
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Koyeli Girigoswami | -- | 5153 | 2023-01-10 04:01:10 | | | |
| 2 | Koyeli Girigoswami | Meta information modification | 5153 | 2023-01-10 04:07:27 | | | | |
| 3 | Peter Tang | Meta information modification | 5153 | 2023-01-10 04:54:02 | | |
Video Upload Options
Nanoparticles (NPs) designed for various theranostic purposes have hugely impacted scientific research in the field of biomedicine, bringing forth hopes of a future revolutionized area called nanomedicine. A budding advancement in this area is the conjugation of various cell membranes onto nanoparticles to develop biomimetic cells called ‘Nanodecoys’ (NDs), which can imitate the functioning of natural cells. This technology of coating cell membranes on NPs has enhanced the working capabilities of nano-based techniques by initiating effective navigation within the bodily system. Due to the presence of multiple functional moieties, nanoparticles coated with cell membranes hold the ability to interact with complex biological microenvironments inside the body with ease.
1. Introduction
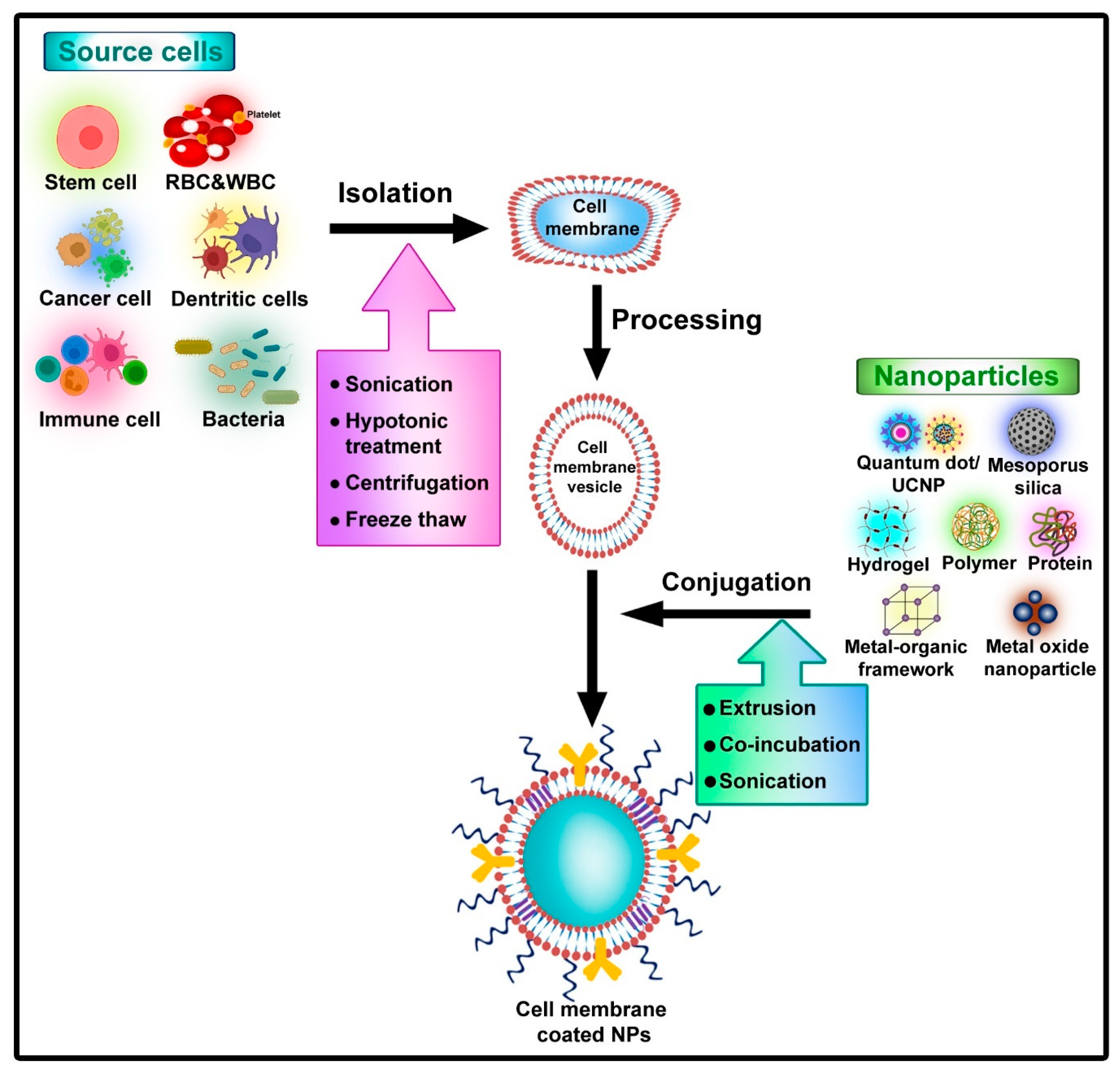
2. Toxicological Aspects of Nano-Based Drug Delivery System
3. Cell Membrane-Coated Nanocarrier System
|
S. No |
Cell Membrane/Extraction and Coating Method |
Nanoparticles |
Surface Modifications |
Drugs |
Target Cell/Disease/Pathogen |
Applications/Functions and Limitations |
Key Features |
References |
|---|---|---|---|---|---|---|---|---|
|
1. |
Macrophage. The extrusion technique was used to coat macrophage membranes on gold–silver nanocages in order to fabricate macrophage-membrane-coated nanoparticles. |
Gold/Silver nanocages |
- |
Rhodamine B |
Osteomyelitis and local infection |
Anti-bacterial photothermal therapy. Using macrophage membranes coated with bacterial pretreatment, this nanosystem can be used for precision/personalized medicine. The unique construction of gold-silver nanocages (hollow interiors and porous walls) makes it possible to load antibacterial drugs within these nanosystems for on-demand controlled release under NIR light. Limitations exist for the clearance of metal nanoparticles from our bodies. |
Improved the bactericidal effect upon irradiation of NIR |
[9] |
|
2. |
Erythrocytes. In order to prepare human RBC nanosponges (hNS), three steps were taken: (i) hypotonic treatment of packed hRBCs to obtain RBC membranes, (ii) nanoprecipitation by adding poly(lactic-co-glycolic) acid (PLGA) in organic solvents to an aqueous phase to prepare polymeric cores, and (iii) sonication of hRBC vesicles onto PLGA cores. |
Polymeric nanoparticles |
- |
- |
Hemolytic toxins |
Neutralizing the effectiveness of pore-forming toxins (PFTs). hNS was tested against four representative PFTs (melittin, listeriolysin O, α-hemolysin, and streptolysin O) in vitro and in vivo for its capacity to absorb and neutralize these toxins. Limitations of this study involve the risk of blood-borne diseases if the isolation process of the erythrocyte is compromised. Scaling up human erythrocyte-derived membranes has ethical issues. |
The nanosponges possessed novel antivirulence applications against hemolytic toxins of various strains of bacteria |
[10] |
|
3. |
Neutrophil. For the synthesis of neutrophil-NPs, purified and activated human peripheral blood neutrophil plasma membrane was coated onto poly(lactic-co-glycolic acid) (PLGA) polymeric cores. |
PLGA |
- |
- |
Rheumatoid arthritis |
Anti-inflammatory strategy. Their prophylactic regimen was used to test the effectiveness of neutrophil nanoparticles in treating early-stage arthritis in CIA mice. The limitation of this study is the scaling up of neutrophil-derived membranes and manufacturing issues. |
The particle neutralized the proinflammatory cytokines, targeted the cartilage matrix, and suppressed the severity of arthritis |
[11] |
|
4. |
Platelet. A repeated freeze-thaw process was used to extract platelet membrane from platelet rich plasma (PRP). Nanoprecipitation was used to prepare the PLGA cores. PLGA nanoparticles (PNP) were prepared by mixing the nanoparticles with PEGylated platelet membrane and sonicating them. PNP loaded with rapamycin (RAP-PNP) was prepared using the same method except that 800 mg of rapamycin was added to the PLGA solution. |
PLGA |
- |
Rapamycin |
Atherosclerosis |
Targeted drug delivery. By mimicking platelets’ inherent adhesion to atherosclerosis plaques, poly(DL-lactide-co-glycolide) nanoparticles (PNP) were explored as a drug delivery system targeting atherosclerosis plaques using the immunosuppressant Rapamycin (RAP). In apolipoprotein E-deficient (ApoE-/-) mice, PNP encapsulating RAP (RAP-PNP) was tested for anti-atherosclerosis activity against atherosclerotic plaques both in vitro and in vivo. The limitation of this study was that the membrane is human-derived, which can have ethical concerns. Moreover, it induces macrophage autophagy, which may interfere with normal homeostasis. |
Target and delay atherosclerotic plaques. A promising platform for the treatment of atherosclerosis |
[12] |
|
5. |
Cancer cell. Adenocarcinoma cells (MCF-7) were sonicated in buffer solution with protease inhibitor cocktail and differentially centrifuged to isolate the membrane. In order to form yolk-shell-structured nanoparticles, they first coated liposomes with a lipid bilayer coating (LM), then wrapped them with MCF-7 cell membrane (CCM) or 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), respectively, to form CCM@LM and L@LM, respectively, using mesoporous silica nanoparticles (MSN). |
Mesoporous silica nanoparticle |
PEGylated liposome |
Doxorubicin and mefuparib hydrochloride |
Cancer chemotherapy |
Targeted drug delivery. NPs coated with CCM and with a yolk-shell structure were evaluated for cancer chemotherapy. In addition to its homologous tumor-targeting ability due to the CCM coating, the resulting formulation (CCM@LM) exhibited a favorable immune escape profile. The limitation of this study is that we have to be careful while isolating the membrane from cancer cells regarding any residual cells’ presence. |
Significantly improved the antitumor effect compared to chemotherapeutic drugs (Doxil) |
[13] |
|
6. |
Erythrocytes. RBC membrane RBCM was formed by breaking up RBCs extracted from nude mice, and then incubating them under low osmotic pressure. As RBCM is sonicated, its size degrades from micro to nano. Perfluorocarbon (PFC) nanoparticles were encapsulated within biocompatible poly(d,l-lactide-co-glycolide), PLGA, resulting in PFC@PLGA nanoparticles, which were then coated with RBCM. |
Perfluorocarbon nanoparticles |
PLGA |
- |
Cancer radiotherapy |
Therapy. By diffusing oxygen through blood vessels, PFC@PLGARBCM with nanoscale sizes could improve the overall oxygenation status of the tumor after i.v. injection and the tumor is relieved from hypoxia, which can enhance the tumor inhibition by radiotherapy (RT). The limitations exist regarding the oxygen supply to the interior part of the tumors, which may inhibit the necrosis of the tumor. |
Delivery of oxygen and favorable for cancer treatment |
[14] |
|
7. |
Platelet. A freeze-thaw process was used to extract platelet membranes. A model drug for rheumatoid arthritis (RA), FK506-loaded nanoparticle cores were prepared by the process of nanoprecipitation. Platelet-mimetic nanoparticles (PNPs) were prepared by mixing PLGA nanoparticles with platelet membrane solutions and sonicating them to fuse the membrane onto the cores of the nanoparticle. |
PLGA nanoparticles |
- |
Model drug- FK506 |
Rheumatoid arthritis |
Targeted drug delivery. CIA mouse model of RA showed significant RA progression control with FK506-PNPs, and preliminary safety studies showed excellent biocompatibility for PNPs. Limitations include the scaling up of the ghost cells, i.e., the platelet membrane requires human platelets, which has ethical concerns. |
Accurate accumulation of formulation in the inflammatory synovial tissue. |
[15] |
|
8. |
Cancer cell. PLGA nanoparticles, containing siRNA and dox was prepared by water in oil emulsion method. Hela human cervix carcinoma cells and MDA-MB-231 human breast cancer cells were suspended in typical hypotonic lysing buffer and lysed in ice bath with repeated freezing and thawing. The membranes were collected using repeated centrifugation. In order to obtain membrane vesicles, the above cancer cell membrane fragments were extruded for 20 passes through a 400 nm polycarbonate membrane. To coat the membrane vesicles onto PLGA cores, nanocores and membrane vesicles were co-extruded through a 200 nm polycarbonate membrane. |
PLGA nanoparticles |
- |
Doxorubicin and PD-L1 siRNA |
Cancer therapy |
Targeted drug delivery. PLGA nanocores loaded with doxorubicin (Dox) and siRNA targeting PD-L1 (si.PD-L1) were constructed, camouflaged, and functionally modified using a cancer cell membrane (CCM). In addition for targeting homologous source cells, CCMNPs also have great potential as a platform for guiding the delivery of homologous-targeting therapeutics. PLGA nanoparticles cloaked in Hela membranes exhibited more powerful cellular internalization when compared with bare PLGA nanoparticles, while MDA-MB-231 cells showed reduced nanoparticle binding. Cell membrane isolation from cancer cells may also contain some unlysed cells which may contaminate the product, making this a limitation of this study. Moreover, the extracellular matrix of cancer cells may impart deleterious effects on normal cells which needs to be addressed. |
Selective accumulation and sustained delivery of drugs |
[16] |
|
9. |
Macrophage. Solvothermal method was used to synthesize Fe3 O4 NPs. Membrane-derived vesicles (MM-vesicles) were prepared using RAW 264.7 cells that were suspended in hypotonic lysing buffer containing EDTA-free mini protease inhibitor tablet. The cells were then subjected to Dounce homogenizer for disruption. After isolating the membranes using centrifugation, the MM-vesicles were extracted by physical extrusion of the pellets. The pellets were passed several times through 400 nm and 200 nm microporous membranes using an Avanti mini extruder. Fe3O4 NPs synthesized earlier were mixed with MM-vesicles and extruded through a 200 nm membrane 11 times and the additional MM-vesicles were removed using an external magnetic field; the resultant Fe3O4@MM NPs solution was left in PBS. |
Magnetic iron oxide |
- |
- |
Breast cancer therapy |
Photothermal therapy. MM-vesicles (macrophage membrane-derived vesicles) were collected from macrophages and then coated on Fe3O4 NPs. A macrophage membrane camouflaged nanoparticle (Fe3O4@MM NPs) inherited good biocompatibility and immune evasion properties and was capable of targeting cancer and converting light to heat. It could be used for enhanced photothermal tumor therapy. The fascinating properties of macrophage membrane coatings in evading immune cells and targeting cancer require further investigation. Limitations include the scaling up of membranes from macrophages. |
Exhibited great biocompatibility and light-to-heat conversion capabilities |
[17] |
|
10. |
Erythrocytes. The Prussian blue nanoparticles (PB NPs) were prepared using the precipitation method using citric acid as a capping agent. The whole blood was collected from the eyeball of female KM mice and centrifuged for plasma removal. The RBCs were hemolyzed using distilled water and the membrane was selected using centrifugation. The vesicles were collected by sonication of the membrane followed by a series of extrusions using 400 nm and 200 nm polycarbonate membranes. Ce6 solution was added to these vesicles for binding and excess Ce6 was removed by centrifugation. To prepare PB@RBC/Ce6 NPs, PB NPs were added to RBC/Ce6 vesicles prepared previously and extruded using 100 nm membrane several times to yield the final product, PB@RBC/Ce6 NPs. |
Prussian blue nanoparticles |
Chlorin e6 |
Dual cancer therapy |
Photothermal and photodynamic therapies. Prussian blue nanoparticles (PB NPs) coated with photosensitizing agent Chlorin e6 (Ce6)-embedded RBC membrane vesicles, named PB@RBC/Ce6 NPs, were synthesized. A nude mouse orthotopic tumor model was used to assess the cytotoxicity and therapeutic efficacy of PB@RBC/Ce6 NPs in vivo and in vitro assay was done using 4T1 cell line. The findings of the study suggested that erythrocyte membranes are efficient carriers of the photosensitizer Ce6 due to hydrophobic interaction. They could impart efficient PTT with higher biocompatibility and higher endocytosis in tumor sites imparted synergistic PDT and PTT-mediated cell killing to inhibit cancerous tumor growth. Limitations of this study may be the ethical considerations in the scaling up of the erythrocyte membranes. |
Produced a notable effect in boosting the necrosis and showed a synergistic therapeutic effect |
[18] |
4. Applications
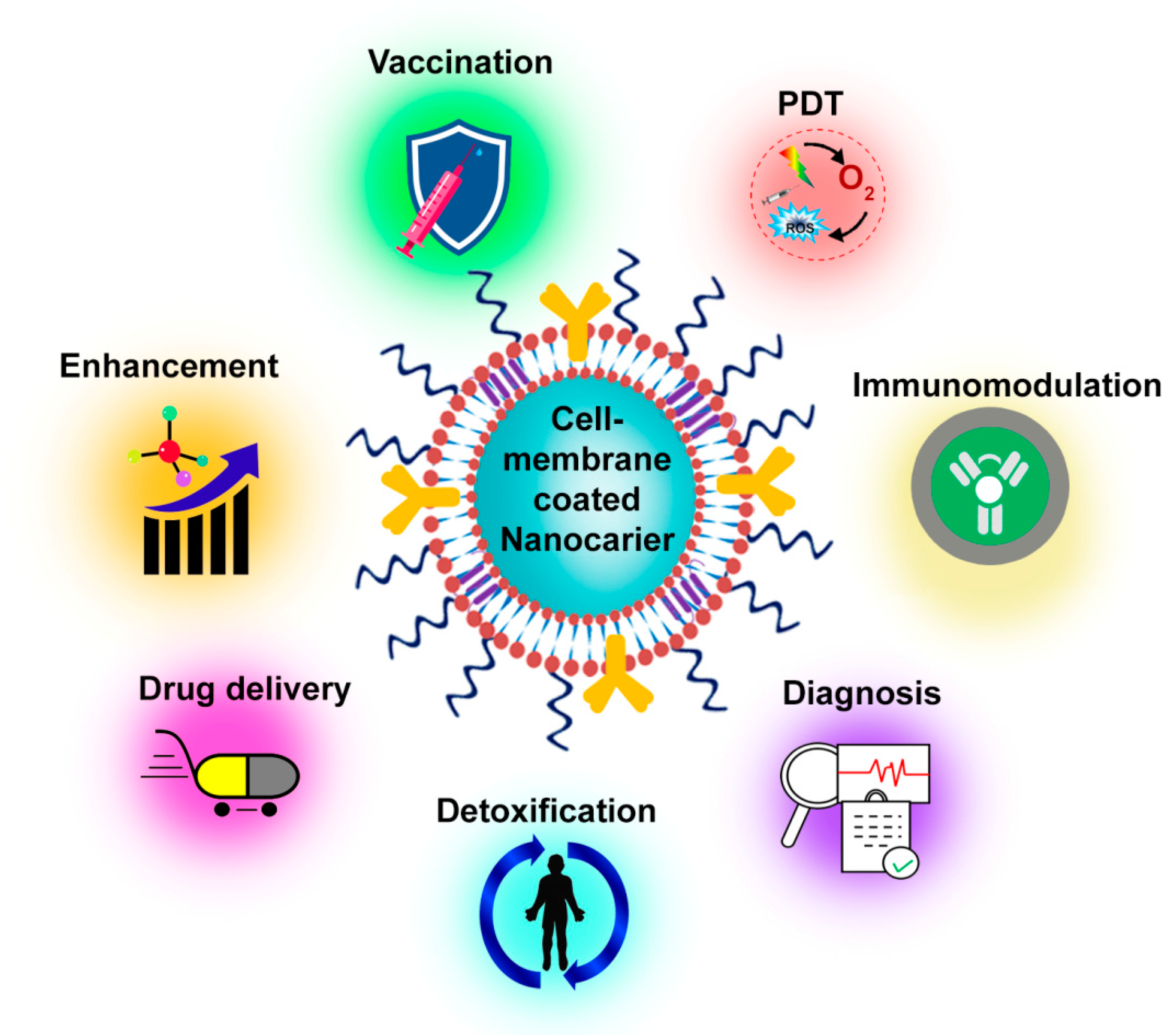
4.1. Bioimaging
4.2. Drug Delivery
4.3. Photodynamic Therapy (PDT)
4.4. Theranostics
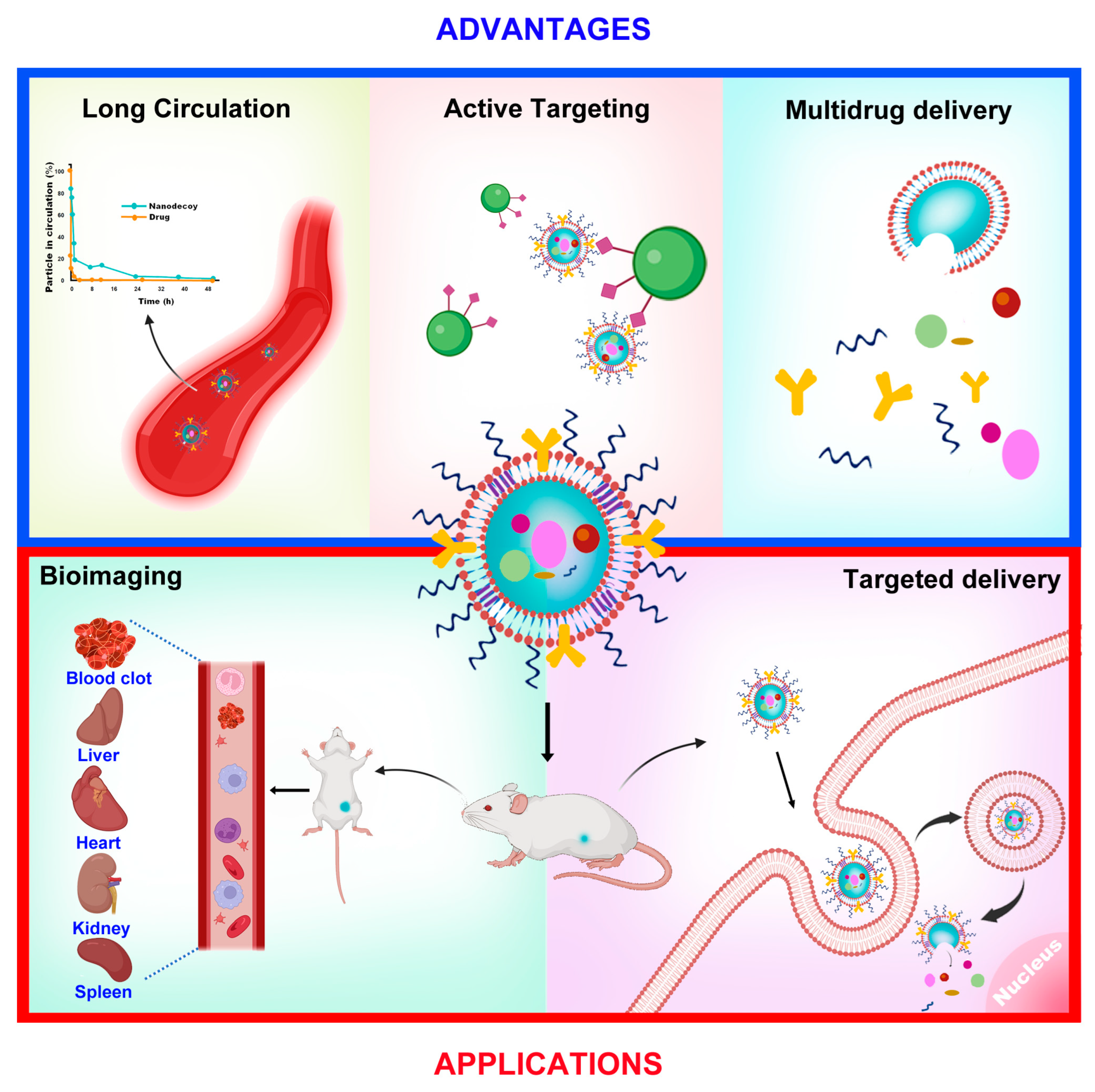
4.5. Other Applications
References
- Liu, W.L.; Zou, M.Z.; Qin, S.Y.; Cheng, Y.J.; Ma, Y.H.; Sun, Y.X.; Zhang, X.Z. Recent advances of cell membrane-coated nanomaterials for biomedical applications. Adv. Funct. Mater. 2020, 30, 2003559.
- Li, H.; Jin, K.; Luo, M.; Wang, X.; Zhu, X.; Liu, X.; Jiang, T.; Zhang, Q.; Wang, S.; Pang, Z. Size dependency of circulation and biodistribution of biomimetic nanoparticles: Red blood cell membrane-coated nanoparticles. Cells 2019, 8, 881.
- Li, S.-D.; Huang, L. Pharmacokinetics and biodistribution of nanoparticles. Mol. Pharm. 2008, 5, 496–504.
- Liang, T.; Zhang, R.; Liu, X.; Ding, Q.; Wu, S.; Li, C.; Lin, Y.; Ye, Y.; Zhong, Z.; Zhou, M. Recent advances in macrophage-mediated drug delivery systems. Int. J. Nanomed. 2021, 16, 2703.
- Shiraishi, K.; Yokoyama, M. Toxicity and immunogenicity concerns related to PEGylated-micelle carrier systems: A review. Sci. Technol. Adv. Mater. 2019, 20, 324–336.
- Xuan, M.; Shao, J.; Li, J. Cell membrane-covered nanoparticles as biomaterials. Natl. Sci. Rev. 2019, 6, 551–561.
- Ghosh, S.; Girigoswami, K.; Girigoswami, A. Membrane-encapsulated camouflaged nanomedicines in drug delivery. Nanomedicine 2019, 14, 2067–2082.
- Li, J.; Wang, Y.; Yang, J.; Liu, W. Bacteria activated-macrophage membrane-coated tough nanocomposite hydrogel with targeted photothermal antibacterial ability for infected wound healing. Chem. Eng. J. 2021, 420, 127638.
- Wang, C.; Wang, Y.; Zhang, L.; Miron, R.J.; Liang, J.; Shi, M.; Mo, W.; Zheng, S.; Zhao, Y.; Zhang, Y. Pretreated macrophage-membrane-coated gold nanocages for precise drug delivery for treatment of bacterial infections. Adv. Mater. 2018, 30, 1804023.
- Chen, Y.; Chen, M.; Zhang, Y.; Lee, J.H.; Escajadillo, T.; Gong, H.; Fang, R.H.; Gao, W.; Nizet, V.; Zhang, L. Broad-spectrum neutralization of pore-forming toxins with human erythrocyte membrane-coated nanosponges. Adv. Healthc. Mater. 2018, 7, 1701366.
- Zhang, Q.; Dehaini, D.; Zhang, Y.; Zhou, J.; Chen, X.; Zhang, L.; Fang, R.H.; Gao, W.; Zhang, L. Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat. Nanotechnol. 2018, 13, 1182–1190.
- Song, Y.; Huang, Z.; Liu, X.; Pang, Z.; Chen, J.; Yang, H.; Zhang, N.; Cao, Z.; Liu, M.; Cao, J. Platelet membrane-coated nanoparticle-mediated targeting delivery of Rapamycin blocks atherosclerotic plaque development and stabilizes plaque in apolipoprotein E-deficient (ApoE−/−) mice. Nanomed. Nanotechnol. Biol. Med. 2019, 15, 13–24.
- Nie, D.; Dai, Z.; Li, J.; Yang, Y.; Xi, Z.; Wang, J.; Zhang, W.; Qian, K.; Guo, S.; Zhu, C. Cancer-cell-membrane-coated nanoparticles with a yolk–shell structure augment cancer chemotherapy. Nano Lett. 2019, 20, 936–946.
- Gao, M.; Liang, C.; Song, X.; Chen, Q.; Jin, Q.; Wang, C.; Liu, Z. Erythrocyte-membrane-enveloped perfluorocarbon as nanoscale artificial red blood cells to relieve tumor hypoxia and enhance cancer radiotherapy. Adv. Mater. 2017, 29, 1701429.
- He, Y.; Li, R.; Liang, J.; Zhu, Y.; Zhang, S.; Zheng, Z.; Qin, J.; Pang, Z.; Wang, J. Drug targeting through platelet membrane-coated nanoparticles for the treatment of rheumatoid arthritis. Nano Res. 2018, 11, 6086–6101.
- Chen, M.; Chen, M.; He, J. Cancer cell membrane cloaking nanoparticles for targeted co-delivery of doxorubicin and PD-L1 siRNA. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1635–1641.
- Meng, Q.-F.; Rao, L.; Zan, M.; Chen, M.; Yu, G.-T.; Wei, X.; Wu, Z.; Sun, Y.; Guo, S.-S.; Zhao, X.-Z. Macrophage membrane-coated iron oxide nanoparticles for enhanced photothermal tumor therapy. Nanotechnology 2018, 29, 134004.
- Sun, L.; Li, Q.; Hou, M.; Gao, Y.; Yang, R.; Zhang, L.; Xu, Z.; Kang, Y.; Xue, P. Light-activatable Chlorin e6 (Ce6)-imbedded erythrocyte membrane vesicles camouflaged Prussian blue nanoparticles for synergistic photothermal and photodynamic therapies of cancer. Biomater. Sci. 2018, 6, 2881–2895.
- Rao, L.; Meng, Q.-F.; Bu, L.-L.; Cai, B.; Huang, Q.; Sun, Z.-J.; Zhang, W.-F.; Li, A.; Guo, S.-S.; Liu, W. Erythrocyte membrane-coated upconversion nanoparticles with minimal protein adsorption for enhanced tumor imaging. ACS Appl. Mater. Interfaces 2017, 9, 2159–2168.
- Rao, L.; Bu, L.L.; Cai, B.; Xu, J.H.; Li, A.; Zhang, W.F.; Sun, Z.J.; Guo, S.S.; Liu, W.; Wang, T.H. Cancer cell membrane-coated upconversion nanoprobes for highly specific tumor imaging. Adv. Mater. 2016, 28, 3460–3466.
- Zhang, X.; He, S.; Ding, B.; Qu, C.; Zhang, Q.; Chen, H.; Sun, Y.; Fang, H.; Long, Y.; Zhang, R. Cancer cell membrane-coated rare earth doped nanoparticles for tumor surgery navigation in NIR-II imaging window. Chem. Eng. J. 2020, 385, 123959.
- Chen, H.; Sha, H.; Zhang, L.; Qian, H.; Chen, F.; Ding, N.; Ji, L.; Zhu, A.; Xu, Q.; Meng, F. Lipid insertion enables targeted functionalization of paclitaxel-loaded erythrocyte membrane nanosystem by tumor-penetrating bispecific recombinant protein. Int. J. Nanomed. 2018, 13, 5347.
- Li, R.; He, Y.; Zhu, Y.; Jiang, L.; Zhang, S.; Qin, J.; Wu, Q.; Dai, W.; Shen, S.; Pang, Z. Route to rheumatoid arthritis by macrophage-derived microvesicle-coated nanoparticles. Nano Lett. 2018, 19, 124–134.
- Wang, H.; Wu, J.; Williams, G.R.; Fan, Q.; Niu, S.; Wu, J.; Xie, X.; Zhu, L.-M. Platelet-membrane-biomimetic nanoparticles for targeted antitumor drug delivery. J. Nanobiotechnol. 2019, 17, 60.
- Pallavi, P.; Sharmiladevi, P.; Haribabu, V.; Girigoswami, K.; Girigoswami, A. A Nano Approach to Formulate Photosensitizers for Photodynamic Therapy. Curr. Nanosci. 2022, 18, 675–689.
- Wang, H.; Zhang, C.; Zhang, Y.; Tian, R.; Cheng, G.; Pan, H.; Cui, M.; Chang, J. An efficient delivery of photosensitizers and hypoxic prodrugs for a tumor combination therapy by membrane camouflage nanoparticles. J. Mater. Chem. B 2020, 8, 2876–2886.
- Peng, L.-H.; Wang, M.-Z.; Chu, Y.; Zhang, L.; Niu, J.; Shao, H.-T.; Yuan, T.-J.; Jiang, Z.-H.; Gao, J.-Q.; Ning, X.-H. Engineering bacterial outer membrane vesicles as transdermal nanoplatforms for photo-TRAIL–programmed therapy against melanoma. Sci. Adv. 2020, 6, eaba2735.
- Zhao, Y.; Wang, J.; Cai, X.; Ding, P.; Lv, H.; Pei, R. Metal–organic frameworks with enhanced photodynamic therapy: Synthesis, erythrocyte membrane camouflage, and aptamer-targeted aggregation. ACS Appl. Mater. Interfaces 2020, 12, 23697–23706.
- Girigoswami, A.; Yassine, W.; Sharmiladevi, P.; Haribabu, V.; Girigoswami, K. Camouflaged nanosilver with excitation wavelength dependent high quantum yield for targeted theranostic. Sci. Rep. 2018, 8, 16459.
- Haribabu, V.; Girigoswami, K.; Sharmiladevi, P.; Girigoswami, A. Water–Nanomaterial Interaction to Escalate Twin-Mode Magnetic Resonance Imaging. ACS Biomater. Sci. Eng. 2020, 6, 4377–4389.
- Sharmiladevi, P.; Akhtar, N.; Haribabu, V.; Girigoswami, K.; Chattopadhyay, S.; Girigoswami, A. Excitation wavelength independent carbon-decorated ferrite nanodots for multimodal diagnosis and stimuli responsive therapy. ACS Appl. Bio Mater. 2019, 2, 1634–1642.
- Sharmiladevi, P.; Girigoswami, K.; Haribabu, V.; Girigoswami, A. Nano-enabled theranostics for cancer. Mater. Adv. 2021, 2, 2876–2891.
- Gowtham, P.; Haribabu, V.; Prabhu, A.D.; Pallavi, P.; Girigoswami, K.; Girigoswami, A. Impact of nanovectors in multimodal medical imaging. Nanomed. J. 2022, 9, 107–130.
- Jagannathan, N.R. Potential of Magnetic Resonance (MR) Methods in Clinical Cancer Research. In Biomedical Translational Research; Springer: Berlin/Heidelberg, Germany, 2022; pp. 339–360.
- Amsaveni, G.; Farook, A.S.; Haribabu, V.; Murugesan, R.; Girigoswami, A. Engineered multifunctional nanoparticles for DLA cancer cells targeting, sorting, MR imaging and drug delivery. Adv. Sci. Eng. Med. 2013, 5, 1340–1348.
- Akhtar, N.; Wu, P.-W.; Chen, C.L.; Chang, W.-Y.; Liu, R.-S.; Wu, C.T.; Girigoswami, A.; Chattopadhyay, S. Radiolabeled Human Protein-Functionalized Upconversion Nanoparticles for Multimodal Cancer Imaging. ACS Appl. Nano Mater. 2022, 5, 7051–7062.
- Jaiganesh, T.; Rani, J.D.V.; Girigoswami, A. Spectroscopically characterized cadmium sulfide quantum dots lengthening the lag phase of Escherichia coli growth. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 92, 29–32.
- Kavya, J.; Amsaveni, G.; Nagalakshmi, M.; Girigoswami, K.; Murugesan, R.; Girigoswami, A. Silver nanoparticles induced lowering of BCl2/Bax causes Dalton’s Lymphoma tumour cell death in mice. J. Bionanoscience 2013, 7, 276–281.
- Rao, L.; Cai, B.; Bu, L.-L.; Liao, Q.-Q.; Guo, S.-S.; Zhao, X.-Z.; Dong, W.-F.; Liu, W. Microfluidic electroporation-facilitated synthesis of erythrocyte membrane-coated magnetic nanoparticles for enhanced imaging-guided cancer therapy. Acs Nano 2017, 11, 3496–3505.
- Li, J.; Wang, X.; Zheng, D.; Lin, X.; Wei, Z.; Zhang, D.; Li, Z.; Zhang, Y.; Wu, M.; Liu, X. Cancer cell membrane-coated magnetic nanoparticles for MR/NIR fluorescence dual-modal imaging and photodynamic therapy. Biomater. Sci. 2018, 6, 1834–1845.
- Mu, X.; Li, J.; Yan, S.; Zhang, H.; Zhang, W.; Zhang, F.; Jiang, J. siRNA delivery with stem cell membrane-coated magnetic nanoparticles for imaging-guided photothermal therapy and gene therapy. ACS Biomater. Sci. Eng. 2018, 4, 3895–3905.
- Kroll, A.V.; Fang, R.H.; Zhang, L. Biointerfacing and applications of cell membrane-coated nanoparticles. Bioconjugate Chem. 2017, 28, 23–32.
- Gong, H.; Zhang, Q.; Komarla, A.; Wang, S.; Duan, Y.; Zhou, Z.; Chen, F.; Fang, R.H.; Xu, S.; Gao, W. Nanomaterial biointerfacing via mitochondrial membrane coating for targeted detoxification and molecular detection. Nano Lett. 2021, 21, 2603–2609.
- Park, J.H.; Jiang, Y.; Zhou, J.; Gong, H.; Mohapatra, A.; Heo, J.; Gao, W.; Fang, R.H.; Zhang, L. Genetically engineered cell membrane–coated nanoparticles for targeted delivery of dexamethasone to inflamed lungs. Sci. Adv. 2021, 7, eabf7820.




