.; Trabelsi-Ayadi, M.; Ayari, F. Optimization of continuous electrocoagulation-adsorption combined process for the treatment of a textile effluent. Chin. J. Chem. Eng. 2021, 44, 310–320.
- Muryanto, M.; Marlina, E.; Sari, A.A.; Harimawan, A.; Sudarno, S. Treatment of beverage industry wastewater using a combination of electrocoagulation and adsorption processes. AIP Conf. Proc. 2018, 2024, 020004.
- Jalil, S.N.A.; Amri, N.; Ajien, A.A.; Ismail, N.F.; Ballinger, B. A hybrid electrocoagulation-adsorption process for fluoride removal from semiconductor wastewater. J. Physics Conf. Ser. 2019, 1349, 012056.
- Anugrah, P.; Said, M.; Bahrin, D. Produced Water Treatment using Electrocoagulation Combination Method with Aluminum (Al) and Iron (Fe) Electrodes and Activated Carbon Adsorption Treatment. Int. J. Adv. Sci. Eng. Inf. Technol. 2022, 12, 703–711.
- Ziouvelou, A.; Tekerlekopoulou, A.G.; Vayenas, D.V. A hybrid system for groundwater denitrification using electrocoagulation and adsorption. J. Environ. Manag. 2019, 249, 109355.
- Thakur, C. Unification electrocoagulation-adsorption treatment for removal of COD and surfactant from automobile wastewater. Int. J. Chem. React. Eng. 2021, 19, 961–968.
- Elabbas, S.; Adjeroud, N.; Mandi, L.; Berrekhis, F.; Pons, M.N.; Leclerc, J.P.; Ouazzani, N. Eggshell adsorption process coupled with electrocoagulation for improvement of chromium removal from tanning wastewater. Int. J. Environ. Anal. Chem. 2020, 13, 1–13.
- Pizutti, J.T.; Santos, R.D.C.D.; Hemkemeier, M.; Piccin, J.S. Electrocoagulation coupled adsorption for anaerobic wastewater post-treatment and reuse purposes. Desalination Water Treat. 2019, 160, 144–152.
- Bellebia, S.; Kacha, S.; Bouyakoub, A.Z.; Derriche, Z. Experimental investigation of chemical oxygen demand and turbidity removal from cardboard paper mill effluents using combined electrocoagulation and adsorption processes. Environ. Prog. Sustain. Energy 2012, 31, 361–370.
- Ouaissa, Y.A.; Chabani, M.; Amrane, A.; Bensmaili, A. Removal of Cr(VI) from Model Solutions by a Combined Electrocoagulation Sorption Process. Chem. Eng. Technol. 2012, 36, 147–155.
- Barhoumi, A.; Ncib, S.; Chibani, A.; Brahmi, K.; Bouguerra, W.; Elaloui, E. High-rate humic acid removal from cellulose and paper industry wastewater by combining electrocoagulation process with adsorption onto granular activated carbon. Ind. Crop. Prod. 2019, 140, 111715.
- De Carvalho, H.P.; Huang, J.; Zhao, M.; Liu, G.; Dong, L.; Liu, X. Improvement of Methylene Blue removal by electrocoagulation/banana peel adsorption coupling in a batch system. Alex. Eng. J. 2015, 54, 777–786.
- Zhu, M.; Yin, X.; Chen, W.; Yi, Z.; Tian, H. Removal of sulphate from mine waters by electrocoagulation/rice straw activated carbon adsorption coupling in a batch system: Optimization of process via response surface methodology. J. Water Reuse Desalination 2018, 9, 163–172.
- Cherifi, M.; Guenfoud, S.; Bendaia, M.; Hazourli, S.; Laefer, D.F.; Leclerc, J.P.; Mecibah, W. Comparative study between electrocoagulation used separately and coupled with adsorption for dairy wastewater treatment using response surface methodology design. Desalination Water Treat. 2021, 223, 235–245.
- Wang, X.; Ni, J.; Pang, S.; Li, Y. Removal of malachite green from aqueous solutions by electrocoagulation/peanut shell adsorption coupling in a batch system. Water Sci. Technol. 2017, 75, 1830–1838.
- Yang, G.C.C.; Tang, P.-L.; Yen, C.-H. Removal of micropollutants from municipal wastewater by graphene adsorption and simultaneous electrocoagulation/electrofiltration process. Water Sci. Technol. 2017, 75, 1882–1888.
- Narayanan, N.V.; Ganesan, M. Use of adsorption using granular activated carbon (GAC) for the enhancement of removal of chromium from synthetic wastewater by electrocoagulation. J. Hazard. Mater. 2009, 161, 575–580.
- Castañeda-Díaz, J.; Pavón-Silva, T.; Gutiérrez-Segura, E.E.; Colín-Cruz, A. Electrocoagulation-Adsorption to Remove Anionic and Cationic Dyes from Aqueous Solution by PV-Energy. J. Chem. 2017, 2017, 5184590.
- Rubí-Juárez, H.; Barrera-Díaz, C.; Ureña-Nuñez, F. Adsorption-assisted electrocoagulation of real car wash wastewater with equilibrium and kinetic studies. Pollut. Res. 2017, 36, 175–184.
- Claude, N.J.; Shanshan, L.; Khan, J.; Yifeng, W.; Dongxu, H.; Xiangru, L. Waste tea residue adsorption coupled with electrocoagulation for improvement of copper and nickel ions removal from simulated wastewater. Sci. Rep. 2022, 12, 1–18.
- Bazrafshan, E.; Alipour, M.R.; Mahvi, A.H. Textile wastewater treatment by application of combined chemical coagulation, electrocoagulation, and adsorption processes. Desalination Water Treat. 2015, 57, 9203–9215.
- Neczaj, E.; Grosser, A. Circular Economy in Wastewater Treatment Plant—Challenges and Barriers. Proceedings 2018, 2, 614.
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions, Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. 2014. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM:2022:34:FIN (accessed on 13 October 2022).
- Widiastuti, N.; Wu, H.; Ang, M.; Zhang, D.-K. The potential application of natural zeolite for greywater treatment. Desalination 2008, 218, 271–280.
- Verstraete, W.; Vlaeminck, S.E. ZeroWasteWater: Short-cycling of wastewater resources for sustainable cities of the future. Int. J. Sustain. Dev. World Ecol. 2011, 18, 253–264.
- Molinos-Senante, M.; Hernandez-Sancho, F.; Sala-Garrido, R. Tariffs and Cost Recovery in Water Reuse. Water Resour. Manag. 2012, 27, 1797–1808.
- Rajaniemi, K.; Tuomikoski, S.; Lassi, U. Electrocoagulation Sludge Valorization—A Review. Resources 2021, 10, 127.
- Rajaniemi, K.; Hu, T.; Nurmesniemi, E.-T.; Tuomikoski, S.; Lassi, U. Phosphate and Ammonium Removal from Water through Electrochemical and Chemical Precipitation of Struvite. Processes 2021, 9, 150.
- Un, U.T.; Onpeker, S.E.; Ozel, E. The treatment of chromium containing wastewater using electrocoagulation and the production of ceramic pigments from the resulting sludge. J. Environ. Manag. 2017, 200, 196–203.
- Sharma, P.; Joshi, H. Utilization of electrocoagulation-treated spent wash sludge in making building blocks. Int. J. Environ. Sci. Technol. 2015, 13, 349–358.
- Golder, A.; Samanta, A.; Ray, S. Anionic reactive dye removal from aqueous solution using a new adsorbent—Sludge generated in removal of heavy metal by electrocoagulation. Chem. Eng. J. 2006, 122, 107–115.
- Ghanbari, F.; Zirrahi, F.; Olfati, D.; Gohari, F.; Hassani, A. TiO2 nanoparticles removal by electrocoagulation using iron electrodes: Catalytic activity of electrochemical sludge for the degradation of emerging pollutant. J. Mol. Liq. 2020, 310, 113217.
- Oladejo, J.; Shi, K.; Luo, X.; Yang, G.; Wu, T. A Review of Sludge-to-Energy Recovery Methods. Energies 2018, 12, 60.
- Phalakornkule, C.; Sukkasem, P.; Mutchimsattha, C. Hydrogen recovery from the electrocoagulation treatment of dye-containing wastewater. Int. J. Hydrogen Energy 2010, 35, 10934–10943.
- Zwain, H.M.; Vakili, M.; Dahlan, I. Waste Material Adsorbents for Zinc Removal from Wastewater: A Comprehensive Review. Int. J. Chem. Eng. 2014, 2014, 347912.
- Yao, Y.; Gao, B.; Chen, J.; Yang, L. Engineered Biochar Reclaiming Phosphate from Aqueous Solutions: Mechanisms and Potential Application as a Slow-Release Fertilizer. Environ. Sci. Technol. 2013, 47, 8700–8708.
- Wu, Y.; Luo, H.; Wang, H.; Zhang, L.; Liu, P.; Feng, L. Fast adsorption of nickel ions by porous graphene oxide/sawdust composite and reuse for phenol degradation from aqueous solutions. J. Colloid Interface Sci. 2014, 436, 90–98.
- Paul, S.C.; Mbewe, P.B.; Kong, S.Y.; Šavija, B. Agricultural Solid Waste as Source of Supplementary Cementitious Materials in Developing Countries. Materials 2019, 12, 1112.
- Blázquez, G.; Martín-Lara, M.A.; Dionisio-Ruiz, E.; Tenorio, G.; Calero, M. Copper biosorption by pine cone shell and thermal decomposition study of the exhausted biosorbent. J. Ind. Eng. Chem. 2012, 18.




