Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | J. FERNANDO AYALA-ZAVALA | -- | 1635 | 2023-01-11 18:43:59 | | | |
| 2 | Conner Chen | + 3 word(s) | 1638 | 2023-01-12 02:56:16 | | | | |
| 3 | Conner Chen | + 2 word(s) | 1640 | 2023-01-13 01:14:51 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Palomares-Navarro, J.J.; Bernal-Mercado, A.T.; González-Aguilar, G.A.; Ortega-Ramirez, L.A.; Martínez-Téllez, M.A.; Ayala-Zavala, J.F. Salmonella Biofilm Formation Process. Encyclopedia. Available online: https://encyclopedia.pub/entry/40071 (accessed on 11 March 2026).
Palomares-Navarro JJ, Bernal-Mercado AT, González-Aguilar GA, Ortega-Ramirez LA, Martínez-Téllez MA, Ayala-Zavala JF. Salmonella Biofilm Formation Process. Encyclopedia. Available at: https://encyclopedia.pub/entry/40071. Accessed March 11, 2026.
Palomares-Navarro, Julian J., Ariadna T. Bernal-Mercado, Gustavo A. González-Aguilar, Luis A. Ortega-Ramirez, Miguel A. Martínez-Téllez, Jesús F. Ayala-Zavala. "Salmonella Biofilm Formation Process" Encyclopedia, https://encyclopedia.pub/entry/40071 (accessed March 11, 2026).
Palomares-Navarro, J.J., Bernal-Mercado, A.T., González-Aguilar, G.A., Ortega-Ramirez, L.A., Martínez-Téllez, M.A., & Ayala-Zavala, J.F. (2023, January 11). Salmonella Biofilm Formation Process. In Encyclopedia. https://encyclopedia.pub/entry/40071
Palomares-Navarro, Julian J., et al. "Salmonella Biofilm Formation Process." Encyclopedia. Web. 11 January, 2023.
Copy Citation
Salmonella can form biofilms that contribute to its resistance in food processing environments. Biofilms are a dense population of cells that adhere to the surface, creating a matrix composed of extracellular polymeric substances (EPS) consisting mainly of polysaccharides, proteins, and extracellular DNA (eDNA).
biofilm formation
exopolymeric substances
control biofilm
1. Introduction
Gastrointestinal infections are caused by consuming contaminated food with enteric pathogens [1]. Salmonella is one of the most prevalent causes of foodborne disease and a significant cause of diarrheal illnesses, resulting in 1.35 million infections per year in the United States of America [2][3]. Salmonella can sense, adapt, and survive stressful environmental conditions, persisting and resisting disinfection due to their biofilm formation. Biofilms are bacterial communities rounded by a self-produced matrix of extracellular polymeric substances (EPS) [4]. Biofilms have been linked to food product contamination and foodborne illness, causing critical problems in public health [5]. These communities represent a considerable problem for the food industry because the EPS matrix offers protection against the cleaning and disinfection processes, and the contaminated surface could lead to cross-contamination [6].
Salmonella can easily adhere to and form biofilms on different abiotic and biotic surfaces [4][7]. Salmonella adhesion is the initial stage in biofilm formation; cells can attach to a surface in minutes or hours, depending on environmental conditions [8]. The EPS of the Salmonella biofilm matrix have been classified based on their function beyond their composition. Structural EPS represent this classification’s largest and most relevant group in protection against disinfectants. These structural EPS are composed of polymers, such as cellulose, curli, colanic acid, and proteinaceous O-antigen [9].
Biofilms are hard to eradicate because the EPS matrix’s three-dimensional network houses bacteria and protects them from the action of disinfectants [10]. This matrix limits diffusion and inactivates xenobiotic agents inside the biofilm, affecting the disinfection process and compromising food safety. Therefore, recent studies have focused on targeting the formation of the EPS matrix in Pseudomonas, Staphylococcus, and Escherichia [11]. However, this paradigm has been little explored in Salmonella [12]. In general, the main components of Salmonella biofilms are polysaccharides, such as cellulose, and protein structures, such as curli and adhesive fimbriae; the inhibition of their synthesis could be an interesting target for biofilm control.
For Salmonella control, the inappropriate and intensive use of disinfecting agents could induce bacterial resistance and, in some cases, affect food contact surfaces [13]. Therefore, the need to research alternatives for disinfecting agents has been emphasized. Terpenes found in plant sources are a promising alternative due to their effect on bacterial growth, biofilm formation, and enzyme activity [14]. Some studies have reported that terpenes significantly reduce Salmonella biofilm formation [15][16][17][18][19]. This effect has been correlated with the decrease in biofilm exopolysaccharide production in Enterobacter cloacae, Staphylococcus aureus, Salmonella Typhimurium, Escherichia coli O157:H7, Listeria monocytogenes, and Streptococcus sobrinus [20][21][22][23][24][25]. However, many details of how this terpene inhibits glucan synthesis are not considered. In other bacterial species, it was observed that terpenes, due to their structure, could potentially interact with glucosyltransferase enzymes that participate in the synthesis of glucans. Ortega-Ramirez et al. [26] showed that citral and geraniol inhibited glycosyltransferase activity, reducing glucans production in E. coli biofilms. In contrast, terpenes have also been shown to regulate the expression of genes related to EPS synthesis, such as cellulose, curli, or colanic acid [20].
There is evidence of the efficacy of terpenes in inhibiting EPS synthesis and biofilms of pathogenic bacteria; however, there is still a lack of knowledge of their specific mode of action in Salmonella [27]. It is also important to characterize the EPS synthesis during biofilm development and quantify changes in the EPS content under different conditions, such as temperature, nutrients, pH, and contact surfaces, to design more complex and practical systems when exploring the terpenes’ mode of action. In addition, the time-dependent antibiofilm activity of terpenes is not normally studied, and it can be useful to define times of action. Finally, exploring molecular inhibition mechanisms of EPS synthesis at genetic and post-translational levels will generate more solid knowledge of the terpenes’ activity.
2. Salmonella Biofilm Formation Process
Salmonella may produce biofilms. These complex communities adhere to various surfaces, contributing to the bacteria’s resistance and persistence in both host and non-host situations. Salmonella biofilm formation is a dynamic and complex process that implies subsequential steps, where sessile cells exhibit a different physiological state than their planktonic counterparts (Figure 1). Salmonella strains can form biofilms starting with the adherence of planktonic cells to surfaces [6]. The initial cell adhesion to surfaces is influenced by different factors, such as the type of surface, texture (rough or smooth), charge, polarity, pH, temperature, and medium nutrients [6][28].
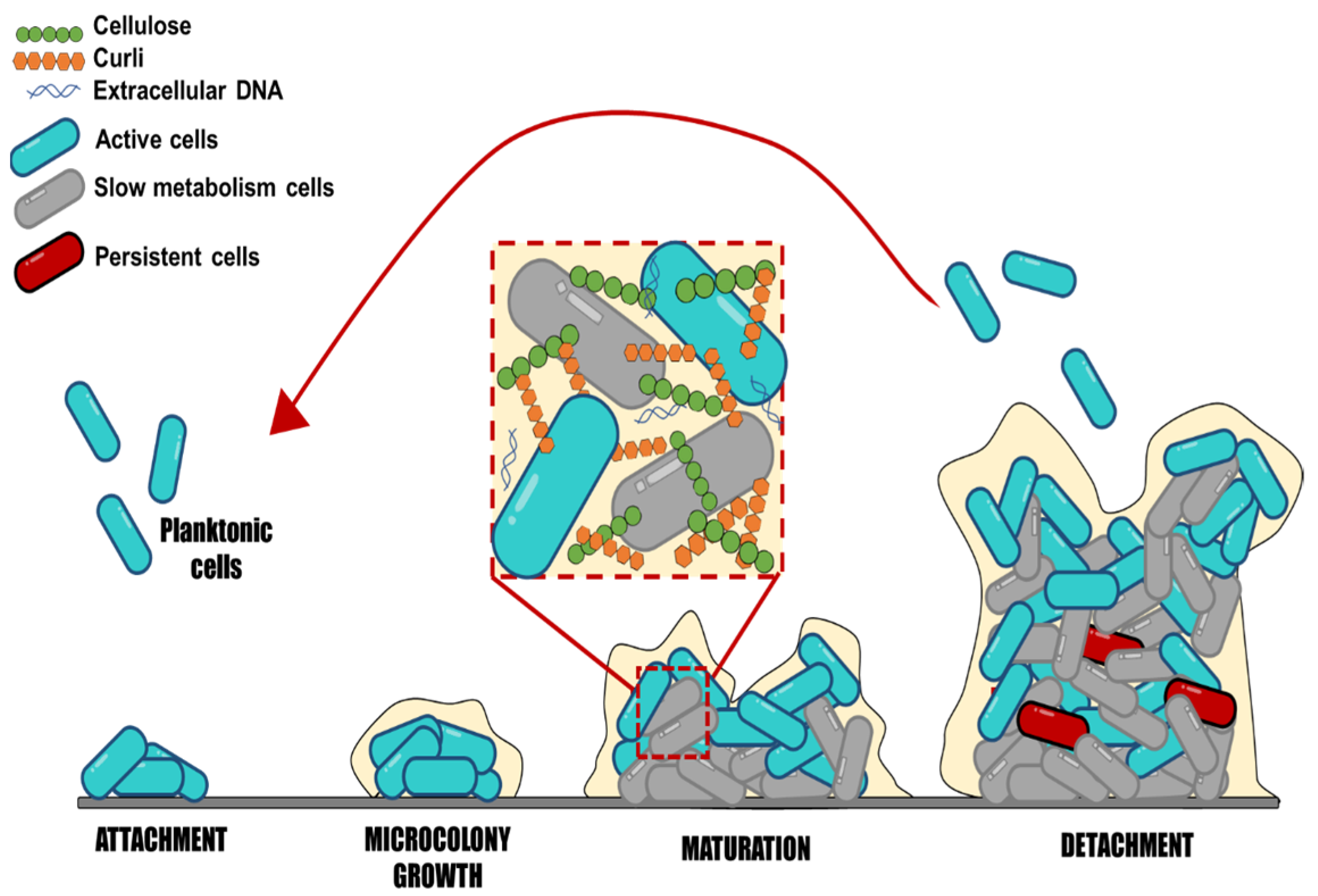
Figure 1. Schematic representation of biofilm formation, step by step. This scheme represents the stages of biofilm development: Attachment, microcolony growth, biofilm maturation, and detachment of embedded cells.
Moraes et al. [29] showed that the different strains (S. Enteritidis, S. Infantis, S. Typhimurium, and S. Heidelberg) have different adhesion abilities and biofilm formation on stainless steel surfaces under different pH, temperature, and NaCl concentrations. All strains demonstrated adherence at pH 4, up to 4% NaCl, and at temperatures of 20 °C and 35 °C. For all studied strains, the chance of adhesion was reduced in conditions where NaCl concentrations reached >8%, 8 °C, and pH 5. Moreover, Salmonella can adhere to gallstones, animal epithelial cells, plant surfaces, and abiotic surfaces [8]. Numerous studies have reported that Salmonella enterica can adhere and form biofilm on plastic, glass, and stainless steel [30][31][32]. These materials are commonly used in kitchens, toilets, slaughterhouses, farms, and the food industry. A study showed that Salmonella adheres differently depending on the surface and temperature conditions, affecting how long they survive in a processing environment [31]. The results showed that at 25 °C, more isolates formed strong and moderate biofilms on plastic surfaces than on stainless steel; at 15 °C, fewer isolates formed strong biofilms than at 25 °C, and plastic surfaces were more prone to adherence than stainless steel at this temperature. The study of Salmonella attachment in different conditions may offer important information to design control actions and reduce biofilms in processing environments.
Considering the ability of Salmonella to survive on abiotic surfaces, this bacterium can represent a potential danger for consumers by contaminating food products. There is limited comprehension of the effect of surfaces, times, and temperatures used in food industries, as well as the influence of low-nutrient conditions, such as food contact surfaces that may have organic residue on adhesion and biofilm formation of Salmonella. It is crucial to describe bacterial adherence, the capacity to form biofilms, and the sanitizer resistance of Salmonella to design efficient control measures and hygiene procedures. Some strategies could directly inhibit virulence factors, such as adhesion, EPS secretion, flagella inhibition, and protein synthesis involved in bacterial metabolism or quorum sensing.
Salmonella biofilm formation continues with the irreversible adhesion caused by the secretion of EPS composed of polysaccharides, proteins, and DNA, which form the matrix biofilm and increase the cell-surface and cell-cell interactions [4]. The EPS matrix accounts for 90% of the biomass, while microorganism cells contribute the rest, 10%, emphasizing the significance of the EPS matrix. Salmonella biofilm’s main components consist of polysaccharides, cellulose, colanic acid, anionic O-antigen capsule, proteins such as the amyloid fibers called curli, flagella, surface protein components, and fatty acids [9]. The amount of each component within Salmonella biofilms is still unknown, representing an excellent area for further studies. It is essential to point out that the exact composition of the biofilms cannot be generalized for all cases; it would be interesting to know how the EPS components of the biofilm vary among serotypes or by modifying environmental factors and how this influences disinfection processes. For example, Kim et al. [33] concluded that the optimal condition for total cell mass and EPS synthesis after 9 days of Salmonella Typhimurium biofilm maturation was at 15 °C for the rdar (red, dry, and rough) and bdar (brown, dry, and rough) strains compared to 25 and 37 °C. It is necessary to establish the most specific contribution of each type of EPS in biofilm formation, and how this and the environmental factors influence the mechanisms of resistance to and survival of disinfectant processes, because only the contribution of the entire matrix is known.
During biofilm maturation, the EPS matrix creates a three-dimensional network essential to biofilm lifestyle and virulence development; this network protects bacterial cells from environmental stresses, such as antimicrobials and immune system cells. The biofilm biomass provides a hydrated viscous environment that protects cells from various stressors, including desiccation, disinfectants, antibiotics, temperature, and oxygen content [34]. It may also prevent the loss of enzymes, nutrients, and molecules that could favor the microenvironment for bacteria within the biofilm [35]. However, the lack of knowledge detected on this point is regarding biofilm characteristics to consider its maturity level; these characteristics may involve measuring the number of adhered cells, EPS content, and variations among serotypes at different times. Salmonella’s lifetime is finished when cells leave, disperse from the biofilm, and revert to planktonic mode [36]. The dispersion of bacteria in biofilms should also be explored to a greater extent, because once they are released from the biofilm, they can colonize new sites and persist in the medium, making disinfection processes more difficult. Still, limited information is available on the time at which biofilm dispersal begins, as well as what environmental factors promote it.
Structural EPS is the largest and most relevant group of substances that interferes in bacterial disinfection tasks. They primarily consist of neutral polysaccharides and protein parts that aid construction and surface colonization [37]. These EPS contribute to the formation processes, highlighting cellulose as one of the main components, followed by other components, such as curli, cholic acid, and protein O antigen material [9]. For all the stated above, biofilms are extremely difficult to remove from surfaces in the food industry. A lack of knowledge is detected around the potential variations in biofilm development, composition, and resistance among Salmonella strains with different morphotypes. In this context, it will also be interesting to determine the ideal conditions for biofilm formation and development.
References
- Sell, J.; Dolan, B. Common gastrointestinal infections. Prim. Care Clin. Off. Pract. 2018, 45, 519–532.
- Gordon, M.A. Salmonella infections in immunocompromised adults. J. Infect. 2008, 56, 413–422.
- CDC. Salmonella Homepage. 2016. Available online: https://www.cdc.gov/salmonella/index.html (accessed on 15 January 2021).
- Merino, L.; Procura, F.; Trejo, F.M.; Bueno, D.J.; Golowczyc, M.A. Biofilm formation by Salmonella sp. in the poultry industry: Detection, control and eradication strategies. Food Res. Int. 2019, 119, 530–540.
- Galiè, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the Food Industry: Health Aspects and Control Methods. Front. Microbiol. 2018, 9, 898.
- Van Houdt, R.; Michiels, C.W. Biofilm formation and the food industry, a focus on the bacterial outer surface. J. Appl. Microbiol. 2010, 109, 1117–1131.
- Moraes, J.O.; Cruz, E.A.; Pinheiro, Í.; Oliveira, T.C.; Alvarenga, V.; Sant’Ana, A.S.; Magnani, M. An ordinal logistic regression approach to predict the variability on biofilm formation stages by five Salmonella enterica strains on polypropylene and glass surfaces as affected by pH, temperature and NaCl. Food Microbiol. 2019, 83, 95–103.
- Sadekuzzaman, M.; Yang, S.; Mizan, M.; Ha, S. Current and Recent Advanced Strategies for Combating Biofilms. Compr. Rev. Food Sci. Food Saf. 2015, 14, 491–509.
- Maruzani, R.; Sutton, G.; Nocerino, P.; Marvasi, M. Exopolymeric substances (EPS) from Salmonella enterica: Polymers, proteins and their interactions with plants and abiotic surfaces. J. Microbiol. 2018, 57, 1–8.
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575.
- Powell, L.; Pritchard, M.F.; Ferguson, E.; Powell, K.A.; Patel, S.U.; Rye, P.; Sakellakou, S.-M.; Buurma, N.J.; Brilliant, C.; Copping, J.M.; et al. Targeted disruption of the extracellular polymeric network of Pseudomonas aeruginosa biofilms by alginate oligosaccharides. Npj Biofilms Microbiomes 2018, 4, 1–10.
- Marshall, J.; Flechtner, A.D.; La Perle, K.M.; Gunn, J.S. Visualization of Extracellular Matrix Components within Sectioned Salmonella Biofilms on the Surface of Human Gallstones. PLoS ONE 2014, 9, e89243.
- Friedman, M. Antibiotic-Resistant Bacteria: Prevalence in Food and Inactivation by Food-Compatible Compounds and Plant Extracts. J. Agric. Food Chem. 2015, 63, 3805–3822.
- Lahiri, D.; Dash, S.; Dutta, R.; Nag, M. Elucidating the effect of anti-biofilm activity of bioactive compounds extracted from plants. J. Biosci. 2019, 44, 52.
- Silva-Espinoza, B.A.; Palomares-Navarro, J.J.; Tapia-Rodriguez, M.R.; Cruz-Valenzuela, M.R.; González-Aguilar, G.A.; Silva-Campa, E.; Pedroza-Montero, M.; Almeida-Lopes, M.; Miranda, R.; Ayala-Zavala, J.F. Combination of ultraviolet light-C and clove essential oil to inactivate Salmonella typhimurium biofilms on stainless steel. J. Food Saf. 2020, 40, e12788.
- Trevisan, D.A.C.; Da Silva, A.F.; Negri, M.; Filho, B.A.D.A.; Junior, M.M.; Patussi, E.V.; Campanerut-Sá, P.A.Z.; Mikcha, J.M.G. Antibacterial and antibiofilm activity of carvacrol against Salmonella enterica serotype Typhimurium. Braz. J. Pharm. Sci. 2018, 54.
- Lira, M.C.; Rodrigues, J.B.; Almeida, E.T.D.C.; Ritter, A.C.; Tondo, E.; Torres, S.M.; Schaffner, D.; de Souza, E.L.; Magnani, M. Efficacy of oregano and rosemary essential oils to affect morphology and membrane functions of noncultivable sessile cells of Salmonella Enteritidis 86 in biofilms formed on stainless steel. J. Appl. Microbiol. 2019, 128, 376–386.
- Miladi, H.; Zmantar, T.; Kouidhi, B.; Chaabouni, Y.; Mahdouani, K.; Bakhrouf, A.; Chaieb, K. Use of carvacrol, thymol, and eugenol for biofilm eradication and resistance modifying susceptibility of Salmonella enterica serovar Typhimurium strains to nalidixic acid. Microb. Pathog. 2017, 104, 56–63.
- Abarkapa, I.; Čolović, R.; Đuragić, O.; Popović, S.; Kokić, B.; Milanov, D.; Pezo, L. Anti-biofilm activities of essential oils rich in carvacrol and thymol against Salmonella Enteritidis. Biofouling 2019, 35, 361–375.
- Liu, F.; Jin, P.; Sun, Z.; Du, L.; Wang, D.; Zhao, T.; Doyle, M.P. Carvacrol oil inhibits biofilm formation and exopolysaccharide production of Enterobacter cloacae. Food Control. 2020, 119, 107473.
- Kim, Y.-G.; Lee, J.-H.; Gwon, G.; Kim, S.-I.; Park, J.G.; Lee, J. Essential Oils and Eugenols Inhibit Biofilm Formation and the Virulence of Escherichia coli O157:H7. Sci. Rep. 2016, 6, 36377.
- Alni, R.H.; Ghorban, K.; Dadmanesh, M. Combined effects of Allium sativum and Cuminum cyminum essential oils on planktonic and biofilm forms of Salmonella typhimurium isolates. 3 Biotech 2020, 10, 315.
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Inhibition mechanism of cardamom essential oil on methicillin-resistant Staphylococcus aureus biofilm. LWT 2020, 122, 109057.
- Zhang, C.; Li, C.; Abdel-Samie, M.A.; Cui, H.; Lin, L. Unraveling the inhibitory mechanism of clove essential oil against Listeria monocytogenes biofilm and applying it to vegetable surfaces. LWT 2020, 134, 110210.
- Li, M.; Lai, G.; Wang, J.; Ye, D. The inhibition of eugenol on glucan is essential for the biofilm eradication effect on caries-related biofilm in an artificial mouth model. Nat. Prod. Res. 2012, 26, 1152–1155.
- Ortega-Ramirez, L.A.; Gutiérrez-Pacheco, M.M.; Vargas-Arispuro, I.; González-Aguilar, G.A.; Martínez-Téllez, M.A.; Ayala-Zavala, J.F. Inhibition of Glucosyltransferase Activity and Glucan Production as an Antibiofilm Mechanism of Lemongrass Essential Oil against Escherichia coli O157:H7. Antibiotics 2020, 9, 102.
- El Hag, M.; Feng, Z.; Su, Y.; Wang, X.; Yassin, A.; Chen, S.; Peng, D.; Liu, X. Contribution of the csgA and bcsA genes to Salmonella enterica serovar Pullorum biofilm formation and virulence. Avian Pathol. 2017, 46, 541–547.
- Abdallah, F.B.; Chaieb, K.; Zmantar, T.; Kallel, H.; Bakhrouf, A. Adherence assays and slime production of Vibrio alginolyticus and Vibrio parahaemolyticus. Braz. J. Microbiol. 2009, 40, 394–398.
- Moraes, J.O.; Cruz, E.A.; Souza, E.G.; Oliveira, T.C.; Alvarenga, V.O.; Peña, W.E.; Sant’Ana, A.S.; Magnani, M. Predicting adhesion and biofilm formation boundaries on stainless steel surfaces by five Salmonella enterica strains belonging to different serovars as a function of pH, temperature and NaCl concentration. Int. J. Food Microbiol. 2018, 281, 90–100.
- Dantas, S.T.A.; Rossi, B.F.; Bonsaglia, E.C.R.; Castilho, I.G.; Hernandes, R.T.; Fernandes, A.; Rall, V.L.M. Cross-Contamination and Biofilm Formation by Salmonella enterica Serovar Enteritidis on Various Cutting Boards. Foodborne Pathog. Dis. 2018, 15, 81–85.
- Obe, T.; Richards, A.K.; Shariat, N.W. Differences in biofilm formation of Salmonella serovars on two surfaces under two temperature conditions. J. Appl. Microbiol. 2021, 132, 2410–2420.
- Da Silva, F.F.M.; Monte, F.J.Q.; de Lemos, T.L.G.; Do Nascimento, P.G.G.; de Medeiros Costa, A.K.; De Paiva, L.M.M. Eugenol derivatives: Synthesis, characterization, and evaluation of antibacterial and antioxidant activities. Chem. Cent. J. 2018, 12, 34.
- Kim, S.-H.; Jyung, S.; Kang, D.-H. Comparative study of Salmonella Typhimurium biofilms and their resistance depending on cellulose secretion and maturation temperatures. LWT 2022, 154, 112700.
- Steenackers, H.; Hermans, K.; Vanderleyden, J.; De Keersmaecker, S.C. Salmonella biofilms: An overview on occurrence, structure, regulation and eradication. Food Res. Int. 2012, 45, 502–531.
- Izadi, P.; Eldyasti, A. Holistic insights into extracellular polymeric substance (EPS) in anammosx bacterial matrix and the potential sustainable biopolymer recovery: A review. Chemosphere 2021, 274, 129703.
- Rumbaugh, K.P.; Sauer, K. Biofilm dispersion. Nat. Rev. Microbiol. 2020, 18, 571–586.
- Solano, C.; García, B.; Valle, J.; Berasain, C.; Ghigo, J.M.; Gamazo, C.; Lasa, I. Genetic analysis of Salmonella enteritidis biofilm formation: Critical role of cellulose. Mol. Microbiol. 2002, 43, 793–808.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
3 times
(View History)
Update Date:
13 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





