1. Polylactic Acid (PLA)
The PLA is one of the most widely used polymer materials for polymeric scaffolds in tissue engineering application because it has natural advantages of good mechanical properties, degradability, biocompatibility, and low cost
[1]. However, the PLA has some limitations. For example, its hydrophobicity may undermine its biocompatibility. Human cells may be damaged if they are exposed to lactic acid, a degradation product of the PLA, for long periods of time
[2]. Different parameters may result in the PLA exhibiting different mechanical properties, such as crystallinity, molecular weight, and processing
[3]. The properties of the PLA can also be changed by tuning the material formulation, such as adding plasticizers, preparing blends, or composites with other materials. Thus, some chemical constituents are normally added into the PLA to improve various properties
[4].
Llorens et al.
[5] prepared the PLA nanofibers equipped with polybiguanide (PHMB) and with an average diameter between 560 and 630 nm by using the electrostatic spinning method. The PHMB-loaded PLA scaffolds have antimicrobial properties. On the one hand, the loading of PHMB increases the hydrophobicity of the scaffold, making it difficult for bacteria to adhere. On the other hand, the PHMB, as a cationic oligomer, has strong antibacterial activity. Scaffolds prepared by the electrostatic spinning method have porous structures that facilitate cell adhesion and proliferation. However, high concentrations of the PHMB are toxic to human cells, so it is important to control the concentration of the drug in the scaffolds and the rate of scaffold degradation. The experimental results showed that the concentration of PHMB did not affect the growth of cells when it was lower than 1.5 wt%. The PHMB-loaded PLA scaffolds showed biocompatibility in terms of the adhesion and proliferation of fibroblast and epithelial cell lines. Moreover, the slow and controlled drug release allowed the addition of PHMB in the scaffold at higher than safe concentrations.
Han et al.
[2] proposed an innovative method for the preparation of PLA/chitosan (CS) composite films by using non-solvent induce phase separation (NIPS). The PLA/CS films prepared using the NIPS are more hydrophilic than those prepared using the casting method, which will result in better biocompatibility and a faster degradation rate of the films. The films prepared using the NIPS can also adjust the pore size of the porous structure to regulate the degradation rate. The PLA-based films were tested for their antibacterial activity against
E. coli. The results showed that the antibacterial ability of the pure PLA film is not obvious due to the polymer structure of the PLA. The addition of CS greatly increases the antimicrobial activity, indicating that the CS is the main antimicrobial component in the film.
The porous film was experimentally demonstrated to be degradable, self-supporting, antibacterial, and transparent. The degradation of CS releases small alkaline molecules, which can neutralize the acidic degradation products of PLA and avoid some inflammation, resulting from acidic degradation to a certain extent.
Douglass et al.
[6] combined the nitric oxide (NO) donor S-nitrosoglutathione (GSNO) with polyhydroxybutyrate (PHB) and the fiber-grade PLA for the manufacture of antimicrobial NO releasing nanofibers. In this study, the PLA was first mixed with the PHB in solution, stirred for 2 h, and then the GSNO was added into the solution. The fibers are formed by electrostatic spinning after 4 h. The fiber grade PLA synthesized with different length−diameter ratios overcomes some of its disadvantages, such as poor heat resistance and fragility. When the PLA was mixed with the PHB by a ratio of 3:1, the blends showed the best plasticity and maintained a certain tensile strength. The GSNO was added as a source of NO release because of the great role of NO in regulating antimicrobial behavior in blood vessels. Compared to PLA/PHB fibers, GSNO-containing fibers showed a significant reduction in colony forming units (CFU) measurements of
S. aureus after both 2 and 24 h of exposure. After 2 h exposure, the bacterial survival rate of fibers containing GSNO was significantly lower than that of PLA/PHB fibers. The bacterial cell membrane on PLA/PHB remains intact, while that on PLA/PHB + 20 wt% GSNO is disrupted. This is due to the release of NO that disrupts the cell membrane.
Although the release of NO helps to kill bacteria, high concentrations of NO will be toxic to mammalian cells. Therefore, mouse fibroblasts were exposed to a 24 h leachate of fibers. The result shows that none of the fibers caused a significant decrease in cell viability compared to cells that were not exposed to the leachate. This demonstrates that nanofibers are not cytotoxic to mammalian cells. Therefore, NO-releasing nanofibers are a promising coating for blood-contacting medical devices.
Sharif et al.
[7] synthesized composite scaffolds of the PLA/PCL blended with nano-hydroxyapatite (n(HA)) and cefixime-β cyclodextrin (Cfx-βCD) by electrospinning. In this study, the PLA and the PCL were mixed with the HA, Cfx, and Cfx-βCD to form composites film by electrospinning. Their antibacterial ability and effects on cell growth were compared. The antibacterial activity of the membrane was determined by using the
S. aureus strain. After adding Cfx, the number of bacteria on the membrane decreased significantly within 24 h. The number of bacteria on the membrane continued to decrease when HA was added to the composite membrane. The best antibacterial activity was PLA-PCL-βCD-Cfx (PPH-βCD-Cfx) composite membrane.
The mouse pre-osteblast cell line (MC3T3) was cultured on the membrane to evaluate the cell viability on different membranes. The MC3T3 can attach and proliferate on all three membranes.
The addition of HA improved the osteoconduction of the composite. The βCD realizes the control of drug release as a carrier of antibiotics. The membranes were shown to have good antibacterial properties and to promote cell proliferation and attachment.
2. Polycaprolactone (PCL)
The PCL is a medical synthetic polymer with biocompatible and biodegradable properties. Due to its flexibility, low density, and easy processing, it has been widely used in tissue engineering scaffolds. In addition, it has good mechanical strength, rigidity, and heat resistance
[8]. Its breakdown products form naturally occurring metabolites, which are readily metabolized by the body and eliminated without toxicity
[9].
In previous research study, natural antimicrobial compounds, such as curcumin, piperine, eugenol, and rutin, were loaded into electrospun nanofibers based on the PCL
[10]. A wound dressing was prepared by electrospinning. The SEM images showed that the nanofibers prepared by electrospinning were more uniform after adding curcumin and piperine. The diameter of the fiber can vary with the change in curcumin concentration. Novel three-component systems of curcumin–piperonin–eugenol (PCPiEu) and curcumin–piperonin–rutin (PCPiR) were designed and prepared. The growth of
S. aureus in the presence of different wound dressings was studied. The growth of bacteria on pure PCL is tremendous, and so pure PCL has no antibacterial activity. With either the addition of curcumin or piperine, the number of bacteria was greatly reduced. In addition, the PC has almost no antibacterial activity against Enterococcus faecalis (Gram-negative), while both PCPiEu and PCPiR have a killing rate of more than 95%.
The MTT test was performed on different wound dressings using human fibroblasts to evaluate the cytotoxicity of wound dressings. The experiment proved that the pure PCL exhibited the highest amount of cell proliferation, which proves that the PCL has high biocompatibility. The result shows that the cell viability of piperine based on PCL samples is the lowest, which proves that piperine has cytotoxicity. The cell viability of PCR samples was higher than 100%, indicating that it was favorable for cell growth. In the three-component samples, the cell viability of PCPiEu and PCPiR was more than 90%. It seems that a better effect can be achieved by adjusting the amount of these natural compounds in the sample.
In general, the three-component wound dressing obtained good results in both the antibacterial test and the cytotoxicity test. In particular, it showed good antibacterial activity against Gram-negative bacteria. Although the mechanical properties of the system decreased compared with the PCL, the three-component wound dressing showed broad-spectrum antibacterial activity.
In another study, graphene (GP), bioglass, and zinc-doped bioglass were added to the PCL filaments, and their antimicrobial activity was analyzed comparatively. Materials for research were produced using the 3D-printing technique. The experimental results showed that the addition of a small amount of GP (0.5%) to PCL filaments resulted in a significant increase in antimicrobial activity compared to the pure PCL. This is because the structure of the GP may cause damage to the cell membranes of microbes and thus eliminate them
[11].
Recently, PCL nanofibers containing Atropa belladonna were fabricated using the electrospinning technique
[12]. The fruits, roots, and stems of belladonna are used in the treatment of many diseases and have strong antioxidant and anticancer properties. In this study, Atropa belladonna extract was used to encapsulate Ag nanoparticles (AgNPs).
This study concludes that both AgNPs and eAgNPs improved the antibacterial activity of PCL nanofibers against Gram-negative and Gram-positive bacteria. In addition, the cell viability of the PCL doped with eAgNPs was higher compared to the neat PCL. The main reasons for the greater cell viability of the PCL doped with eAgNPs may be that it is more hydrophilic than the pure PCL, the toxicity of AgNPs is reduced by coating on the surface of the nanoparticles, and the positive effect of free radicals in the Atropa belladonna structure on cell proliferation.
Felice et al.
[13] synthesized PCL scaffolds compounded with hydroxyapatite (HA) and different concentrations of zinc oxide (ZnO) for bone tissue engineering by electrospinning techniques. The ZnO is an inorganic material with osteoinductive and osteoconductive properties. It has been reported that Zn
2+ can induce osteoblast differentiation. In addition, ZnO is considered an effective antimicrobial agent against broad-spectrum microorganisms. The addition of HA is beneficial to increase the osteoconductivity of the scaffold and can shorten the degradation time of the PCL. To assess the antimicrobial activity of the scaffolds, a series of samples were immersed in the PBS at 37 °C for 0 and 30 days for in vitro degradation, respectively. Sterile samples were immersed in broth containing
S. aureus for 18 h at 35 ± 2 °C, and the surviving CFUs were counted. The surviving CFU of samples after 0 and 30 days of degradation were compared after 18 h of bacterial incubation, respectively. The results showed that the presence of ZnO in the samples degraded for 0 days led to a reduction in the initial bacterial load compared with the negative control group. The higher the concentration of ZnO, the higher the antibacterial activity. On the PCL scaffold containing 6% ZnO, this corresponds to a 96% reduction in bacteria. On the PCL scaffold containing the HA and 1% ZnO, an almost 99% reduction of the initial bacterial load was achieved. Notably, on the scaffold containing the HA, the antibacterial activity decreased instead with increasing the ZnO concentration.
Human fetal osteoblast cell line (HFOb) proliferation was assessed after 3, 7, and 14 days of incubation on PCL, PCL:HA, and PCL:HA:ZnO nanofiber scaffolds. On the PCL and PCL:HA:ZnO 1%, cell proliferation increased exponentially with time. In contrast, cell proliferation in the other samples was constant from day 3 or from day 7. In addition, higher concentrations of ZnO may lead to reduced cell proliferation.
These scaffolds have been shown to have an antibacterial effect against S. aureus. This activity rises with increasing levels of the ZnO. However, high concentrations of the ZnO may reduce cell proliferation. Therefore, low-concentration ZnO scaffolds may be promising regenerative medicine products with antibacterial ability.
3. Polyglycolic Acid (PGA)
The PGA is a semi-crystalline synthetic polymer with good biocompatibility and biodegradability. Once it degrades, the non-crystalline part first will degrade to glycolic acid which can be readily metabolized by the body; the crystalline part then will degrade to harmless water and carbon dioxide
[14]. Like the PLA, it produces acidic degradation products that may trigger inflammation. The PGA has high mechanical strength and high crystallinity. However, the PGA has poor toughness and a relatively high price. Therefore, the PGA is always blended with other polymer materials.
Shuai et al.
[15] prepared polymer scaffolds by laser sintering. The PGA solution and the PLLA solution were mixed, stirred, and ultrasonically dispersed. Meanwhile, the graphene oxide (GO) solution and the nano Ag solution were mixed, stirred, and ultrasonically dispersed. Finally, the two solutions were mixed together, filtered, and dried to obtain powders. The powders were sintered layer by layer to form 3D scaffolds. The SEM images show that AgNPs and GO are evenly distributed. Both the GO and nano Ag are easy to form agglomeration, so uniform dispersion is very important for the antibacterial activity of polymer scaffolds. The
E. coli suspension was placed together with scaffolds with different GO and Ag ratios for 24 h, and the antibacterial activity of the scaffolds was evaluated by turbidimetry. Only when GO exists, the antibacterial effect is not obvious. The antibacterial effect was significantly improved only when Ag was present. When GO and Ag were simultaneously present, the antibacterial effect was further increased.
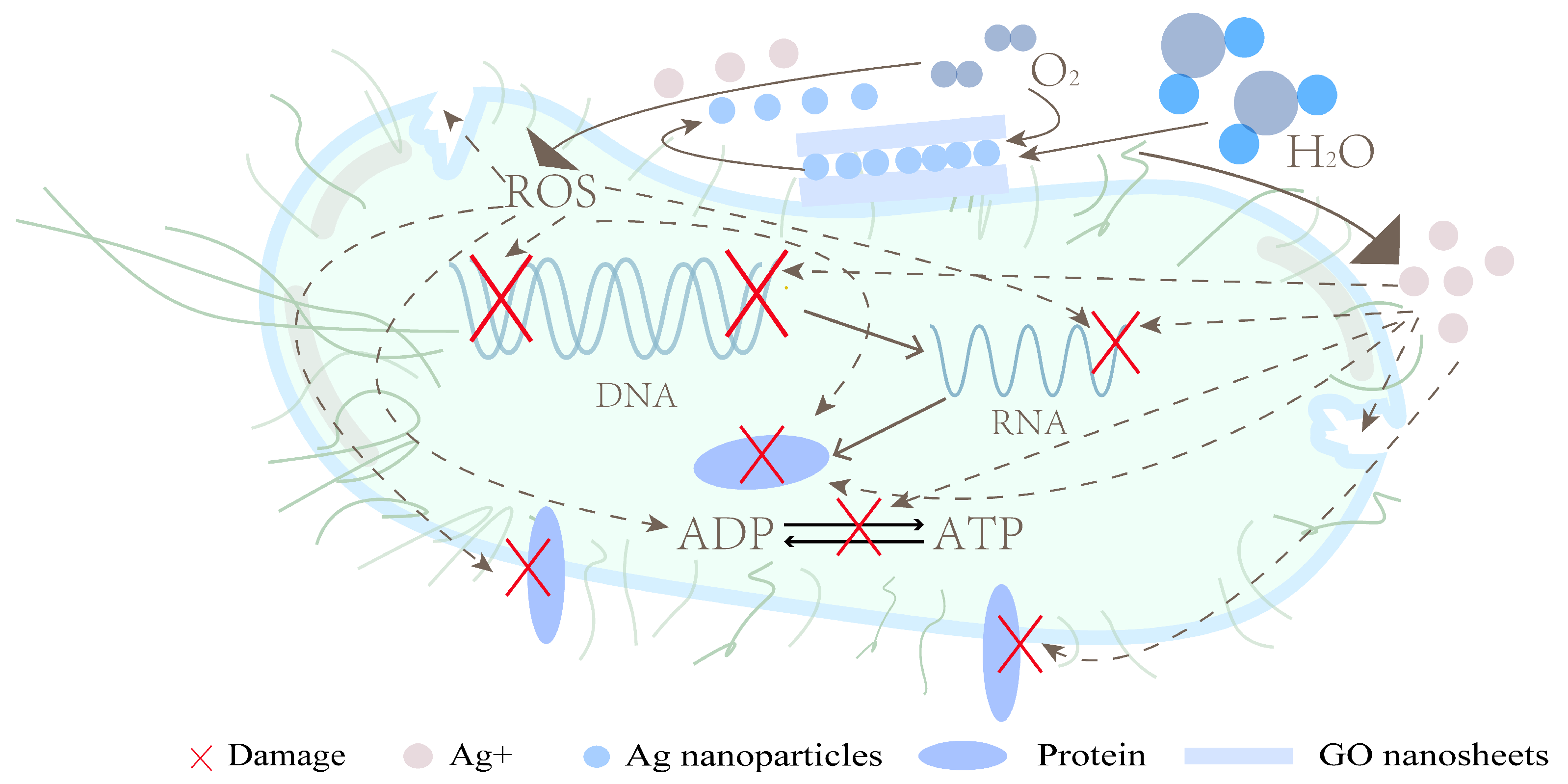
The GO-Ag nanosystem showed a synergistic antibacterial effect by combining the trapping effect of GO nanosheets with the killing effect of Ag. The GO can interact with bacterial cell membranes and adsorb on bacterial cells, resulting in an increased concentration of AgNPs around the bacteria. The antibacterial effect of AgNPs mainly depends on the release of Ag+ and the promotion of reactive oxygen species (ROS) production. Figure 1 shows the synergistic antibacterial mechanism of the GO-Ag.
Figure 1. Synergistic antibacterial mechanism of GO-Ag.
Among them, the scaffolds containing 1 wt% GO and 1 wt% Ag showed good cytocompatibility without affecting MG63 cell adhesion, viability, and proliferation because the presence of the GO has a positive effect on cell adhesion, and excessive Ag has a negative effect.
Wu et al.
[16] found that total alkaloids from Semen Strychnine (TASS) was loaded into polyetheretherketone (PEEK)/PGA composite scaffolds prepared by 3D printing technology to obtain long-lasting antibacterial activity. The PEEK and PGA were dissolved in ethanol solution at a mass ratio of 6:4, and then different levels of TASS were dissolved in the PEEK/PGA suspension. The TASS-PEEK/PGA suspension was stirred magnetically for 2 h, and then the suspension was dried at 50 °C until the precipitate was of constant weight. Finally, the precipitate was ground homogeneously, and the scaffolds were prepared by selective laser sintering technique. The TASS has antibacterial, anti-inflammatory, and analgesic properties. The relationship between the antimicrobial activity of the scaffolds and the TASS content was investigated. As the TASS content increases, the antibacterial activity hinders both
S. aureus and
E. coli from proliferating.
However, the effective TASS level is close to the toxic dose, so making the TASS localized and controllable in release is critical. The human fetal osteoblastic cell line (hFOB 1.19 cells) was used to assess the cytotoxicity of the scaffolds. Different concentrations of TASS-PEEK/PGA were incubated with hFOB 1.19 cells for 1, 3, and 5 days. Compared to PEEK/PGA, 2.5% TASS-PEEK/PGA had a slight effect on cell survival. The 7.5% TASS-PEEK/PGA had fewer cells on the third day of incubation than on the first day, but the number of cells increased again on the fifth day. This may be due to faster drug release and higher TASS levels in the first three days. However, the slow release of the drug after that promoted the cell growth.
4. Poly(Lactic-Co-Glycolic Acid) (PLGA)
The PLGA is a copolymer of the PLA and the PGA, which biodegrades faster than the PLA due to the presence of the PGA
[17]. However, the surface properties of PLGA are not ideal for cell growth
[18]. PLGA has been approved by the US Food and Drug Administration (FDA) for use as an implant, and PLGA has excellent mechanical properties and degradability. Surface modification of PLGA scaffolds is therefore a promising approach, which would provide useful surface properties to the polymer without changing the native properties
[19].
Jing et al.
[20] prepared nanoparticles based on PLGA-CS conjugates and PLGA-alendronate (Alen) conjugates. The PLGA-CS combines the good biocompatibility and biodegradability of PLGA and CS, facilitating drug delivery. Alen, a potent anti-osteoporosis drug, was used as a model drug for modifying the PLGA in this experiment. The cytotoxicity of the nanoparticles was assessed by CCK-8 assay. The cell survival rate was higher than 95% when nanoparticles with different concentrations were placed together with MC3T3 cells for 24 h. This indicates that the nanoparticles have no significant cytotoxicity to MC3T3 cells. To assess the specific cellular uptake of nanoparticles, nanoparticles with Alen (NP4) or without Alen (NP5) were incubated with HDF cells and MC3T3 cells for 3 and 24 h, respectively. The results showed that the uptake of NP4 into MC3T3 cells was significantly greater than that of NP5 after 24 h. This demonstrated that the Alen-modified nanoparticles had specific uptake into MC3T3 cells. Although the antimicrobial activity of nanoparticles was not tested in the article, the addition of CS may give the nanoparticles some antimicrobial activity.
De Faria et al.
[21] prepared PLGA and CS blend fibers by using electrostatic spinning method. The PLGA-CS mats were functionalized with GO-Ag through a chemical reaction between the carboxyl group of GO and the primary amine functional group on PLGA-CS fibers.
To evaluate the antibacterial activity of PLGA-CS after modification with GO-Ag, unmodified PLGA-CS was used as a control. The GO-Ag modified PLGA-CS and PLGA-CS were exposed to E. coli, P. aeruginosa (Gram-negative), and S. aureus (Gram-positive) for 3 h. The result showed that the antibacterial activity was greatly improved after GO-Ag modification. The inactivation of E. coli and P. aeruginosa reached more than 98%, while the inactivation of S. aureus was lower at 79.4 ± 6.1%. This may be due to the thicker peptidoglycan layer of Gram-positive bacteria, which played a protective role against S. aureus cells.
Azzazy et al.
[22] designed PLGA nanoparticles with CS coating and loaded with harmala alkaloid-rich fraction (HARF) (H/CS/PLGA) by the emulsion–solvent evaporation method. HARF has been reported to increase collagen and fibroblasts in the microenvironment near the wound, which allows it to accelerate wound healing. The lactic acid produced by the degradation of PLGA accelerates reparative angiogenesis, while CS has antibacterial, bioadhesive, and hemostatic properties. To evaluate the cytotoxicity of these nanoparticles, human skin fibroblasts were treated with different concentrations of H/CS/PLGA NPs to test cell viability. The cell viability was higher than 85% for all concentrations and did not differ significantly from the untreated cells.
5. Summary of Antimicrobial Strategies for Degradable Synthetic Polymers
Antimicrobial applications of biodegradable synthetic polymers and mammalian cells used for testing biocompatibility are shown in Table 1.
Table 1. Summary of antimicrobial applications and biocompatibility testing of degradable synthetic polymers.
These material systems were tested for their antimicrobial efficacy and biocompatibility. In PLA/PHMB, the complete growth inhibition of both bacteria occurred when the PHMB concentration was above 1.5 wt% (bacterial growth was lower than 1% compared to the PLA control). In contrast, when the PHMB concentration was below 0.75 wt%, bacterial growth was not significantly inhibited, but bacterial growth on the scaffold was still lower than that of the control. For the E. coli, it was about 30% lower than the control, and for the M. luteus, it was about 20% lower than the control. The antibacterial effect of the system was demonstrated to be dependent on the loading concentration of PHMB. For fibroblast and epithelial cell adhesion, it was 275% and 175% when the PHMB concentration was 1.5 wt% compared to the control, while it decreased to 175% and 100% when PHMB was increased to 2.5 wt%. This indicates that the system has good biocompatibility in a range of concentrations. Overall, low concentrations of PHMB inhibited bacterial adhesion and colonization due to the controlled and sustained release of PHMB. In contrast, significant inhibition of bacterial growth requires a concentration of PHMB higher than 0.75 wt%. In PLA/CS, the average antibacterial rate increased from 84.90% to 99.77% when PLA:CS was increased from 8:1 to 3:1. This indicates that the antimicrobial effect of the system depends on the relative content of CS. In the PLA/GSNO/PHB system, the bacterial adhesion rate of S. aureus was about 72.9% and 79.7% after 2 and 24 h exposure to the fiber. Only about 20% of the bacteria remained viable after 2 h of exposure. Mouse fibroblasts showed greater than 90% viability compared to the control group without fiber exposure. In PLA/PCL/n(HA)/cfx-βCD, the growth of S. aureus was reduced by 90% within 24 h. The cell viability of MC3T3 cells increased continuously from day 3 to day 7 and surpassed that of the control at day 14, contributing to cell proliferation. Antibacterial testing of S. aureus in PCL/curcumin/piperine/eugenol/rutin showed that the dressing containing piperine killed 100% of the bacteria and the dressing containing curcumin killed more than 90% of the bacteria. Two three-component systems, PCPiEu and PCPiR, achieved bactericidal rates of approximately 80%. Antibacterial tests on E. faecalis showed that curcumin had no antibacterial activity against this bacterium, while the three-component systems PCPiEu and PCPiR exhibited bactericidal rates of 99.47% and 96.88%. In cellular tests performed on human fibroblasts, the cell viability of the dressing containing only piperine was only 16.5%, whereas the three-component systems PCPiEu and PCPiR showed 94.2% and 98.5% cell viability. Therefore, the two three-component systems showed good overall performance in terms of antimicrobial and biocompatibility. In PCL/Atropa/AgNPs, the antimicrobial effect was brought about by AgNPs, and the antimicrobial effect was slightly reduced by the addition of Atropa. In contrast, in the cytotoxicity test of HaCaT cells, the cell survival rate was increased by about 30% with the addition of Atropa than with the addition of AgNPs only. In PCL/HA/ZnO, PCL/HA/ZnO 1% reduced the initial S. aureus bacterial load by almost 99%. In PGA/PLLA/GO/Ag, antimicrobial tests were conducted using E. coli. GO showed good synergistic bactericidal effect with Ag. GO itself had no significant bactericidal effect, while the addition of GO increased the bactericidal rate of PGA/PLLA/GO/Ag by 17.1% over PGA/PLLA/Ag to 95.4%. With the increase of Ag content to 1.5%, the bactericidal rate reached 99.9%. However, the 1.5% Ag content made the scaffolds less cytocompatible. While 1% content of Ag still maintained good biocompatibility. In PGA/PEEK/TASS, the antimicrobial rate of the scaffold reached 55.71% for E. coil and 15.84% for S. aureus when the TASS content was 7.5%. In the biocompatibility test, a TASS content of 2.5% better promoted the proliferation and differentiation of human fetal osteoblasts. Among PGA/PLGA/Ag@AuNPs, Ag@AuNPs showed strong bactericidal rates, reaching over 90% and 99.9999% against E. coli and S. aureus, respectively, and the bactericidal rate continued to increase with the increase of nanoparticles. The degradation of the outer layer of the scaffold does not release large amounts of Au and AgNPs, so the scaffold has good biocompatibility. In PLGA/CS/GO/Ag, the killing rate of both E. coli and P. aeru-ginosa was over 98%, while the inactivation rate of S. aureus was only about 79.4%.
It seems that all these antimicrobial methods have good bactericidal effect; however, they still have some drawbacks. For example, good bactericidal methods are often accompanied by strong cytotoxicity, which requires control of its concentration and release rate. Most of the methods are effective against only one type of bacteria but are not effective against others. More antimicrobial material systems need to be investigated in order to achieve an antimicrobial effect while promoting cell proliferation.