Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mengzhou Xue | -- | 2083 | 2023-01-13 10:58:54 | | | |
| 2 | Camila Xu | Meta information modification | 2083 | 2023-01-13 11:12:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Li, Z.; Liu, Y.; Wei, R.; Yong, V.W.; Xue, M. Zinc in Neurological Diseases. Encyclopedia. Available online: https://encyclopedia.pub/entry/40156 (accessed on 07 February 2026).
Li Z, Liu Y, Wei R, Yong VW, Xue M. Zinc in Neurological Diseases. Encyclopedia. Available at: https://encyclopedia.pub/entry/40156. Accessed February 07, 2026.
Li, Zhe, Yang Liu, Ruixue Wei, V. Wee Yong, Mengzhou Xue. "Zinc in Neurological Diseases" Encyclopedia, https://encyclopedia.pub/entry/40156 (accessed February 07, 2026).
Li, Z., Liu, Y., Wei, R., Yong, V.W., & Xue, M. (2023, January 13). Zinc in Neurological Diseases. In Encyclopedia. https://encyclopedia.pub/entry/40156
Li, Zhe, et al. "Zinc in Neurological Diseases." Encyclopedia. Web. 13 January, 2023.
Copy Citation
Zinc is a trace element essential for human survival, and its deficiency has been linked to various adverse effects, such as growth retardation, impaired functioning of the immune system, and cognitive dysfunction.
zinc
brain
stroke
neurotoxicity
1. Introduction
Zinc is a trace element essential for human survival, and its deficiency has been linked to various adverse effects, such as growth retardation, impaired functioning of the immune system, and cognitive dysfunction [1][2]. Zinc is associated with the synthesis and activity of numerous proteins and enzymes, including matrix metalloproteinases (MMPs), deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) polymerases, and insulin [3]. Zinc insufficiency is one of the world’s major public health issues since it is linked to several diseases [4][5][6][7]. Maintaining zinc homeostasis is crucial for normal brain function, and zinc deficiency or overload may contribute to brain injury and exacerbate neurological conditions [8].
Zinc plays a role in both physiological and pathological processes in CNS [9][10]. A major site of storage of zinc is the telencephalon, especially in the cortical areas, hippocampus, and amygdala [11]. Accordingly, zinc has far-reaching effects on cognition, emotional stability, and memory [12]. Therefore, maintaining zinc homeostasis is essential for brain health, and it appears relevant to investigate its potential contributions to many neurological diseases.
2. Distribution of Zinc in the Brain
At 2–3 g in total, zinc is an abundant transition metal found in high concentrations in the mammalian brain and is distributed unequally throughout different organs and tissues [13]. In adults, 60% of zinc is found in skeletal muscles; 30% in bones; 5% in the liver and skin; 1.5% in the brain, kidney, and heart; and less than 2% in other tissues [14]. Only a small fraction of zinc circulates in the blood, approximately 80% of which is loosely bound to albumin, and 20% is tightly bound to α2-macroglobulin [15]. Zinc is crucial for the development and physiology of the mammalian brain [16]. The amount of zinc in brain tissue is second only to iron in terms of trace metal concentration [17]. In this regard, while the iron content in the normal brain is around 0.04 mg/g with a concentration of ~ 720 μM, that of zinc is about 10 μg/g with a concentration of 150 μM [18][19]. Timm-Danscher and Nissl stainings were used to analyze the distribution of zinc in the rat brain and revealed that zinc was highly deposited in neuron-rich areas, primarily the hippocampus and cerebral cortex [20][21].
The brain stores zinc in three different forms: vesicular zinc, protein-bound zinc, and zinc ions (Zn2+) [20]. In the CNS, around 85% of zinc is tightly linked to proteins that perform both functional and structural purposes, such as zinc-related metalloenzymes, MMPs, and zinc transcription factors [22][23][24]. Vesicular zinc is mostly present in the synaptic vesicles at the axon terminals of glutamatergic neurons, where it is released in a calcium- and the impulse-dependent way [25]. The concentration of Zn2+ in the brain is roughly ten times higher than that in serum, and it is required for neural regulation, synaptic plasticity, learning, and memory [22].
Given that zinc is unable to diffuse across the cell membrane, specialized transporters or membrane channels are required for its entry into cells [26]. Zinc transporters mainly include metallothioneins (MTs), Zrt-Irt-like proteins (ZIPs), and the zinc transporter family (ZnTs). Zinc enters neurons through three main pathways: α-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate-acid /kainite (Ca2+-A/K) AMPA/KA channels [27], voltage-dependent channels, and N-methyl-D-aspartate (NMDA) receptor-gated channels [28]. In particular, AMPA/KA gated channels can pass more zinc in neurons [29][30][31].
2.1. Role of Zinc in Neurogenesis
Neurogenesis is particularly robust in embryonic and neonatal periods. Zinc plays critical roles in neuronal proliferation and differentiation, neuronal migration, and axonal growth during neurodevelopment [18]. It is necessary for the synthesis of a wide range of proteins, hormones, and growth factors [32]. During embryogenesis, stem cells and neuronal progenitor cells proliferate and differentiate in the neural tube, one of the first brain structures. Zinc has been recognized to be essential for DNA polymerase and other crucial enzymes in the developing brain [21][33]. As a result, zinc shortage during pregnancy leads to abnormal hippocampal proliferation and neuronal differentiation in the fetus, which appears to impact future memory and learning processes and even neurological abnormalities [34][35].
However, neurogenesis is not limited to the developmental phase, and zinc is required for the proliferation, differentiation, and death of neurons throughout the human brain’s life cycle [32]. Wistar females, during pregnancy and lactation, received a drinking water solution of ZnSO4 at an estimated dose of 16 mg/kg. Behavioral tests showed enhanced spatial memory and increased blood zinc levels in the pups [36]. The US recommended dietary allowance for zinc for women during pregnancy is 11.5 ± 1.75 mg/d [37]. Zinc deficiency (<56 μg/dL) during pregnancy and breastfeeding can impede the growth of newborns, increasing the risk of dwarfism, impaired learning memory function, and delayed mental development, whereas zinc supplementation can improve the development of premature newborns [38][39].
2.2. Role of Zinc in Promoting Redox Homeostasis
Several age-related chronic illnesses, including atherosclerosis, cancer, and dementia, display oxidative stress as a contributing component. Reactive oxygen species (ROS) are chemically reactive chemical entities containing oxygen, such as O2·-, H2O2, and OH, which are constantly produced in vivo under aerobic conditions [40][41]. Zinc is a redox-inert metal that participates in redox-regulated signaling. It acts as an antioxidant by accelerating the action of copper/zinc superoxide dismutase [42], maintaining membrane structure, and promoting the synthesis of metallothionein (MT), a metal-binding protein [43]. Zinc helps to maintain cellular redox equilibrium via a variety of processes, including zinc’s dynamic interaction with sulfur in protein cysteine clusters [44], control of oxidant generation and metal-induced oxidative damage [45], and controlling redox signaling by altering enzyme activity, binding interactions, and molecular chaperone activity [46].
Given the complexity of the pathways and events that disruption of zinc homeostasis might influence, the negative effects of these changes are not to be underestimated. The events involved in the regulation of cellular oxidative/antioxidant homeostasis by zinc are diverse and interrelated, including (i) regulation of oxidant production and metal-induced oxidative damage; (ii) regulation of glutathione (GSH) metabolism and overall thiol redox status by zinc; and (iii) direct or indirect regulation of redox signaling [40]. Thus, the dysregulation of zinc homeostasis may have significant adverse effects. Deficits in zinc are associated with oxidative stress, impaired GSH metabolism, tubulin oxidation, and disruptions in redox-sensitive signaling in the developing nervous system [47]. These alterations may lead to altered organ cellularity, organization, and connectivity, thereby increasing the risk of diseases later in life [48][49].
2.3. Role of Zinc on Immunity in the CNS
Patients with zinc deficiency show increased susceptibility to various pathogens [50]. Regarding the human immune system, the innate and adaptive immune systems are both regulated by zinc-finger-bearing transcription factors. Hence, a direct, as well as an indirect role of zinc in altering intracellular signaling, can be anticipated [51]. The role of zinc in immune function is also connected to zinc transporter proteins. Overall, even a minor zinc shortage may elevate pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin 1 beta (IL-1β), and IL-6 [52][53][54].
Microglia are an innate immune cell type found in the CNS. In response to insults, such as infection or other detrimental molecules, microglia are activated and secrete MMPs, ROS, and other pro-inflammatory molecules [55][56]. In models of ischemic stroke and neurodegenerative diseases, zinc chelator TPEN has been shown to prevent neuronal loss by inhibiting microglial activation, which is triggered when neurons release zinc [57]. PARP-1, which can chelate zinc, has been shown to minimize neuronal death in rats with ischemic stroke [58] and other neurodegenerative diseases [59].
3. The Role of Zinc in Stroke
3.1. Ischemic Stroke
As the primary cause of disability, stroke ranks just behind heart disease as the second biggest cause of mortality globally [55]. Ischemic stroke accounts for approximately 80% of all strokes and may be caused by a combination of events such as cardiogenic embolism, obstruction of tiny blood arteries in the brain, and atherosclerosis influencing cerebral circulation [60]. Ischemic stroke is a thrombo-inflammatory disease in which platelets and immune cells accumulate at sites of ischemic vascular damage, disrupting the permeability of the BBB, triggering other processes such as neuroinflammation, microglia activation, and excitotoxicity; these collectively contribute to neuronal death [55][61].
Zinc may provide anti-atherogenic properties by preventing metabolic and physiological dysregulation of the vascular endothelium due to its antioxidant and membrane-stabilizing characteristics [62][63]. The supplement, along with zinc in zinc-deficient endothelial cells, induces a partial repair of the endothelial cell barrier, but supplementation with calcium and magnesium does not achieve the same effect [64]. As a consequence, zinc appears to be required for endothelial integrity, and a deficit might compromise endothelial barrier function [65].
During ischemic stroke, zinc is released from synaptic vesicles of glutamatergic neurons, and the abnormal accumulation of zinc stimulates the activation of microglia [66] and ultimately influences cell survival and function in part through mitochondrial dysfunction [67]. In ischemic stroke, zinc release can directly produce ROS or activate NADPH oxidase to produce ROS, leading to brain injury [68] (Figure 1). Zinc is considered an independent risk factor for ischemic stroke [63][69], and it has been observed to accumulate in the synaptic gap of neurons in animal models of ischemic stroke, speeding up the development of cerebral infarction [70]. As a result, inhibiting zinc over-release may prevent brain injury after ischemic stroke.
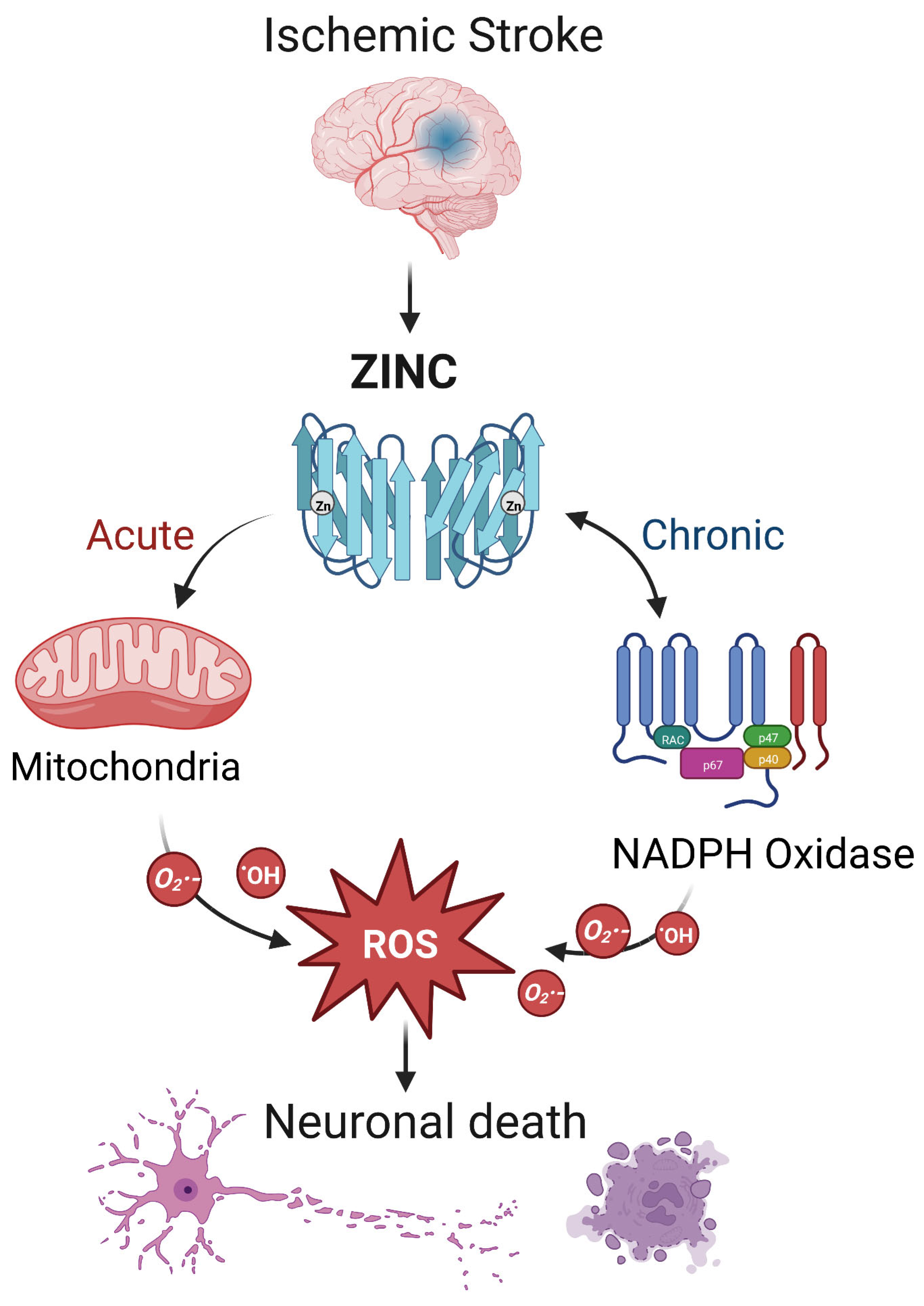
Figure 1. The interaction between zinc and ROS in ischemic stroke. In ischemic stroke, zinc is released from sources such as the synaptic vesicles of glutamatergic neurons. Acutely, zinc may elevate ROS through action on mitochondria. In the chronic phase, zinc accumulation can activate neuronal NADPH oxidases to generate additional oxidative species. The resultant ROS may produce neurotoxicity, further increasing zinc accumulation and ischemic brain injury. Note that while researchers show a central role for ROS, other mechanisms of zinc toxicity exist and are described in the text.
In vitro and animal experiments support a causal relationship between zinc dysregulation and neuronal damage after ischemic stroke. The injection of the zinc chelator ZnEDTA 14 days after middle cerebral artery occlusion (MCAO) in adult male rats led to a significant decrease in infarct volume and neuronal damage and improvement in neurological function [71]. In MCAO rats, zinc-induced CDK5-Tyr15 phosphorylation activates CDK5 in the hippocampus, which exacerbates neuronal death in ischemic stroke [72]. In an experimental model of ischemic stroke, normobaric hyperoxia treatment reduces zinc accumulation in penumbral tissues, thus reducing ischemic injury [73].
Some noteworthy findings about zinc levels have been reported from clinical investigations of patients diagnosed with an ischemic stroke. Serum zinc concentrations were considerably lower in ischemic stroke patients compared to age- and sex-matched healthy controls [74]. Calcium, copper, and iron levels did not change significantly between patients with ischemic stroke and healthy controls; the researchers postulated that low blood zinc concentration is associated with an increased risk of ischemic stroke based on their findings [75]. In a case-cohort study, blood zinc concentrations were inversely linked with the incidence of ischemic stroke, especially in women. This might be related to the function of zinc in the metabolism of sex hormones and the reproductive cycle, but the mechanism underlying the possible interaction is still unclear and warrants further investigation [76]. It appears that ischemic stroke could be a condition that would benefit from preventative zinc supplementation [75].
3.2. Intracerebral Hemorrhage (Hemorrhagic Stroke)
Intracerebral hemorrhage (ICH) is a non-traumatic hemorrhage in the brain parenchyma. ICH is particularly catastrophic, with a high mortality rate of up to 50% of the survivors. Over 70% are dependent on functioning aids a year after the event [77][78][79]. Despite clinical advances, including the continued evolution of minimally invasive surgical procedures to remove the blood clot, the prognosis of ICH remains poor [80][81][82]. A number of factors play a role in the pathogenesis of ICH, including hypertension, diabetes, lipid metabolism problems, and genetics, but also changes in the levels of essential trace elements and heavy metals [69][83][84][85].
Zinc is involved in coagulation’s intrinsic pathway, where it binds directly to the XII factor and increases its sensitivity to enzymatic activation [86]. Therefore, both animal models and patients with zinc deficiency have been shown to have prolonged bleeding times as a result of impaired coagulation cascades and fibrin formation [87]. Similarly, after platelets are activated at the site of injury, zinc binds to fibrinogen and fibrin, promoting the formation of a fibrin network [87][88]. Patients with ICH had lower zinc levels than normal controls (0.13 ± 0.02 vs. 3.17 ± 0.74 μg/dI); p < 0.001) [89]. It has been determined that plasma zinc levels and the risk of first-ever stroke in hypertensive patients are significantly correlated with the risk of ICH in a recent study [90]. Previously, a retrospective cohort study examined the relationship between hypozincemia and the severity of aneurysmal subarachnoid hemorrhage. The findings indicated that zinc deficiency (plasma zinc concentration < 10 μmol/L) is associated with a more severe course of the disease. As a result, the authors concluded that zinc deficiency independently contributes to an unfavorable outcome [91].
References
- Prasad, A.S. Discovery of human zinc deficiency: Its impact on human health and disease. Adv. Nutr. 2013, 4, 176–190.
- Sanna, A.; Firinu, D.; Zavattari, P.; Valera, P. Zinc Status and Autoimmunity: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 68.
- McAllister, B.B.; Dyck, R.H. A new role for zinc in the brain. eLife 2017, 6, e31816.
- Nakatani, S.; Mori, K.; Shoji, T.; Emoto, M. Association of Zinc Deficiency with Development of CVD Events in Patients with CKD. Nutrients 2021, 13, 1680.
- Begum, F.; Me, H.M.; Christov, M. The Role of Zinc in Cardiovascular Disease. Cardiol. Rev. 2022, 30, 100–108.
- Vujasinovic, M.; Hedstrom, A.; Maisonneuve, P.; Valente, R.; von Horn, H.; Lohr, J.M.; Haas, S.L. Zinc deficiency in patients with chronic pancreatitis. World J. Gastroenterol. 2019, 25, 600–607.
- Swardfager, W.; Herrmann, N.; McIntyre, R.S.; Mazereeuw, G.; Goldberger, K.; Cha, D.S.; Schwartz, Y.; Lanctot, K.L. Potential roles of zinc in the pathophysiology and treatment of major depressive disorder. Neurosci. Biobehav. Rev. 2013, 37, 911–929.
- Wessells, K.R.; Brown, K.H. Estimating the global prevalence of zinc deficiency: Results based on zinc availability in national food supplies and the prevalence of stunting. PLoS ONE 2012, 7, e50568.
- Adamo, A.M.; Zago, M.P.; Mackenzie, G.G.; Aimo, L.; Keen, C.L.; Keenan, A.; Oteiza, P.I. The role of zinc in the modulation of neuronal proliferation and apoptosis. Neurotox. Res. 2010, 17, 1.
- Portbury, S.D.; Adlard, P.A. Zinc Signal in Brain Diseases. Int. J. Mol. Sci. 2017, 18, 2506.
- Choi, S.; Hong, D.K.; Choi, B.Y.; Suh, S.W. Zinc in the Brain: Friend or Foe? Int. J. Mol. Sci. 2020, 21, 8941.
- Bertoni-Freddari, C.; Fattoretti, P.; Casoli, T.; Di Stefano, G.; Giorgetti, B.; Balietti, M. Brain aging: The zinc connection. Exp. Gerontol. 2008, 43, 389–393.
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784.
- Solomons, N.W. Update on zinc biology. Ann Nutr Metab 2013, 62 (Suppl. 1), 8–17.
- King, J.C.; Shames, D.M.; Woodhouse, L.R. Zinc homeostasis in humans. J. Nutr. 2000, 130, 1360S–1366S.
- Bitanihirwe, B.K.; Cunningham, M.G. Zinc: The brain’s dark horse. Synapse 2009, 63, 1029–1049.
- Marger, L.; Schubert, C.R.; Bertrand, D. Zinc: An underappreciated modulatory factor of brain function. Biochem. Pharmacol. 2014, 91, 426–435.
- Sensi, S.L.; Paoletti, P.; Bush, A.I.; Sekler, I. Zinc in the physiology and pathology of the CNS. Nat. Rev. Neurosci. 2009, 10, 780–791.
- Li, Z.; Liu, Y.; Wei, R.; Khan, S.; Zhang, R.; Zhang, Y.; Yong, V.W.; Xue, M. Iron Neurotoxicity and Protection by Deferoxamine in Intracerebral Hemorrhage. Front. Mol. Neurosci. 2022, 15, 927334.
- Frederickson, C.J. Neurobiology of zinc and zinc-containing neurons. Int. Rev. Neurobiol. 1989, 31, 145–238.
- Suh, S.W.; Won, S.J.; Hamby, A.M.; Yoo, B.H.; Fan, Y.; Sheline, C.T.; Tamano, H.; Takeda, A.; Liu, J. Decreased brain zinc availability reduces hippocampal neurogenesis in mice and rats. J. Cereb. Blood Flow Metab. 2009, 29, 1579–1588.
- Koh, J.Y. Zinc and disease of the brain. Mol. Neurobiol. 2001, 24, 99–106.
- Parisi, A.F.; Vallee, B.L. Zinc metalloenzymes: Characteristics and significance in biology and medicine. Am. J. Clin. Nutr. 1969, 22, 1222–1239.
- Liu, Y.; Mu, Y.; Li, Z.; Yong, V.W.; Xue, M. Extracellular matrix metalloproteinase inducer in brain ischemia and intracerebral hemorrhage. Front. Immunol. 2022, 13, 986469.
- Toth, K. Zinc in neurotransmission. Annu. Rev. Nutr. 2011, 31, 139–153.
- Gibson, R.S.; Ferguson, E.L. Assessment of dietary zinc in a population. Am. J. Clin. Nutr. 1998, 68, 430S–434S.
- Kerchner, G.A.; Canzoniero, L.M.; Yu, S.P.; Ling, C.; Choi, D.W. Zn2+ current is mediated by voltage-gated Ca2+ channels and enhanced by extracellular acidity in mouse cortical neurones. J. Physiol. 2000, 528 Pt 1, 39–52.
- Koh, J.Y.; Choi, D.W. Zinc toxicity on cultured cortical neurons: Involvement of N-methyl-D-aspartate receptors. Neuroscience 1994, 60, 1049–1057.
- Marin, P.; Israel, M.; Glowinski, J.; Premont, J. Routes of zinc entry in mouse cortical neurons: Role in zinc-induced neurotoxicity. Eur. J. Neurosci. 2000, 12, 8–18.
- Karakas, E.; Simorowski, N.; Furukawa, H. Structure of the zinc-bound amino-terminal domain of the NMDA receptor NR2B subunit. EMBO J. 2009, 28, 3910–3920.
- Weiss, J.H.; Sensi, S.L. Ca2+-Zn2+ permeable AMPA or kainate receptors: Possible key factors in selective neurodegeneration. Trends Neurosci. 2000, 23, 365–371.
- Kumar, V.; Kumar, A.; Singh, K.; Avasthi, K.; Kim, J.J. Neurobiology of zinc and its role in neurogenesis. Eur. J. Nutr. 2021, 60, 55–64.
- Levenson, C.W.; Morris, D. Zinc and neurogenesis: Making new neurons from development to adulthood. Adv. Nutr. 2011, 2, 96–100.
- Chudasama, Y.; Wright, K.S.; Murray, E.A. Hippocampal lesions in rhesus monkeys disrupt emotional responses but not reinforcer devaluation effects. Biol. Psychiatr. 2008, 63, 1084–1091.
- Goeldner, C.; Reiss, D.; Wichmann, J.; Meziane, H.; Kieffer, B.L.; Ouagazzal, A.M. Nociceptin receptor impairs recognition memory via interaction with NMDA receptor-dependent mitogen-activated protein kinase/extracellular signal-regulated kinase signaling in the hippocampus. J. Neurosci. 2008, 28, 2190–2198.
- Piechal, A.; Blecharz-Klin, K.; Pyrzanowska, J.; Widy-Tyszkiewicz, E. Maternal zinc supplementation improves spatial memory in rat pups. Biol. Trace Elem. Res. 2012, 147, 299–308.
- National Research Council. Recommended Dietary Allowances, 10th ed.; Reports funded by National Institutes of Health; The National Academies Collection: Washington, DC, USA, 1989.
- Yu, X.; Chen, W.; Wei, Z.; Ren, T.; Yang, X.; Yu, X. Effects of maternal mild zinc deficiency and different ways of zinc supplementation for offspring on learning and memory. Food Nutr. Res. 2016, 60, 29467.
- Wang, H.; Hu, Y.F.; Hao, J.H.; Chen, Y.H.; Su, P.Y.; Wang, Y.; Yu, Z.; Fu, L.; Xu, Y.Y.; Zhang, C.; et al. Maternal zinc deficiency during pregnancy elevates the risks of fetal growth restriction: A population-based birth cohort study. Sci. Rep. 2015, 5, 11262.
- Oteiza, P.I. Zinc and the modulation of redox homeostasis. Free Radic. Biol. Med. 2012, 53, 1748–1759.
- Zhao, X.; Drlica, K. Reactive oxygen species and the bacterial response to lethal stress. Curr. Opin. Microbiol. 2014, 21, 1–6.
- Maret, W. The redox biology of redox-inert zinc ions. Free Radic. Biol. Med. 2019, 134, 311–326.
- Hernandez-Camacho, J.D.; Vicente-Garcia, C.; Parsons, D.S.; Navas-Enamorado, I. Zinc at the crossroads of exercise and proteostasis. Redox Biol. 2020, 35, 101529.
- Hempe, J.M.; Cousins, R.J. Cysteine-rich intestinal protein binds zinc during transmucosal zinc transport. Proc. Natl. Acad. Sci. USA 1991, 88, 9671–9674.
- Baltaci, A.K.; Yuce, K.; Mogulkoc, R. Zinc Metabolism and Metallothioneins. Biol. Trace Elem. Res. 2018, 183, 22–31.
- Maret, W. Zinc coordination environments in proteins as redox sensors and signal transducers. Antioxid. Redox Sign. 2006, 8, 1419–1441.
- Mehta, A.J.; Yeligar, S.M.; Elon, L.; Brown, L.A.; Guidot, D.M. Alcoholism causes alveolar macrophage zinc deficiency and immune dysfunction. Am. J. Respir. Crit. Care Med. 2013, 188, 716–723.
- Joshi, P.C.; Mehta, A.; Jabber, W.S.; Fan, X.; Guidot, D.M. Zinc deficiency mediates alcohol-induced alveolar epithelial and macrophage dysfunction in rats. Am. J. Respir. Cell Mol. Biol. 2009, 41, 207–216.
- Ayton, S.; Lei, P.; Appukuttan, A.T.; Renoir, T.; Foliaki, S.; Chen, F.; Adlard, P.A.; Hannan, A.J.; Bush, A.I. Brain Zinc Deficiency Exacerbates Cognitive Decline in the R6/1 Model of Huntington’s Disease. Neurotherapeutics 2020, 17, 243–251.
- Ibs, K.H.; Rink, L. Zinc-altered immune function. J. Nutr. 2003, 133, 1452S–1456S.
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017, 9, 1286.
- Gammoh, N.Z.; Rink, L. Zinc in Infection and Inflammation. Nutrients 2017, 9, 624.
- Wessels, I.; Haase, H.; Engelhardt, G.; Rink, L.; Uciechowski, P. Zinc deficiency induces production of the proinflammatory cytokines IL-1beta and TNFalpha in promyeloid cells via epigenetic and redox-dependent mechanisms. J. Nutr. Biochem. 2013, 24, 289–297.
- Kahmann, L.; Uciechowski, P.; Warmuth, S.; Plumakers, B.; Gressner, A.M.; Malavolta, M.; Mocchegiani, E.; Rink, L. Zinc supplementation in the elderly reduces spontaneous inflammatory cytokine release and restores T cell functions. Rejuvenation Res. 2008, 11, 227–237.
- Xue, M.; Yong, V.W. Neuroinflammation in intracerebral haemorrhage: Immunotherapies with potential for translation. Lancet Neurol. 2020, 19, 1023–1032.
- Liu, Y.; Li, Z.; Khan, S.; Zhang, R.; Wei, R.; Zhang, Y.; Xue, M.; Yong, V.W. Neuroprotection of minocycline by inhibition of extracellular matrix metalloproteinase inducer expression following intracerebral hemorrhage in mice. Neurosci. Lett. 2021, 764, 136297.
- Haase, H.; Rink, L. Functional significance of zinc-related signaling pathways in immune cells. Annu. Rev. Nutr. 2009, 29, 133–152.
- Chen, J.; Li, X.; Xu, S.; Zhang, M.; Wu, Z.; Zhang, X.; Xu, Y.; Chen, Y. Delayed PARP-1 Inhibition Alleviates Post-stroke Inflammation in Male Versus Female Mice: Differences and Similarities. Front. Cell. Neurosci 2020, 14, 77.
- Kauppinen, T.M.; Suh, S.W.; Higashi, Y.; Berman, A.E.; Escartin, C.; Won, S.J.; Wang, C.; Cho, S.H.; Gan, L.; Swanson, R.A. Poly(ADP-ribose)polymerase-1 modulates microglial responses to amyloid beta. J. Neuroinflamm. 2011, 8, 152.
- Maida, C.D.; Norrito, R.L.; Daidone, M.; Tuttolomondo, A.; Pinto, A. Neuroinflammatory Mechanisms in Ischemic Stroke: Focus on Cardioembolic Stroke, Background, and Therapeutic Approaches. Int. J. Mol. Sci. 2020, 21, 6454.
- Zhang, X.; Zhang, Y.; Wang, F.; Liu, Y.; Yong, V.W.; Xue, M. Necrosulfonamide Alleviates Acute Brain Injury of Intracerebral Hemorrhage via Inhibiting Inflammation and Necroptosis. Front. Mol. Neurosci. 2022, 15, 916249.
- Hennig, B.; Toborek, M.; McClain, C.J. Antiatherogenic properties of zinc: Implications in endothelial cell metabolism. Nutrition 1996, 12, 711–717.
- Grungreiff, K.; Gottstein, T.; Reinhold, D. Zinc Deficiency-An Independent Risk Factor in the Pathogenesis of Haemorrhagic Stroke? Nutrients 2020, 12, 3548.
- Hennig, B.; Wang, Y.; Ramasamy, S.; McClain, C.J. Zinc deficiency alters barrier function of cultured porcine endothelial cells. J. Nutr. 1992, 122, 1242–1247.
- Shuttleworth, C.W.; Weiss, J.H. Zinc: New clues to diverse roles in brain ischemia. Trends Pharmacol. Sci. 2011, 32, 480–486.
- Bu, S.; Lv, Y.; Liu, Y.; Qiao, S.; Wang, H. Zinc Finger Proteins in Neuro-Related Diseases Progression. Front. Neurosci. 2021, 15, 760567.
- Mammadova-Bach, E.; Braun, A. Zinc Homeostasis in Platelet-Related Diseases. Int. J. Mol. Sci. 2019, 20, 5258.
- Zhou, X.; Cooper, K.L.; Sun, X.; Liu, K.J.; Hudson, L.G. Selective Sensitization of Zinc Finger Protein Oxidation by Reactive Oxygen Species through Arsenic Binding. J. Biol. Chem. 2015, 290, 18361–18369.
- Zhang, Y.; Khan, S.; Liu, Y.; Siddique, R.; Zhang, R.; Yong, V.W.; Xue, M. Gap Junctions and Hemichannels Composed of Connexins and Pannexins Mediate the Secondary Brain Injury Following Intracerebral Hemorrhage. Biology 2021, 11, 27.
- Weiss, J.H.; Sensi, S.L.; Koh, J.Y. Zn(2+): A novel ionic mediator of neural injury in brain disease. Trends Pharmacol. Sci. 2000, 21, 395–401.
- Lee, J.M.; Zipfel, G.J.; Park, K.H.; He, Y.Y.; Hsu, C.Y.; Choi, D.W. Zinc translocation accelerates infarction after mild transient focal ischemia. Neuroscience 2002, 115, 871–878.
- Tuo, Q.Z.; Liuyang, Z.Y.; Lei, P.; Yan, X.; Shentu, Y.P.; Liang, J.W.; Zhou, H.; Pei, L.; Xiong, Y.; Hou, T.Y.; et al. Zinc induces CDK5 activation and neuronal death through CDK5-Tyr15 phosphorylation in ischemic stroke. Cell Death Dis. 2018, 9, 870.
- Dong, W.; Qi, Z.; Liang, J.; Shi, W.; Zhao, Y.; Luo, Y.; Ji, X.; Liu, K.J. Reduction of zinc accumulation in mitochondria contributes to decreased cerebral ischemic injury by normobaric hyperoxia treatment in an experimental stroke model. Exp. Neurol. 2015, 272, 181–189.
- Munshi, A.; Babu, S.; Kaul, S.; Shafi, G.; Rajeshwar, K.; Alladi, S.; Jyothy, A. Depletion of serum zinc in ischemic stroke patients. Methods Find. Exp. Clin. Pharmacol. 2010, 32, 433–436.
- Mattern, L.; Chen, C.; McClure, L.A.; Brockman, J.; Cushman, M.; Judd, S.; Kahe, K. Serum Zinc Levels and Incidence of Ischemic Stroke: The Reasons for Geographic and Racial Differences in Stroke Study. Stroke 2021, 52, 3953–3960.
- Howard, V.J.; Madsen, T.E.; Kleindorfer, D.O.; Judd, S.E.; Rhodes, J.D.; Soliman, E.Z.; Kissela, B.M.; Safford, M.M.; Moy, C.S.; McClure, L.A.; et al. Sex and Race Differences in the Association of Incident Ischemic Stroke With Risk Factors. JAMA Neurol. 2019, 76, 179–186.
- Shao, Z.; Tu, S.; Shao, A. Pathophysiological Mechanisms and Potential Therapeutic Targets in Intracerebral Hemorrhage. Front. Pharmacol. 2019, 10, 1079.
- Keep, R.F.; Hua, Y.; Xi, G. Intracerebral haemorrhage: Mechanisms of injury and therapeutic targets. Lancet Neurol. 2012, 11, 720–731.
- Li, Z.; Liu, Y.; Wei, R.; Khan, S.; Xue, M.; Yong, V.W. The combination of deferoxamine and minocycline strengthens neuroprotective effect on acute intracerebral hemorrhage in rats. Neurol. Res. 2021, 43, 854–864.
- Hanley, D.F.; Thompson, R.E.; Rosenblum, M.; Yenokyan, G.; Lane, K.; McBee, N.; Mayo, S.W.; Bistran-Hall, A.J.; Gandhi, D.; Mould, W.A.; et al. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): A randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet 2019, 393, 1021–1032.
- Bai, Q.; Xue, M.; Yong, V.W. Microglia and macrophage phenotypes in intracerebral haemorrhage injury: Therapeutic opportunities. Brain 2020, 143, 1297–1314.
- Liu, Y.; Bai, Q.; Yong, V.W.; Xue, M. EMMPRIN Promotes the Expression of MMP-9 and Exacerbates Neurological Dysfunction in a Mouse Model of Intracerebral Hemorrhage. Neurochem. Res. 2022, 47, 2383–2395.
- Levenson, C.W. Trace metal regulation of neuronal apoptosis: From genes to behavior. Physiol. Behav. 2005, 86, 399–406.
- Zhang, Y.; Khan, S.; Liu, Y.; Wu, G.; Yong, V.W.; Xue, M. Oxidative Stress Following Intracerebral Hemorrhage: From Molecular Mechanisms to Therapeutic Targets. Front. Immunol. 2022, 13, 847246.
- Bai, Q.; Sheng, Z.; Liu, Y.; Zhang, R.; Yong, V.W.; Xue, M. Intracerebral haemorrhage: From clinical settings to animal models. Stroke Vasc. Neurol. 2020, 5, 388–395.
- Bernardo, M.M.; Day, D.E.; Halvorson, H.R.; Olson, S.T.; Shore, J.D. Surface-independent acceleration of factor XII activation by zinc ions. II. Direct binding and fluorescence studies. J. Biol. Chem. 1993, 268, 12477–12483.
- Taylor, K.A.; Pugh, N. The contribution of zinc to platelet behaviour during haemostasis and thrombosis. Metallomics 2016, 8, 144–155.
- Vu, T.T.; Fredenburgh, J.C.; Weitz, J.I. Zinc: An important cofactor in haemostasis and thrombosis. Thromb. Haemost. 2013, 109, 421–430.
- Karadas, S.; Sayin, R.; Aslan, M.; Gonullu, H.; Kati, C.; Dursun, R.; Duran, L.; Gonullu, E.; Demir, H. Serum levels of trace elements and heavy metals in patients with acute hemorrhagic stroke. J. Membr. Biol. 2014, 247, 175–180.
- Zhang, J.; Cao, J.; Zhang, Y.; Li, H.; Zhang, H.; Huo, Y.; Li, J.; Liu, X.; Wang, X.; Qin, X.; et al. Baseline Plasma Zinc and Risk of First Stroke in Hypertensive Patients: A Nested Case-Control Study. Stroke 2019, 50, 3255–3258.
- Arleth, T.; Olsen, M.H.; Orre, M.; Rasmussen, R.; Bache, S.; Eskesen, V.; Frikke-Schmidt, R.; Moller, K. Hypozincaemia is associated with severity of aneurysmal subarachnoid haemorrhage: A retrospective cohort study. Acta Neurochir. (Wien) 2020, 162, 1417–1424.
More
Information
Subjects:
Others
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Entry Collection:
Neurodegeneration
Revisions:
2 times
(View History)
Update Date:
13 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




