Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Elena A. Filonova | -- | 2625 | 2023-01-09 11:07:17 | | | |
| 2 | Elena A. Filonova | + 1 word(s) | 2626 | 2023-01-09 11:14:30 | | | | |
| 3 | Dean Liu | Meta information modification | 2626 | 2023-01-10 01:32:55 | | | | |
| 4 | Dean Liu | -1 word(s) | 2625 | 2023-01-10 01:35:41 | | | | |
| 5 | Dean Liu | + 2 word(s) | 2627 | 2023-01-11 06:57:38 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pikalova, E.Y.; Kalinina, E.G.; Pikalova, N.S.; Filonova, E.A. Application of High-Entropy Alloys in SOFC Technology. Encyclopedia. Available online: https://encyclopedia.pub/entry/39909 (accessed on 01 March 2026).
Pikalova EY, Kalinina EG, Pikalova NS, Filonova EA. Application of High-Entropy Alloys in SOFC Technology. Encyclopedia. Available at: https://encyclopedia.pub/entry/39909. Accessed March 01, 2026.
Pikalova, Elena Y., Elena G. Kalinina, Nadezhda S. Pikalova, Elena A. Filonova. "Application of High-Entropy Alloys in SOFC Technology" Encyclopedia, https://encyclopedia.pub/entry/39909 (accessed March 01, 2026).
Pikalova, E.Y., Kalinina, E.G., Pikalova, N.S., & Filonova, E.A. (2023, January 09). Application of High-Entropy Alloys in SOFC Technology. In Encyclopedia. https://encyclopedia.pub/entry/39909
Pikalova, Elena Y., et al. "Application of High-Entropy Alloys in SOFC Technology." Encyclopedia. Web. 09 January, 2023.
Copy Citation
The mechanisms of the stabilization of a high-entropy state in such materials, as well as the effect of structural and charge factors on the stability of the resulting homogeneous solid solution are performed. An introduction to the synthesis methods for HEAs (high-entropy alloys) is given.
solid oxide fuel cells (SOFC)
high-entropy alloys
entropy of mixing
1. Introduction
Solid oxide fuel cells (SOFCs) are highly efficient electrochemical devices that directly convert fuel energy into electricity in a manner that is ecologically friendly. As such, they have attracted much attention from scientific groups and manufacturers [1]. In order to optimize the efficiency of SOFCs and extend their lifetime, increase the types of fuel that can be used, reduce operating temperatures [2], expand the possibility of their use in multigeneration [3][4] and hybrid [5] systems, and successfully market them [6], new advanced SOFC materials must be developed, as well as cost-effective methods of fabrication [7][8]. Currently, a new concept for designing materials based on the possibility of regulating the phase stability and functional properties of multicomponent solid solutions through the control of configurational entropy finds its application in various areas of science and technology [9][10][11]. New high- and medium-entropy materials have recently been developed for SOFC electrolytes [12][13][14], cathodes [15][16][17], anodes [18], and protective coatings for interconnectors [19].
2. High-Entropy Alloys: Theory, Achievements, and Prospects for Use in SOFC Technology
2.1. Theoretical Founditions and Recent Advances in the Development of High-Entropy Alloys
Recently, in the field of materials science a new direction has been widely developed which is related to the study of the unusual properties of high-entropy alloys (HEAs), first proposed in 1995 by Yeh and Huang [20]. Usually, such materials consist of several elements (at least five) taken in an equimolar ratio, for example, the CrMnFeCoNi system, obtained by Cantor et al. [21], and high-entropy CuCoNiCrAlxFe alloys, produced by the Yeh et al. group [22]. Unlike conventional multicomponent alloys, which are mainly multiphase structures, these HEAs were characterized by the appearance of a single-phase solid solution with a uniform and disordered distribution of their constituent elements. The obtained HEAs [21][22] demonstrated structural stability, including at elevated temperatures, high strength while maintaining plasticity, low thermal conductivity, slow diffusion of elements in the alloy, and grain boundary mobility. It was found that the structure of the HEA acquires a noticeable distortion of the crystal lattice which hinders the movement of dislocations and leads to enhanced hardness. Slow diffusion (sluggish diffusion) of elements in HEAs is associated with the occurrence of local stresses and an increase in the migration energy barrier. Slowing down the diffusion processes in HEAs can contribute to the formation of a nanosized grain structure [21]. Due to the distortion of the crystal lattice and mixing of the components, a “cocktail” effect occurs, in that the properties of the resulting material differ significantly from the properties of the individual components included in its composition [23].
To date, hundreds of HEA compositions with a variety of structures have been studied, namely, single-phase solid solutions with face-centered cubic (FCC), body-centered cubic (BCC), hexagonal close-packed (HCP), and orthorhombic structures, as well as HE amorphous metallic glasses [24] (Figure 1a). The main features of HEA’s properties (summarized in Figure 1b) and the scope of their application are presented in a number of recent reviews [10][11][24][25][26][27][28][29][30].
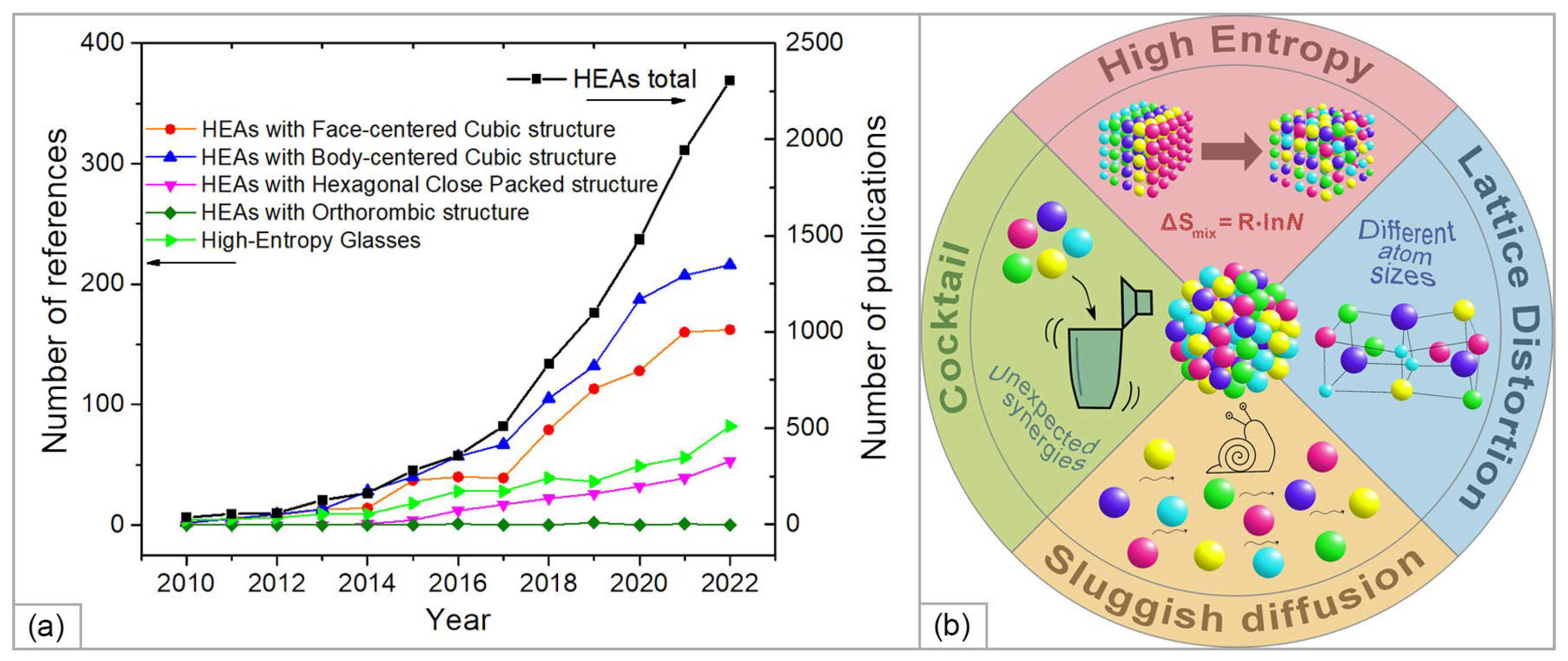
Figure 1. (a) Recent publications on the development and application of high-entropy alloys (HEAs) of different structures (for 2010–2022 years, according to the Scopus data); (b) schematic illustration of the properties and characteristics of HEAs.
A number of alternative names for HEAs have been proposed in the literature, such as multi-principal element alloys (MPEAs) [31] and complex concentrated alloys (CCAs) [32]. In addition, the concept of medium-entropy alloys (MEAs) was introduced for alloys containing four or fewer elements in an equiatomic ratio or close to it [33]. In analyzing the place of HEA among other types of condensed matter, Gelchinski et al. [10] suggested that HEAs can be placed between amorphous compounds and nanocrystals. From one perspective, no regions with a repeating unit cell can be found in their structure in contrast to nanocrystals, where such regions, albeit nanosized, are present. Alternatively, the degree of order in HEAs is higher than in amorphous materials since there are regularly located lattice sites (according to the type of crystal lattice) with disordered atoms of various elements.
Among the most used HEA components are Fe, Ni, Co, Cr, Al, Cu, Mn, and Ti, which are also included in the classical compositions (Figure 2).
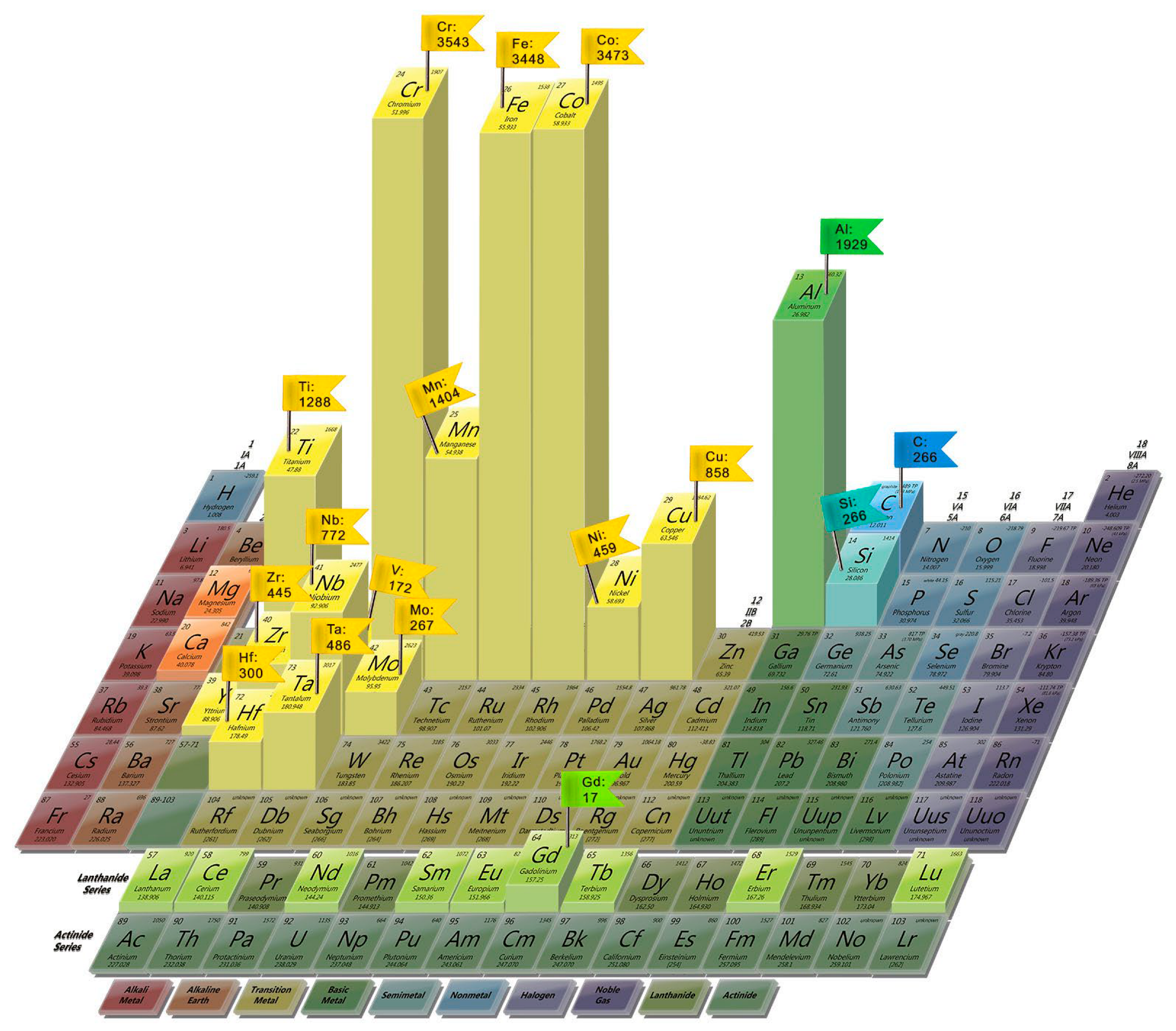
Figure 2. Frequency of use of elements in HEAs, Scopus data from years 2010–2022 (shown as vertical lines with corresponding numbers, elements used less than 10 times shown without numbers).
The basic principle of the synthesis of high-entropy materials is the formation of a homogeneous solid solution from a mixture of components. For example, the transition from a multi-phase to a single-phase system is possible with rapid cooling of the mixture [34]. In this case, a disordered substitutional solid solution is formed, in which atoms are randomly located at the lattice sites, which leads to an increased configurational entropy of the resulting phase. The difference between the atomic radii of the initial HEA components distorts the lattice and the structure of such phases can be considered, as was mentioned above, as an intermediate state between stable crystalline phases and metastable amorphous materials in which there is no long-range order. This raises the question of the long-term stability of the resulting homogeneous solid solution in a high-entropy state and the possible occurrence of local ordering with time.
Thermodynamic stability conditions lead to the requirement for a negative change in the Gibbs energy during the formation of a given material (at constant pressure and temperature):
where ΔG is a Gibbs-free energy change, ΔH is an enthalpy change, ΔS is an entropy change, and T is an absolute temperature. The entropy of an alloy comprises both the configurational entropy and the thermal entropy. The configurational entropy, also called as entropy of mixing, ΔSmix, in a multicomponent equiatomic system depends on the number of components as follows:
where R is the absolute gas constant, and N is the number of the components.
Figure 3a shows the entropy of mixing, calculated according to Equation (2) depending on the number of elements in equimolar alloys [30]. Thus, for equimolar two-, four-, and five-component alloys, the entropy of mixing was equal to 5.76, 11.52, and 13.37 J/K mol, respectively. Based on the entropy approach, alloys can be roughly divided into three categories according to their entropy of mixing on the supposition of random distribution of elements in a solid solution, namely (i) low-entropy alloys (traditional alloys) with one or two main elements with ΔSmix≤5.76 or 0.69 R, (ii) medium-entropy alloys with two to four base elements with entropy of mixing in the range of 0.69 R<ΔSmix<1.61 R, and (iii) high-entropy alloys with five or more base elements with 1.61 R≤ΔSmix, as shown in the inset in Figure 3a.
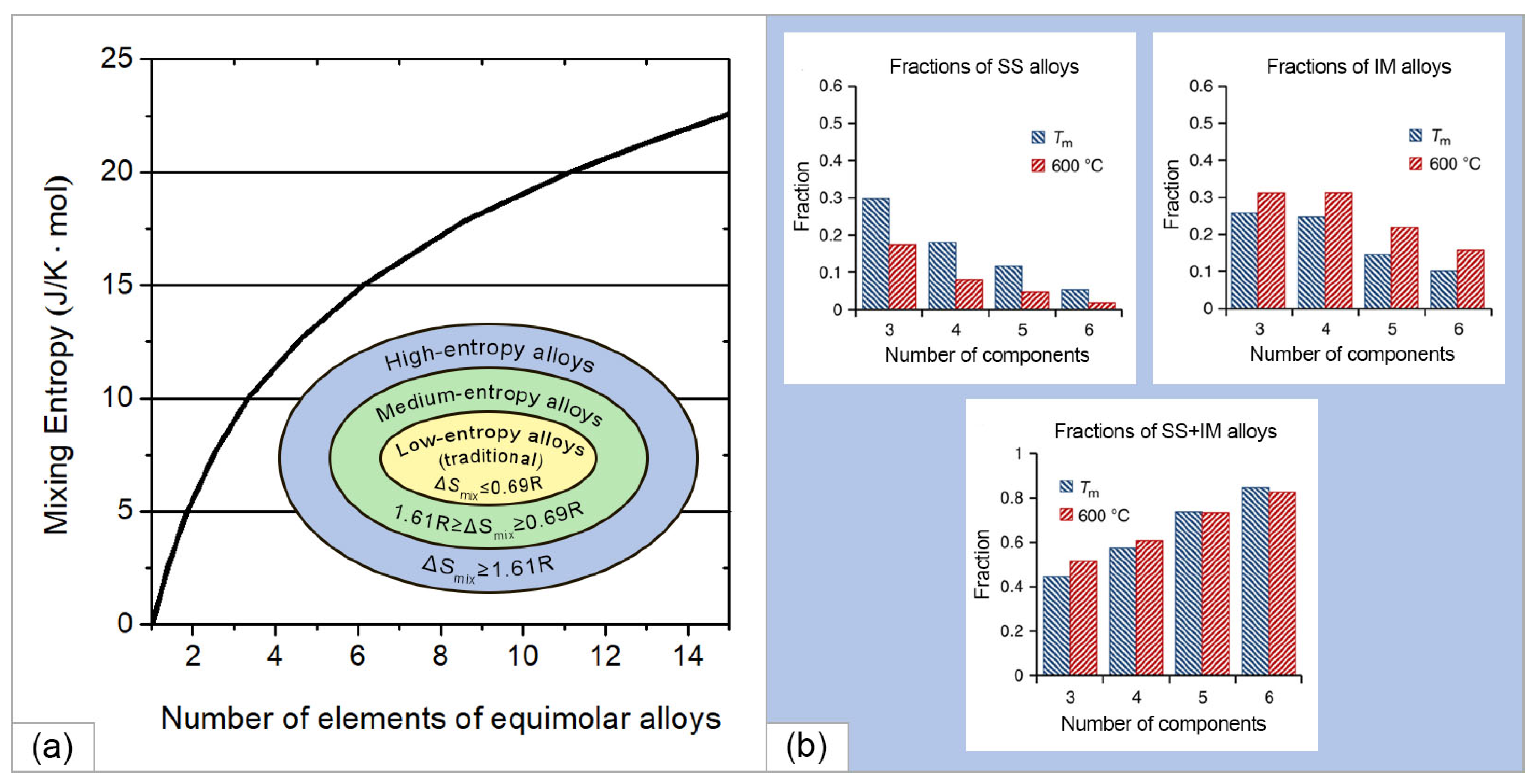
Figure 3. (a) The ratio between the entropy of mixing and the number of elements in a completely disordered equimolar alloy and inset in (a) categories of alloys based on the entropy approach (constructed using the data presented in [30] with permission of the LAVOISIER); (b) calculated fraction of different type of alloys on the number of components at the melting temperature (Tm) and at 600 °C (reproduced from [31]).
An increase in the number of components in an alloy increases the configurational entropy, which reduces the Gibbs energy, which is also facilitated by an increase in temperature. The competing factor is the change in enthalpy; however, at a high entropy of mixing and a total negative change in the Gibbs energy, a homogeneous disordered solid solution is formed instead of a multiphase system. In this case, possible processes of spinodal decomposition, the formation of intermetallic compounds, and the appearance of ordered structures and secondary phases are suppressed. It is also noted in [30] that in HEAs the percentage of individual components in the HEA can vary from 5 to 35%, which significantly expands the range of materials under consideration. It is worth noting the surprising result obtained by Senkov et al. in [31]. The authors evaluated 134,547 alloy systems using a calculated phase diagram (CALPHAD) method and found that the formation of solid solution (SS) or intermetallic alloys (IM) became less likely as the number of elements increased, while the probability of the formation of mixed SS + IM phases increased (Figure 3b). As the number of elements increases, ΔSmix rises slowly while the probability of at least one pair of elements favoring IM formation increases more rapidly, explaining this apparent contradiction with the major principle of HEAs.
The following quantitative empirical factors (Ω and δ criteria) determine the stability of HEAs:
where , is a mixing entropy; ΔHmix is a mixing enthalpy; ci and ri are a concentration and an atomic radius of the i-th element, respectively; and r¯ is an average atomic radius. The Ω value characterizes the ratio between the mixing entropy and the enthalpy change, while the δ value characterizes the difference in the atomic radii of the elements included in the HEA. The Ω and δ criteria values equal to 1.1 and 6.6 were determined in [35] that ensured stability of the TiZrNbMoVx and CoCrFeNiAlNbx alloys.
The difference in the values of the electronegativity of the elements included in the HES was also considered as a stability factor (Δχ criterion):
where is the electronegativity of the i-th element, and χ¯¯ is an average electronegativity. The low value of the Δχ criterion ensures a uniform ability to attract electrons across the lattice, leading to a stable solid solution [36]. Polletti and Battezzati assigned the values of Δχ<6% and δ<6% as a guideline for the selection of elements to predict new HEAs [37]. The stability of a high-entropy state in HEAs is also affected by such parameters as the density of valence (VEC) and free electrons (e/a), i.e., their number per atom [37][38].
In addition to the configuration component, the total value of entropy also includes temperature-dependent contributions, vibrational, electron, and magnetic. Using finite-temperature ab initio methods, Ma et al. [39] revealed that entropy contributions beyond the configurational contribution were crucial for determining phase stability (for hcp, fcc, and bcc structures) and such properties of the Cantor alloy (CoCrFeMnNi) as thermal expansion and bulk modulus. According to their results, electronic and magnetic entropies can contribute up to 50% of the configurational entropy value. Shuo Wang et al. [40], when considering electronic and thermodynamic properties of this alloy in nonmagnetic (NM) and ferrimagnetic (FIM) states, demonstrated that the magnetic properties of the constituent atoms play an important role in the thermodynamics of this HEA. Calorimetric measurements of thermal entropy of the series of the HEAs, including the Cantor alloy by Haas et al. [41], showed that it was a few folds higher than the configurational contribution. However, for alloys with the same crystal structure, the thermal contributions did not depend on the number and concentration of the alloying elements; therefore, it was directly demonstrated that despite the thermal entropy being significantly higher than the configurational entropy, it does not increase the thermal stability of HEAs relative to simple alloys or pure metals.
It is worth mentioning that an increasing number of recent studies have been revealing that the formation of single-phase solid solutions in HEAs show a weak dependence on maximization of ΔSmix through equiatomic ratios of elements. Moreover, the entropy maximum has been shown to be not the most significant parameter in the creation of multicomponent alloys with high functional properties. On the basis of these studies, the review by Li and Raabe [42] demonstrated that by changing the strategy of creating alloys from single-phase equiatomic to two- or multi-phase non-equiatomic, it is possible to obtain high-strength and superplastic HEAs.
2.2. HEA Synthesis Methods
A wide range of synthesis techniques has been developed for HEAs. Methods of synthesis, both bulk and powder-based, have been reviewed in a number of review papers [10][43][44]. The most commonly used solid-state method to obtain HEA powders is that of metal alloying as it is the most simple and most suitable method due to its increased solid solubility, high level of homogeneity, and room-temperature processing [45]. The method includes high-energy planetary ball milling (usually using stainless steel balls) in a protective milling media (Figure 4a). Mechanical alloying is followed by the consolidation of HEAs using, for example, spark plasma sintering (SPS) [46]. The second most commonly used method to obtain full pre-alloyed HEA powders suitable, for example, for additive manufacturing is that of atomizing, using different gas or liquid streams (Figure 4b) [47]. HEAs can also be manufactured via liquid-state synthesis methods using melting and casting techniques such as conventional arc melting [48][49] (Figure 4c), as well as vacuum induction melting, directional solidification, infiltration (Figure 4d), etc. Gas-state HEAs synthesis methods, through the deposition of thin HEAs films, include the plasma spray process (Figure 4e), thermal spraying, and magnetron sputtering (Figure 4f), molecular beam epitaxy, vapor deposition, etc. More detailed descriptions of liquid and gas-state methods are given in the recent reviews [50][51]. Among the most cost-effective methods to form HEA coatings is electrochemical deposition which, it should also be noted, does not require complicated equipment [52]. This provides the opportunity to control the film thickness and content simply by regulating the deposition parameters such as current density and applied potential.
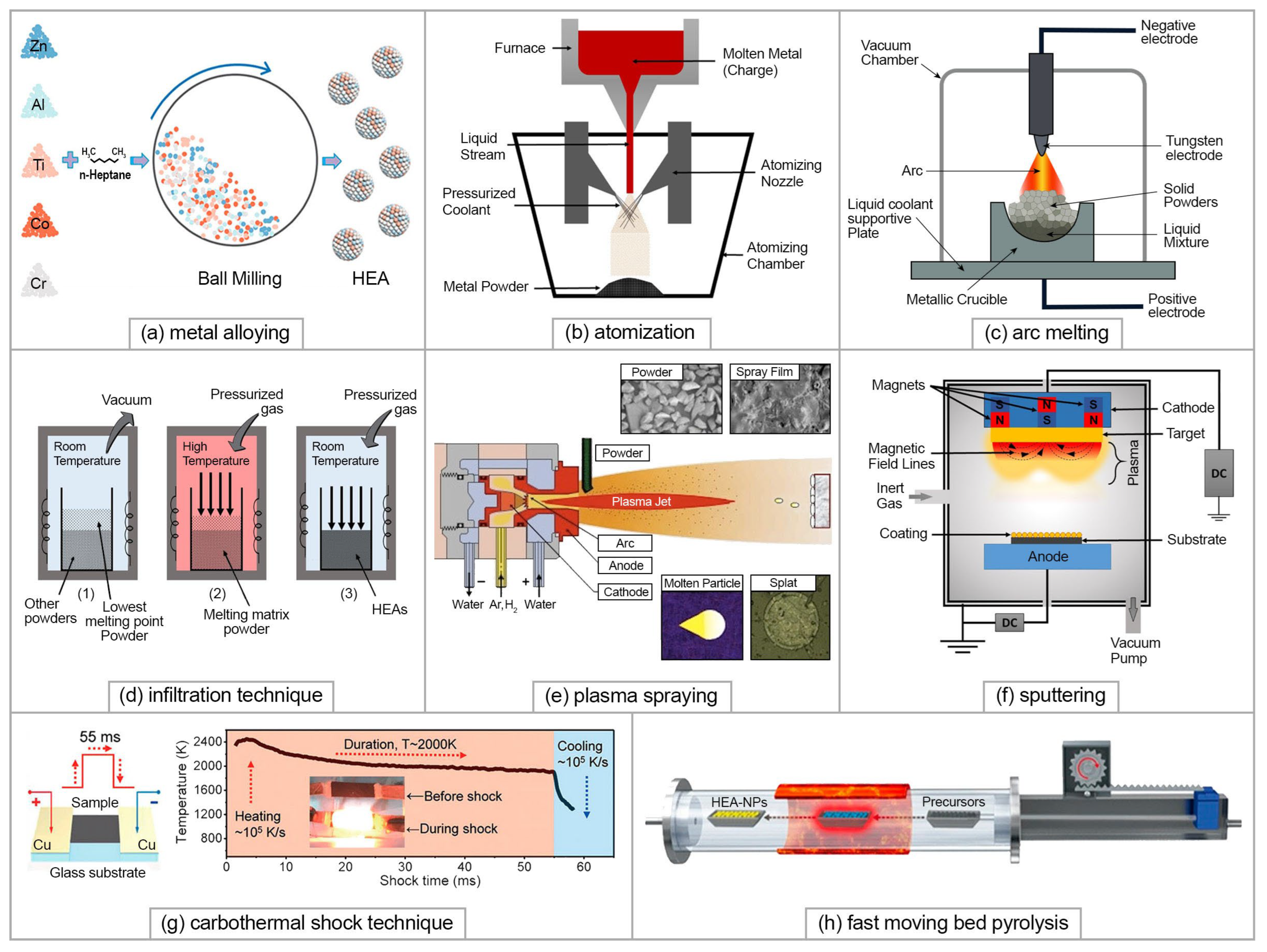
Figure 4. HEA synthesis methods: (a) metal alloying; (b) atomization; (c) arc melting; (d) infiltration; (e) plasma spraying; (f) sputtering; (g) carbothermal shock technique; (h) fast-moving bed pyrolysis ((a,g,h) are reprinted with permission from [28], Copyright (2020) American Chemical Society; (b,d,f) are reproduced from [50] with permission of Springer Nature; (e) is reproduced from [51] with permission of the Royal Society of Chemistry).
Nanosized HEAs are reported to have superior performance in the fields closely related to the fuel cell technology, namely catalysis, energy storage, and conversion [28][53][54]. However, their production is challenging due to the critical conditions that are required for the synthesis, such as heating up to extremally high temperatures followed by rapid cooling to “freeze” the nonequilibrium state. There are a few synthesis techniques which allow HEA nanoparticles to be obtained: a carbothermal shock technique [55] (Figure 4g), sputter deposition [56], solvothermal synthesis [57], and fast-moving bed pyrolysis method [58] (Figure 4h), a microwave heating method that utilizes carbon substrates [59], etc.
Kinetically controlled laser synthesis was reported as a new, highly reproducible, and scalable method to obtain HEA’s nanoparticles. Due to fast kinetics, it allows the formation of a large number of isolated ultrafine nanoparticles with properties close to those of the ablation target used [60]. Recently, Wang et al. proposed using laser scanning ablation as a simple and general approach to synthesizing both high-entropy alloy and ceramic nanoparticles [61]. The advantage of this method is that it can be implemented at atmospheric temperature and pressure, both for synthesis where there is a substrate or where no substrate is used. The ultrarapid process ensures the synthesis of HEA from up to nine metallic elements regardless of their thermodynamic solubility.
2.3. HEAs Applications
Although conventional alloys are presently used in modern advanced applications, it should be noted that the development of new alloys based on one or two major elements gradually approached the limit of feasible combinations at the end of the twentieth century [62]. This saturation created certain difficulties in meeting material requirements in the face of the anticipated technology-driven performance leap. Under these circumstances, HEA and related materials can provide new advanced features. Studies undertaken in HEA high temperature applications have shown that appropriate composition design and process selection can lead to HEA replacing traditional alloys for such energy-related applications as energy conversion and storage [63], hydrogen storage [27][64], catalysis [28], electrocatalysis [65][66], electrocatalysis for hydrogen evolution, oxygen evolution, and oxygen reduction reaction [67][68], surface electrocatalysis [69], nuclear power [70][71][72], lithium and sodium batteries [73][74], and coatings for energy applications [75].
References
- Sazali, N.; Wan Salleh, W.N.; Jamaludin, A.S.; Mhd Razali, M.N. New Perspectives on Fuel Cell Technology: A Brief Review. Membranes 2020, 10, 99.
- Ramadhani, F.; Hussain, M.A.; Mokhlis, H.; Hajimolana, S. Optimization Strategies for Solid Oxide Fuel Cell (SOFC) Application: A Literature Survey. Renew. Sustain. Energy Rev. 2017, 76, 460–484.
- Lin, G.; Wang, X.; Rezazadeh, A. Electrical Energy Storage from a Combined Energy Process Based on Solid Oxide Fuel Cell and Use of Waste Heat. Sustain. Energy Technol. Assess. 2021, 48, 101663.
- Rupiper, L.N.; Skabelund, B.B.; Ghotkar, R.; Milcarek, R.J. Impact of Fuel Type on the Performance of a Solid Oxide Fuel Cell Integrated with a Gas Turbine. Sustain. Energy Technol. Assess. 2022, 51, 101959.
- Ampah, J.D.; Afrane, S.; Agyekum, E.B.; Adun, H.; Yusuf, A.A.; Bamisile, O. Electric Vehicles Development in Sub-Saharan Africa: Performance Assessment of Standalone Renewable Energy Systems for Hydrogen Refuelling and Electricity Charging Stations (HRECS). J. Clean. Prod. 2022, 376, 134238.
- Mendonça, C.; Ferreira, A.; Santos, D.M.F. Towards the Commercialization of Solid Oxide Fuel Cells: Recent Advances in Materials and Integration Strategies. Fuels 2021, 2, 393–419.
- Medvedev, D.A.; Lyagaeva, J.G.; Gorbova, E.V.; Demin, A.K.; Tsiakaras, P. Advanced Materials for SOFC Application: Strategies for the Development of Highly Conductive and Stable Solid Oxide Proton Electrolytes. Prog. Mater. Sci. 2016, 75, 38–79.
- Pikalova, E.Y.; Kalinina, E.G. Solid Oxide Fuel Cells Based on Ceramic Membranes with Mixed Conductivity: Improving Efficiency. Russ. Chem. Rev. 2021, 90, 703–749.
- Sarkar, A.; Wang, Q.; Schiele, A.; Chellali, M.R.; Bhattacharya, S.S.; Wang, D.; Brezesinski, T.; Hahn, H.; Velasco, L.; Breitung, B. High-Entropy Oxides: Fundamental Aspects and Electrochemical Properties. Adv. Mater. 2019, 31, 1806236.
- Gelchinski, B.R.; Balyakin, I.A.; Yuryev, A.A.; Rempel, A.A. High-Entropy Alloys: Properties and Prospects of Application as Protective Coatings. Russ. Chem. Rev. 2022, 91, RCR5023.
- Akrami, S.; Edalati, P.; Fuji, M.; Edalati, K. High-Entropy Ceramics: Review of Principles, Production and Applications. Mater. Sci. Eng. R Rep. 2021, 146, 100644.
- Gazda, M.; Miruszewski, T.; Jaworski, D.; Mielewczyk-Gryń, A.; Skubida, W.; Wachowski, S.; Winiarz, P.; Dzierzgowski, K.; Łapiński, M.; Szpunar, I.; et al. Novel Class of Proton Conducting Materials—High Entropy Oxides. ACS Mater. Lett. 2020, 2, 1315–1321.
- Spiridigliozzi, L.; Ferone, C.; Cioffi, R.; Accardo, G.; Frattini, D.; Dell’Agli, G. Entropy-Stabilized Oxides Owning Fluorite Structure Obtained by Hydrothermal Treatment. Materials 2020, 13, 558.
- Zhang, F.; Cheng, F.; Cheng, C.; Guo, M.; Liu, Y.; Miao, Y.; Gao, F.; Wang, X. Preparation and Electrical Conductivity of (Zr, Hf, Pr, Y, La) O High Entropy Fluorite Oxides. J. Mater. Sci. Technol. 2022, 105, 122–130.
- Shijie, Z.; Na, L.; Liping, S.; Qiang, L.; Lihua, H.; Hui, Z. A Novel High-Entropy Cathode with the A2BO4-Type Structure for Solid Oxide Fuel Cells. J. Alloy. Compd. 2022, 895, 162548.
- Dąbrowa, J.; Adamczyk, J.; Stępień, A.; Zajusz, M.; Bar, K.; Berent, K.; Świerczek, K. Synthesis and Properties of the Gallium-Containing Ruddlesden-Popper Oxides with High-Entropy B-Site Arrangement. Materials 2022, 15, 6500.
- Han, X.; Yang, Y.; Fan, Y.; Ni, H.; Guo, Y.; Chen, Y.; Ou, X.; Ling, Y. New Approach to Enhance Sr-Free Cathode Performance by High-Entropy Multi-Component Transition Metal Coupling. Ceram. Int. 2021, 47, 17383–17390.
- Ma, G.; Chen, D.; Ji, S.; Bai, X.; Wang, X.; Huan, Y.; Dong, D.; Hu, X.; Wei, T. Medium-Entropy SrV1/3Fe1/3Mo1/3O3 with High Conductivity and Strong Stability as SOFCs High-Performance Anode. Materials 2022, 15, 2298.
- Zhao, Q.; Geng, S.; Zhang, Y.; Chen, G.; Zhu, S.; Wang, F. High-Entropy FeCoNiMnCu Alloy Coating on Ferritic Stainless Steel for Solid Oxide Fuel Cell Interconnects. J. Alloy. Compd. 2022, 908, 164608.
- Huang, K.H.; Yeh, J.W. A Study on the Multicomponent Alloy Systems Containing Equal-Mole Elements. Master’s Thesis, National Tsing Hua University, Hsinchu, Taiwan, 1996.
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural Development in Equiatomic Multicomponent Alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218.
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303.
- Chen, J.; Zhou, X.; Wang, W.; Liu, B.; Lv, Y.; Yang, W.; Xu, D.; Liu, Y. A Review on Fundamental of High Entropy Alloys with Promising High–Temperature Properties. J. Alloy. Compd. 2018, 760, 15–30.
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-Entropy Alloy: Challenges and Prospects. Mater. Today 2016, 19, 349–362.
- Miracle, D.B.; Senkov, O.N. A Critical Review of High Entropy Alloys and Related Concepts. Acta Mater. 2017, 122, 448–511.
- George, E.P.; Raabe, D.; Ritchie, R. High-Entropy Alloys. Nat. Rev. Mater. 2019, 4, 515–534.
- Marques, F.; Balcerzak, M.; Winkelmann, F.; Zepon, G.; Felderhoff, M. Review and Outlook on High-Entropy Alloys for Hydrogen Storage. Energy Environ. Sci. 2021, 14, 5191–5227.
- Xin, Y.; Li, S.; Qian, Y.; Zhu, W.; Yuan, H.; Jiang, P.; Guo, R.; Wang, L. High-Entropy Alloys as a Platform for Catalysis: Progress, Challenges, and Opportunities. ACS Catal. 2020, 10, 11280–11306.
- Cheng, H.; Pan, Z.; Fu, Y.; Wang, X.; Wei, Y.; Luo, H.; Li, X. Review—Corrosion-Resistant High-Entropy Alloy Coatings: A Review. J. Electrochem. Soc. 2021, 168, 111502.
- Yeh, J.W. Recent Progress in High-Entropy Alloys. Eur. J. Control 2006, 31, 633–648.
- Senkov, O.N.; Miller, J.D.; Miracle, D.B.; Woodward, C. Accelerated Exploration of Multi-Principal Element Alloys with Solid Solution Phases. Nat. Commun. 2015, 6, 6529.
- Gorsse, S.; Couzinié, J.-P.; Miracle, D.B. From High-Entropy Alloys to Complex Concentrated Alloys. Comptes Rendus Phys. 2018, 19, 721–736.
- Zhou, Y.; Zhou, D.; Jin, X.; Zhang, L.; Du, X.; Li, B. Design of Non-Equiatomic Medium-Entropy Alloys. Sci. Rep. 2018, 8, 1236.
- Łoński, W.; Spilka, M.; Kądziołka-Gaweł, M.; Gębara, P.; Radoń, A.; Warski, T.; Młynarek-Żak, K.; Babilas, R. The Effect of Cooling Rate on the Structure and Selected Properties of AlCoCrFeNiSix (x = 0; 0.25; 0.5; 0.75) High Entropy Alloys. J. Alloy. Compd. 2022, 905, 164074.
- Zhang, Y.; Yang, X.; Liaw, P.K. Alloy Design and Properties Optimization of High-Entropy Alloys. JOM 2012, 64, 830–838.
- Toda-Caraballo, I.; Rivera-Díaz-del-Castillo, P.E.J. A Criterion for the Formation of High Entropy Alloys Based on Lattice Distortion. Intermetallics 2016, 71, 76–87.
- Poletti, M.G.; Battezzati, L. Electronic and Thermodynamic Criteria for the Occurrence of High Entropy Alloys in Metallic Systems. Acta Mater. 2014, 75, 297–306.
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of Valence Electron Concentration on Stability of Fcc or Bcc Phase in High Entropy Alloys. J. Appl. Phys. 2011, 109, 103505.
- Ma, D.; Grabowski, B.; Körmann, F.; Neugebauer, J.; Raabe, D. Ab Initio Thermodynamics of the CoCrFeMnNi High Entropy Alloy: Importance of Entropy Contributions beyond the Configurational One. Acta Mater. 2015, 100, 90–97.
- Wang, S.; Zhang, T.; Hou, H.; Zhao, Y. The Magnetic, Electronic, and Thermodynamic Properties of High Entropy Alloy CrMnFeCoNi: A First-Principles Study. Phys. Status Solidi B 2018, 255, 1800306.
- Haas, S.; Mosbacher, M.; Senkov, O.; Feuerbacher, M.; Freudenberger, J.; Gezgin, S.; Völkl, R.; Glatzel, U. Entropy Determination of Single-Phase High Entropy Alloys with Different Crystal Structures over a Wide Temperature Range. Entropy 2018, 20, 654.
- Li, Z.; Raabe, D. Strong and Ductile Non-Equiatomic High-Entropy Alloys: Design, Processing, Microstructure, and Mechanical Properties. JOM 2017, 69, 2099–2106.
- Kaushik, N.; Meena, A.; Mali, H.S. High Entropy Alloy Synthesis, Characterisation, Manufacturing & Potential Applications: A Review. Mater. Manuf. Process. 2022, 37, 1085–1109.
- Torralba, J.M.; Alvaredo, P.; García-Junceda, A. High-Entropy Alloys Fabricated via Powder Metallurgy. A Critical Review. Powder Metall. 2019, 62, 84–114.
- Rajendrachari, S. An Overview of High-Entropy Alloys Prepared by Mechanical Alloying Followed by the Characterization of Their Microstructure and Various Properties. Alloys 2022, 1, 116–132.
- Průša, F.; Šenková, A.; Vojtěch, D.; Čapek, J.; Bernatiková, A. High Entropy Alloys Prepared by Combination of Mechanical Alloying and Spark Plasma Sintering. Manuf. Technol. 2016, 16, 1350–1354.
- Wang, P.; Huang, P.; Ng, F.L.; Sin, W.J.; Lu, S.; Nai, M.L.S.; Dong, Z.; Wei, J. Additively Manufactured CoCrFeNiMn High-Entropy Alloy via Pre-Alloyed Powder. Mater. Des. 2019, 168, 107576.
- Ryltsev, R.E.; Estemirova, S.K.; Gaviko, V.S.; Yagodin, D.A.; Bykov, V.A.; Sterkhov, E.V.; Cherepanova, L.A.; Sipatov, I.S.; Balyakin, I.A.; Uporov, S.A. Structural Evolution in TiZrHfNb High-Entropy Alloy. Materialia 2022, 21, 101311.
- Uporov, S.A.; Ryltsev, R.E.; Sidorov, V.A.; Estemirova, S.K.; Sterkhov, E.V.; Balyakin, I.A.; Chtchelkatchev, N.M. Pressure Effects on Electronic Structure and Electrical Conductivity of TiZrHfNb High-Entropy Alloy. Intermetallics 2022, 140, 107394.
- Alshataif, Y.A.; Sivasankaran, S.; Al-Mufadi, F.A.; Alaboodi, A.S.; Ammar, H.R. Manufacturing Methods, Microstructural and Mechanical Properties Evolutions of High-Entropy Alloys: A Review. Met. Mater. Int. 2020, 26, 1099–1133.
- Wang, X.; Guo, W.; Fu, Y. High-Entropy Alloys: Emerging Materials for Advanced Functional Applications. J. Mater. Chem. A 2021, 9, 663–701.
- Shojaei, Z.; Khayati, G.R.; Darezereshki, E. Review of Electrodeposition Methods for the Preparation of High-Entropy Alloys. Int. J. Miner. Metall. Mater. 2022, 29, 1683–1696.
- Zhang, G.; Ming, K.; Kang, J.; Huang, Q.; Zhang, Z.; Zheng, X.; Bi, X. High Entropy Alloy as a Highly Active and Stable Electrocatalyst for Hydrogen Evolution Reaction. Electrochim. Acta 2018, 279, 19–23.
- Dai, W.; Lu, T.; Pan, Y. Novel and Promising Electrocatalyst for Oxygen Evolution Reaction Based on MnFeCoNi High Entropy Alloy. J. Power Sources 2019, 430, 104–111.
- Yao, Y.; Huang, Z.; Xie, P.; Lacey, S.D.; Jacob, R.J.; Xie, H.; Chen, F.; Nie, A.; Pu, T.; Rehwoldt, M.; et al. Carbothermal Shock Synthesis of High-Entropy-Alloy Nanoparticles. Science 2018, 359, 1489–1494.
- Tsai, C.-F.; Wu, P.-W.; Lin, P.; Chao, C.-G.; Yeh, K.-Y. Sputter Deposition of Multi-Element Nanoparticles as Electrocatalysts for Methanol Oxidation. Jpn. J. Appl. Phys. 2008, 47, 5755–5761.
- Bondesgaard, M.; Broge, N.L.N.; Mamakhel, A.; Bremholm, M.; Iversen, B.B. General Solvothermal Synthesis Method for Complete Solubility Range Bimetallic and High-Entropy Alloy Nanocatalysts. Adv. Funct. Mater. 2019, 29, 1905933.
- Gao, S.; Hao, S.; Huang, Z.; Yuan, Y.; Han, S.; Lei, L.; Zhang, X.; Shahbazian-Yassar, R.; Lu, J. Synthesis of High-Entropy Alloy Nanoparticles on Supports by the Fast Moving Bed Pyrolysis. Nat. Commun. 2020, 11, 2016.
- Qiao, H.; Saray, M.T.; Wang, X.; Xu, S.; Chen, G.; Huang, Z.; Chen, C.; Zhong, G.; Dong, Q.; Hong, M.; et al. Scalable Synthesis of High Entropy Alloy Nanoparticles by Microwave Heating. ACS Nano 2021, 15, 14928–14937.
- Shih, C.-Y.; Streubel, R.; Heberle, J.; Letzel, A.; Shugaev, M.V.; Wu, C.; Schmidt, M.; Gökce, B.; Barcikowski, S.; Zhigilei, L.V. Two Mechanisms of Nanoparticle Generation in Picosecond Laser Ablation in Liquids: The Origin of the Bimodal Size Distribution. Nanoscale 2018, 10, 6900–6910.
- Wang, B.; Wang, C.; Yu, X.; Cao, Y.; Gao, L.; Wu, C.; Yao, Y.; Lin, Z.; Zou, Z. General Synthesis of High-Entropy Alloy and Ceramic Nanoparticles in Nanoseconds. Nat. Synth. 2022, 1, 138–146.
- Murty, B.S.; Yeh, J.W.; Ranganathan, S. Applications and Future Directions. In High Entropy Alloys; Elsevier: Amsterdam, The Netherlands, 2014; pp. 159–169.
- Straumal, B.; Korneva, A.; Kuzmin, A.; Klinger, L.; Lopez, G.A.; Vershinin, N.; Straumal, A.; Gornakova, A. High Entropy Alloys for Energy Conversion and Storage: A Review of Grain Boundary Wetting Phenomena. Energies 2022, 15, 7130.
- Dewangan, S.K.; Mohan, M.; Kumar, V.; Sharma, A.; Ahn, B. A Comprehensive Review of the Prospects for Future Hydrogen Storage in Materials-application and Outstanding Issues. Int. J. Energy Res. 2022, 46, 16150–16177.
- Wang, K.; Huang, J.; Chen, H.; Wang, Y.; Yan, W.; Yuan, X.; Song, S.; Zhang, J.; Sun, X. Recent Progress in High Entropy Alloys for Electrocatalysts. Electrochem. Energy Rev. 2022, 5, 17.
- Glasscott, M.W. Classifying and Benchmarking High-Entropy Alloys and Associated Materials for Electrocatalysis: A Brief Review of Best Practices. Curr. Opin. Electrochem. 2022, 34, 100976.
- Sarac, B.; Zadorozhnyy, V.; Ivanov, Y.P.; Spieckermann, F.; Klyamkin, S.; Berdonosova, E.; Serov, M.; Kaloshkin, S.; Greer, A.L.; Sarac, A.S.; et al. Transition Metal-Based High Entropy Alloy Microfiber Electrodes: Corrosion Behavior and Hydrogen Activity. Corros. Sci. 2021, 193, 109880.
- Huo, X.; Yu, H.; Xing, B.; Zuo, X.; Zhang, N. Review of High Entropy Alloys Electrocatalysts for Hydrogen Evolution, Oxygen Evolution, and Oxygen Reduction Reaction. Chem. Rec. 2022, e202200175.
- Pedersen, J.K.; Batchelor, T.A.A.; Yan, D.; Skjegstad, L.E.J.; Rossmeisl, J. Surface Electrocatalysis on High-Entropy Alloys. Curr. Opin. Electrochem. 2021, 26, 100651.
- Wang, Z.; Liu, F.; Guo, Z.; Zhang, J.; Wang, L.; Yan, G. Advance in and Prospect of Moderator Materials for Space Nuclear Reactors. Int. J. Energy Res. 2021, 45, 11493–11509.
- Shi, T.; Lei, P.-H.; Yan, X.; Li, J.; Zhou, Y.-D.; Wang, Y.-P.; Su, Z.-X.; Dou, Y.-K.; He, X.-F.; Yun, D.; et al. Current Development of Body-Centered Cubic High-Entropy Alloys for Nuclear Applications. Tungsten 2021, 3, 197–217.
- Pickering, E.J.; Carruthers, A.W.; Barron, P.J.; Middleburgh, S.C.; Armstrong, D.E.J.; Gandy, A.S. High-Entropy Alloys for Advanced Nuclear Applications. Entropy 2021, 23, 98.
- Sturman, J.W.; Baranova, E.A.; Abu-Lebdeh, Y. Review: High-Entropy Materials for Lithium-Ion Battery Electrodes. Front. Energy Res. 2022, 10, 862551.
- Zheng, S.-M.; Tian, Y.-R.; Liu, Y.-X.; Wang, S.; Hu, C.-Q.; Wang, B.; Wang, K.-M. Alloy Anodes for Sodium-Ion Batteries. Rare Met. 2021, 40, 272–289.
- Dixit, S.; Rodriguez, S.; Jones, M.R.; Buzby, P.; Dixit, R.; Argibay, N.; DelRio, F.W.; Lim, H.H.; Fleming, D. Refractory High-Entropy Alloy Coatings for High-Temperature Aerospace and Energy Applications. J. Therm. Spray Technol. 2022, 31, 1021–1031.
More
Information
Subjects:
Chemistry, Physical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
5 times
(View History)
Update Date:
11 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





