Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jianping Xu | -- | 4715 | 2023-01-05 13:40:57 | | | |
| 2 | Rita Xu | Meta information modification | 4715 | 2023-01-06 02:43:59 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lin, L.; Xu, J. Production of Fungal Pigments. Encyclopedia. Available online: https://encyclopedia.pub/entry/39794 (accessed on 08 February 2026).
Lin L, Xu J. Production of Fungal Pigments. Encyclopedia. Available at: https://encyclopedia.pub/entry/39794. Accessed February 08, 2026.
Lin, Lan, Jianping Xu. "Production of Fungal Pigments" Encyclopedia, https://encyclopedia.pub/entry/39794 (accessed February 08, 2026).
Lin, L., & Xu, J. (2023, January 05). Production of Fungal Pigments. In Encyclopedia. https://encyclopedia.pub/entry/39794
Lin, Lan and Jianping Xu. "Production of Fungal Pigments." Encyclopedia. Web. 05 January, 2023.
Copy Citation
Due to the negative environmental and health effects of synthetic colorants, pigments of natural origins of plants and microbes constitute an abundant source for the food, cosmetic, textile, and pharmaceutical industries. The demands for natural alternatives, which involve natural colorants and natural biological processes for their production, have been growing rapidly. Fungi contain some of the most prolific pigment producers, and they excel in bioavailability, yield, cost-effectiveness, and ease of large-scale cell culture as well as downstream processing.
fungi
pigment biosynthesis
stress factors
pigmentogenesis
1. Introduction
Colours constitute an indispensable part of our life. Most living organisms on our planet display certain coloured hues through absorption and refraction of specific wavelengths of light [1][2]. All pigments possess conjugated moieties, namely chromophores, that facilitate electronic resonances and mediate energy transfers within and between cells. The energy captured and/or reflected by pigments has been shown to be involved in multiple biological processes, ranging from the utilization of solar energy for metabolic needs and photo-protection for the maintenance of life, to camouflage, as well as mate and pollinator attraction.
Fungi are ubiquitously distributed in both natural and anthropogenic environments, featuring a vast diversity of species, morphology, and pigmentation [1][3][4]. They can be favourable or detrimental to humans, the latter of which involves a range of mycoses, especially in immunocompromised individuals with immunological deficiency, HIV infection, and undergoing organ transplantation or cancer therapy [5][6]. The significant roles of fungal pigments in microbial pathogenesis have been extensively investigated and documented [1][5][6][7][8]. Studies using fungal mutants with altered pigmentation have revealed how these pigments may provide a survival advantage for pathogens in host environments [8], for example, to aid in the evasion of the host immune system. Similarly, confronted with external environmental stresses such as ultraviolet light, irradiation, nutrient limitation, and osmotic pressure, fungi have evolved the mechanism of pigmentogenesis as adaptive means for their survival and dissemination. Indeed, as common secondary metabolites, fungal pigments have likely played an underestimated but key role in cell protection and ecological interactions with other organisms [9].
The ecological roles of fungal pigments in the interactions with abiotic and biotic factors are similarly reflected in their relevance to human health and human welfare. For example, fungal pigments have been used as natural colorants in foods and as nutraceuticals (functional foods), gaining increasing popularity in certain regions and age groups, including among the elderly for healthy aging [10]. Indeed, fungal pigments, secondary metabolites with tremendously diverse structures, have shown a diversity of bioactivities, such as anti-tumor, anti-obesity, cholesterol-lowering and/or anti-atherosclerotic, anti-Alzheimer disease, anti-oxidative, and immunosuppressive activities [1]. Together, these beneficial bioactivities of fungal pigments to humans make them attractive pharmacophores in the drug industry.
As described above, a common application of fungal pigments is as food colorants. Indeed, food colorants have been used by humans for thousands of years. Most colorants in early human history were derived from natural sources. However, over the last two centuries, synthetic colorants have been increasingly used. Unfortunately, a variety of detrimental effects have been linked to synthetic food colorants, including but not limited to attention deficit/hyperactivity in children, allergies, cancer, reproductive disorders, and neurological impairment [10]. Such reports have propelled the exploration of natural alternatives [10][11][12]. These natural alternatives include natural colorants as well as the natural biological processes that produce these colorants. For long-term sustainable development of this industry, the targeted materials and processes should have as few negative impacts on the environment as possible.
Among the many synthetic food colorants are the yellow colorants tartrazine and sunset yellow as well as the red colouring compounds carmine and amaranth [13]. These colorants have been managed and controlled based on regulations issued by the World Health Organization (WHO). However, their acceptability by consumers has been declining, predominantly due to their associations with various negative health issues and to their long-term persistence in the ecosystem while posing significant concerns to other organisms in the environment.
2. Pigmented Fungi and Biosynthesis of Pigments
2.1. Carotenoids
‘Carotene’, derived from the Latin word carota, was coined in 1831 by Wackenroder, who extracted and purified the orange pigment from carrot (Daucus carota). The names of carotenoids do not reflect their structural cues but rather are based on the stem name ‘carotene’, which is preceded by Greek prefixes (e.g., α, β, ε) that denote the two end groups [14].
The charming colours and the health-promoting effects of various carotenoids have evoked interest in several related fields. Like carotenoids from other groups of organisms, such as plants, algae, and bacteria, carotenoids of fungal origins are known to have characteristic hues of yellow, orange, and red. This is ascribed to the ubiquitous presence, in their molecular structures, of an aliphatic polyene chain comprising eight isoprene units involving light-absorbing conjugated double bonds, the latter of which is responsible for the physicochemical traits of carotenoids [1][15].
Some carotenoids act as precursors of vitamin A in mammals including humans, specifically those with β-ring end groups, such as β-carotene, zeaxanthin, and β-cryotoxanthin [16]. Indeed, carotenoids are among the most medically significant bio-pigments. Mounting evidence has revealed that carotenoids can lower the risk of cardiovascular diseases, age-related eye disorders such as macular degeneration and cataracts [17], and cancers [1][16][18].
So far, over 200 fungal species have been documented to be able to produce carotenes [19]. Carotenoid-producing fungi are very diverse, including Rhodotorula mucilaginosa [20], Rhodotorula glutinis [21], Blakeslea trispora [22], Phycomyces blakesleeanus, Mucor circinelloides, Fusarium sporotrichioides [23], Rhodosporium paludigenum, Neurospora crassa, and Xanthophyllomyces dendrorhous [24]. Among them, Blakeslea trispora stands out as a major industrial fungus used to produce β-carotene as a colorant in foods, which was authorized by EU Health and Consumer Protection Directorate General in 1995 [19].
Blakeslea trispora is a filamentous fungus capable of producing β-carotene through lycopene cyclization [25]. Lycopene is a red-coloured intermediate of the β-carotene biosynthesis in B. trispora and is converted from isopentenyl pyrophosphate (IPP, C5), the first isoprene unit of the terpenoid synthetic pathway [26]. The fungal isopentenyl pyrophosphate (IPP) is produced via the mevalonate (MVA) pathway, with acetyl-CoA as a starting precursor [1][26].
The early biosynthetic steps of β-carotene involve the sequential additions of IPP (C5) units to yield geranyl pyrophosphate (GPP, C10), farnesyl pyrophosphate (FPP, C15), and geranylgeranyl pyrophosphate (GGPP, C20) [26]. The initial compound bearing the typical aliphatic carotenoid-like structure is phytoene (a colorless molecule), converted from the condensation reaction of two GGPP units catalyzed by phytoene synthase (Figure 1). The successive step is the dehydrogenase-catalyzed conversion of phytoene to lycopene, followed by the sequential cyclization of lycopene to produce γ-carotene, and then to β-carotene, catalyzed by lycopene cyclase (Figure 1) [27][28].
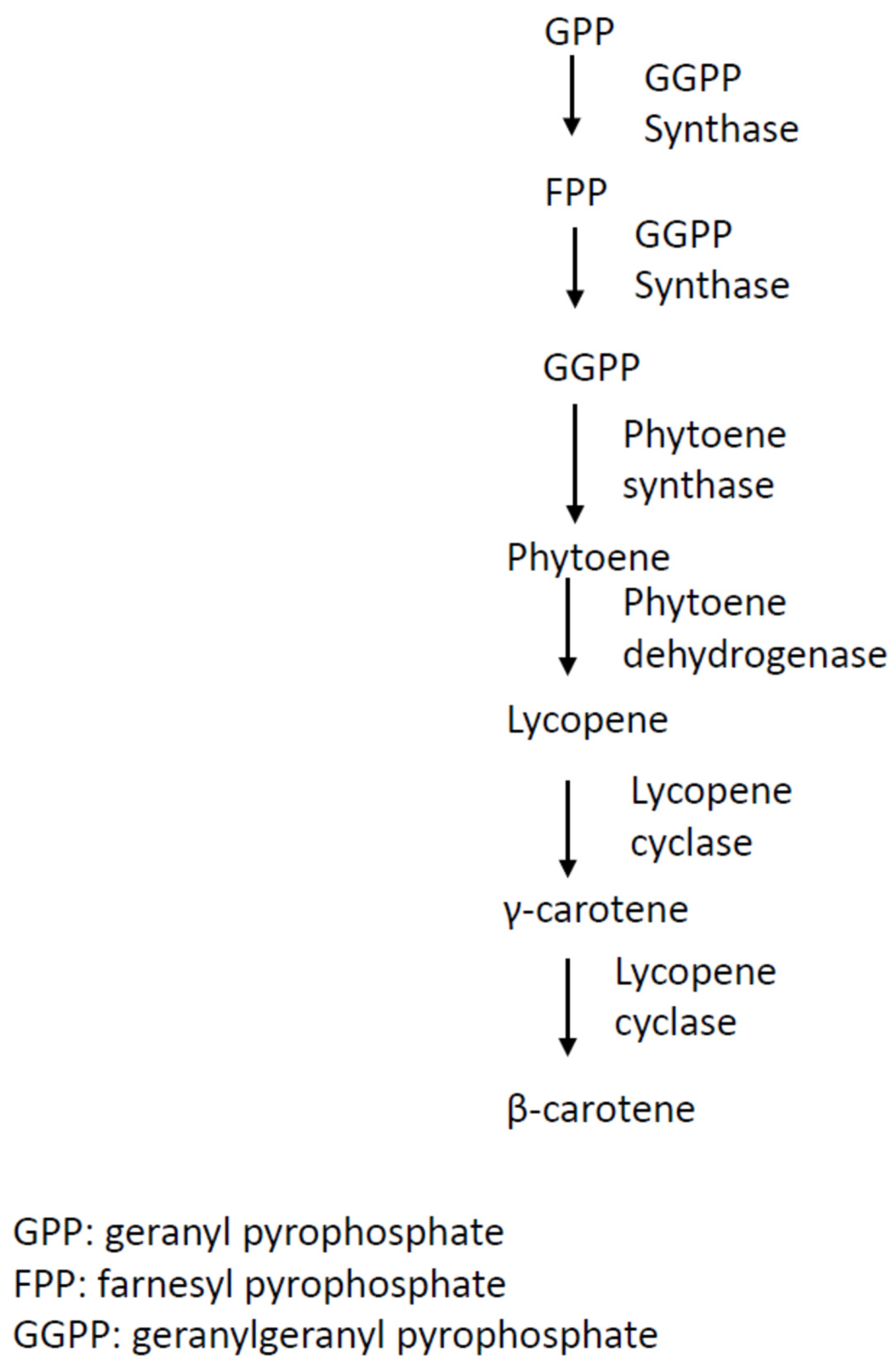
Figure 1. Biosynthesis pathway of β-carotene in Blakeslea trispora. This schematic pathway was drawn based on information reviewed in reference [29].
2.2. Melanins
Melanins of fungal origin are generated by a complicated polymerization process involving polyketides and free radicals, with the resultant compounds displaying dark green, brown, or black hues. They are resistant to chemical degradation by acids and are insoluble in most solvents; they are only susceptible to degradation by oxidation and are soluble in alkaline solvents [30].
Fungal melanins are negatively charged and hydrophobic [31]. They are deposited in fungal cell walls or as extracellular polymers accumulated in the medium around fungal cells. The production and secretion of melanin is associated with fungal resistance against ultra-violet (UV) irradiation, extreme temperature, enzymatic lysis, oxidants, and desiccation; with maintenance of an appropriate balance of metal ions; and with provision of structural rigidity to cell walls [32]. In other words, melanins represent a kind of fungal adaptation strategy for environmental stresses.
On the other hand, in some mammalian pathogenic fungi, exemplified by Cryptococcus neoformans, Aspergillus fumigatus, Candida albicans, Candida glabrata, and Candida parapsilosis, melanins might contribute to fungal virulence, reflecting a resistance mechanism of fungal pathogens to the host immune system, such as macrophages, monocytes, and dendritic cells. Recent investigations have revealed that melanin pigments can lead to antigen masking to circumvent the recognition by host immune system, thereby facilitating the survival of pathogenic fungal species against phagocytosis [6][33]. Studies with the dematiaceous (darkly-pigmented) fungus Wangiella dermatitidis, which causes a rare but potentially lethal human mycosis, have shown that loss of melanin production might result in the abolishment of invasive hyphal forms, increased vulnerability to neutrophil killing, and attenuated virulence in the murine infection models [34].
Apart from their protective role and resistance mechanism towards unfavourable conditions, which renders them promising bio-compounds in medicine and the food industry, fungal melanins are also used in the field of material engineering as a novel biopolymer [35]. Of note, two halophilic fungal species, Trimmatostroma salinum and Phaeotheca triangularis, isolated from the eastern coast of the Adriatic Sea, have been reported to produce melanin with a supply of saturated sodium chloride [36]. In addition, an Antarctic desert-inhabiting fungus, Cryomyces antarcticus, has recently been found to produce melanin pigments possessing high-dose radiation resistance [37]. Collectively, these fungal extremophiles are recognized as prolific sources of melanin with desirable physicochemical properties for industrial and medical applications.
Given that both pigmented (melanized) and albino strains of the same fungi can survive and grow under laboratory settings, melanins are not essential for fungal growth (primary metabolism). Rather, they are used for fungal defense and resistance (secondary metabolism) against external conditions, including adverse milieu within host, substantial temperature oscillation, extensive radiation exposure, oxidative stress, osmotic pressure (high salinity), desiccation, and poor nutrient supply.
Recent studies have shown that fungi usually synthesize three different kinds of melanin: cell wall immobilized DOPA (dihydroxyphenylalanine)-melanin, DHN (dihydroxynaphthalene)-melanin, and extracellular water-soluble pyomelanin (Figure 2). A. fumigatus is recognized to be able to produce the latter two, whereas C. neoformans can biosynthesize the former one [38]. In C. neoformans, DOPA-melanin biosynthesis proceeds through a series of oxidation-reduction reactions, starting from tyrosine or L-3,4 dihydroxyphenylalanine (L-dopa) (see Figure 2a for details). These compounds are subsequently oxidized to DOPA-quinone by a phenol oxidase enzyme such as laccase or tyrosinase, depending on the substrates. The ensuing reactions towards melanin synthesis proceed very quickly, yielding the intermediate dihydroxyindole, which in turn gives rise to melanin via polymerization [39].
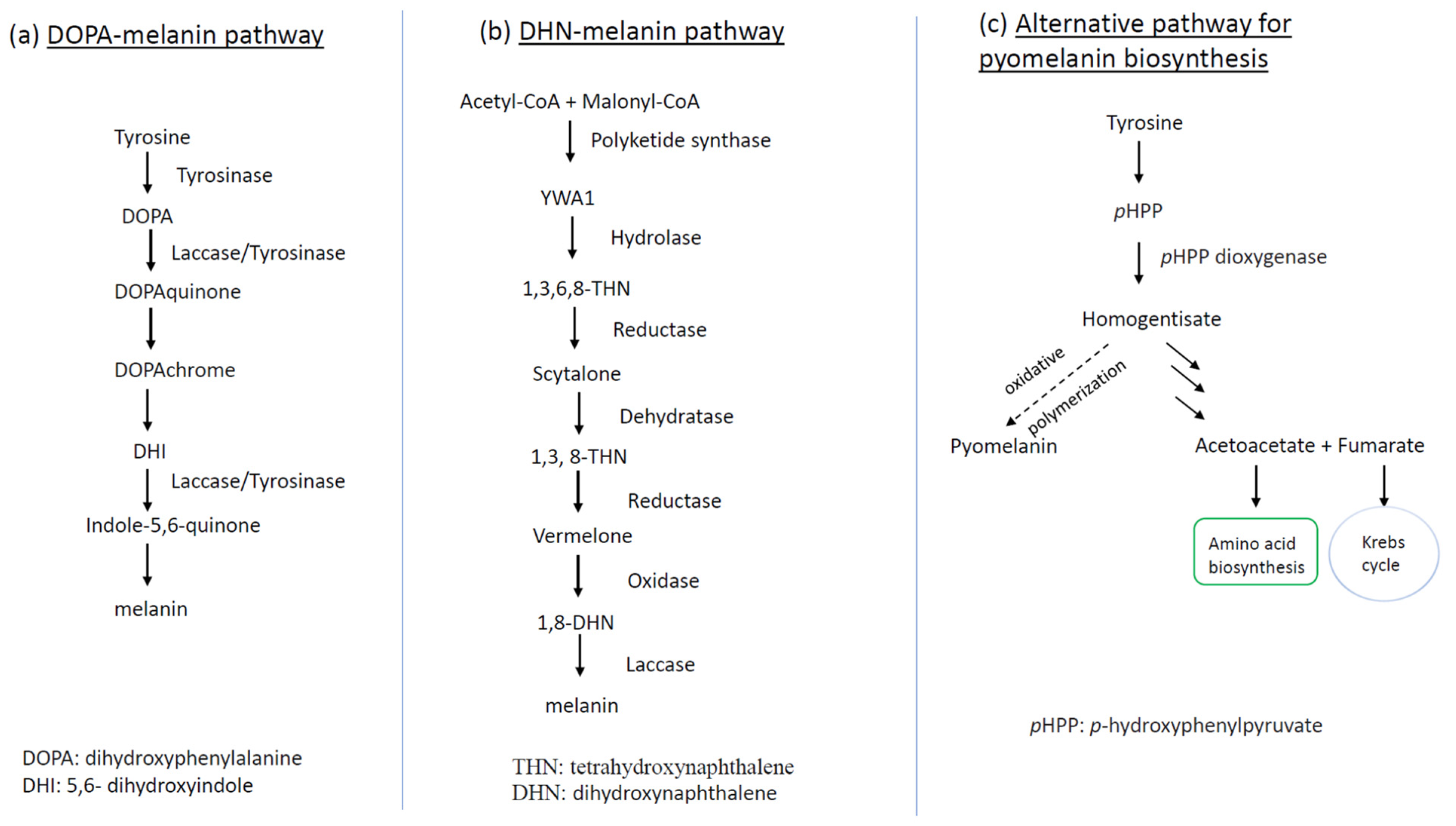
Figure 2. Schematic representation of fungal melanin biosynthetic pathways: (a) DOPA-melanin production route as shown in C. neoformans; (b) biosynthetic pathway of DHN-melanin for deposit in conidial cell wall as manifested by A. fumigatus; (c) alternative pathway of pyomelanin biosynthesis linked to conidial germination of A. fumigatus. The dotted line in (c) means that the step is active only during conidial germination. YWA1: Yellow pigment intermediate of WA polyketide synthase.
Fungi may also synthesize melanin via the DHN pathway, as exemplified by the common mold and opportunistic human pathogen A. fumigatus. The DHN-melanin biosynthetic pathway is encoded in a gene cluster consisting of six genes—abr1, abr2, ayg1, arp1, arp2, and pksP/alb1―located on its second chromosome [40]. The initial gene in the biosynthetic pathway is pksP/alb1, which encodes a polyketide synthase (PKS) catalyzing the conversion (β-ketoacyl condensation) from the acetyl-CoA and malonyl-CoA to the heptaketide napthopyrone YWA1. The second step is hydrolysis, catalyzed by the ayg1-coding enzyme, which converts YWA1 to 1,3,6,8-tetrahydroxynaphthalene (1,3,6,8-THN). Subsequently, the pathway implements a series of reduction steps followed by aromatization/dehydration, which ends in an oxidative polymerization. Briefly speaking, the resultant intermediate 1,3,6,8-THN is reduced again by Arp2p (Arp2 protein), giving rise to vermelone, the latter of which is oxidized by the copper oxidase Abr1p to form the penultimate product 1,8-DHN. The last reaction generates DHN-melanin from 1,8-DHN, catalyzed by the laccase Abr2p [41].
The other type of melanin generated by A. fumigatus is pyomelanin, a pigment with a dark brown hue. In contrast to DHN-melanin, the production of pyomelanin does not have its own biosynthetic pathway but rather proceeds via the polymerization of homogentisate, an intermediate derived from the degradation of L-tyrosine/L-phenylalanine, which is coupled with conidial germination [38][42].
Recent studies with the commercial wood-ear mushroom Auricularia auricula have shown its melanin in association with the mushroom cell wall, which is analogous to that of the model fungus C. neoformans, belonging to DOPA-melanin [43][44]. See Figure 2a for the biosynthesis of melanin in A. auricula.
Strikingly, A. auricula represents the most feasible commercial melanin producer in terms of scalability and its non-pathogenic feature. It has been proposed that fungal melanins, regardless of their precursors and biosynthetic paths, likely share similar functional groups and analogous physicochemical traits [45]. This black mushroom is found to yield up to 10% melanin in fungal dry mass [43]. A. auricula is a popular edible and medicinal mushroom in eastern Asia and is attracting increasing interest from consumers in Europe as well as North America. More recently, A. auricula mushroom wastes have been reported as a rich source of melanin for extraction, with an output of up to 11% of fungal dry mass under optimal conditions [46]. Although the industrial process of fungal melanin production is still in its infancy, in-depth explorations with A. auricula melanin—biosynthetic steps, fermentation, extraction, and isolation—are promising since this mushroom, in terms of a melanin provider, has several advantages over plant- and animal-derived sources, including independence of seasonal variations, low-cost maintenance, expedient operation, and affordable reaction conditions.
Melanin is widely regarded to possess radioprotective properties due to its ability to scavenge free radicals generated by radiation, and the presence of its numerous aromatic oligomers allowing the dissipation of high-energy recoil electrons [45][47]. It is proposed that melanized fungi isolated from extreme environments, such as the leakage areas around the Chernobyl nuclear power plant and Antarctic highlands, could be used for absorption and decontamination of nuclear waste [48].
2.3. Polyketides
Polyketide-based pigments of fungal origins are abundantly produced by many fungi, including most filamentous ascomycete genera. Anthraquinones and naphthoquinones are representative classes of polyketide-based pigments in fungi, displaying various colours [1].
The pigment class of anthraquinones is characterized by a polycyclic aromatic hydrocarbon structure stemming from the merger of three benzene rings. The diversity of anthraquinones results from the presence of different substituents, such as -OH, -CH3, -OCH3, and -CH2OH, and from the reduction of carbonyl groups as well as double bonds in the benzene ring, allowing the formation of hydroxyanthraquinones.
Anthraquinones can generate a broad scope of hues, from yellows to reds and even blue shades. Their wide spectrum of colors is attributed to their relatively short conjugated chromophores. They give weak yellows in simple unsubstituted forms, while allowing the attachment of substituents to generate extensive bathochromic shifts of the absorption maximum and, as a result, give reds and even blues.
Anthraquinones including hydroxyanthraquinones (HAQNs) are commonly produced by fungi, such as Aspergillus spp., Eurotium spp., Fusarium spp., Dreschlera spp., Penicillium spp., Emericella purpurea, Culvularia lunata, Mycosphaerella rubella, and Microsporum spp. [1][49]. Of note is Arpink Red™, the polyketide anthraquinone-based natural food colorant initially described in 2004 [50]. This colorant is commercially produced using the fungus Penicillium oxalicum var. Armeniaca.
Like anthraquinones, naphthoquinone pigments are produced by many fungal species. Most naphthoquinones are coloured, usually varying between yellow, orange, and brown [51]. It has been reported that the biosynthesis of naphthoquinone pigments in some Fusarium species is triggered by environmental stresses, manifested under the conditions of growth inhibition or arrest [52]. Indeed, many fungal secondary metabolites, such as naphthoquinone pigments, are induced by factors of biotic and abiotic origin, including competition from other organisms, the presence of toxic compounds, etc. [53]. Penicillium and the related genus Talaromyces can produce naphthoquinone pigments and are widespread, requiring minimal conditions to grow. These fungi are aerobic, require minimal nutrients, and can grow quickly at room temperature. Thus, these genera are promising candidates for the biosynthesis of pigments in laboratory and industrial settings and are particularly relevant to the modulation of biomass or pigment production [9].
The synthesis of fungal polyketides requires polyketide synthase (PKS) and uses precursors such as acetyl-CoA and malonyl-CoA [54]. Characteristic polyketide pigments produced by fungi are known to be anthraquinones, hydroxyanthraquinones, and naphthoquinones [54].
Bikaverin, belonging to the naphthoquinone class, is a kind of bright red pigment present predominantly in the genus Fusarium, among which Fusarium fujikuroi is a representative species [55]. In F. fujikuroi, bikaverin (viz. 6, 11-dihydroxy-3, 8-dimethoxy-1-methyl-benzo-xanthein-7, 10, 12-trion) is produced via a polyketide biosynthetic pathway (Figure 3a). Genetic analyses with F. fujikuroi have demonstrated that a gene cluster composed of six bik genes, encoding a multifunctional polyketide synthase, is responsible for bikaverin biosynthesis [56]. Within the bik gene cluster situated on chromosome 5 of F. fujikuroi, bik 1–3 are recognized to be biosynthetic genes, bik 4 and bik 5 are the regulatory genes, and bik 6 encodes a transporter of bikaverin (Figure 3b).
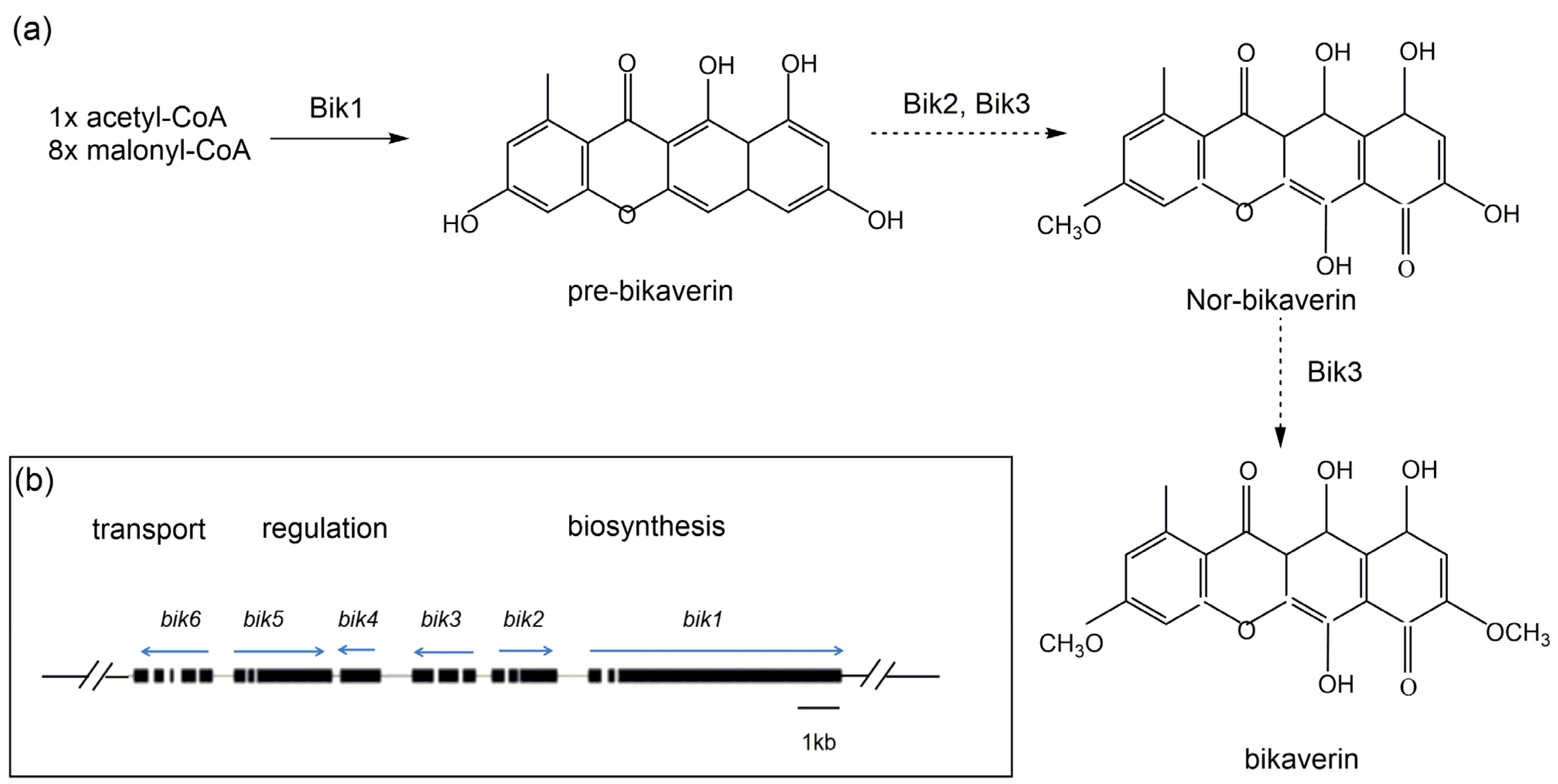
Figure 3. Schematic representation of bikaverin biosynthetic route in Fusarium fujikuroi. (a) The preferred biosynthetic steps are indicated by bold arrows, and some possible conversions are indicated by dashed arrows. (b) Organization of bikaverin (bik) gene cluster in F. fujikuroi. Direction of transcription for each predicted gene is indicted by the arrows [57][58].
Bikaverin is of great interest due to its pharmaceutical values along with its use as a colorant in the textile industry. Its wide-spectrum bioactivities, including anti-microbial and anti-proliferative properties, make it a promising pigment for further development in pharmaceutical discovery and industrial applications [59].
2.4. Azaphilones
Azaphilone pigments are a structurally diverse group of fungal pigments. They are polyketide derivates, that is, pigments with pyrone-quinone structures encompassing a highly oxygenated bicyclic core and a chiral quaternary center [60][61][62]. Azaphilones are structurally diverse due to the easy insertion of nitrogen.
The azaphilone-producing fungi are widespread in nature, including microscopic fungi (also called “mold”), such as species in genera Penicillium, Monascus, Chaetomium, and Talaromyces, as well as fungi devoid of mold appearance such as those in genera Hypoxylon, Daldinia, Creosphaeria (members of the Xylariaceae family), and Bulgaria (Bulgariaceae). Among these genera, Monascus is the best known and has provided colorants for human use for over two thousand years. Azaphilone pigments give rise to yellow, red, or green hues of fruiting bodies and/or mycelia.
The genus Monascus includes three species: M. pilosus, M. purpureus, and M. ruber. They make up most food colorant strains in Asian food [19]. Monascus pigments generally consist of six major azaphilone pigments: ankaflavin and monascin (yellow), monascorubrin and rubropunctatin (orange), as well as monascorubramine and rubropunctamine (violet). However, some strains of Monascus spp. can co-produce citrinin, a mycotoxin displaying hepatotoxic and nephrotoxic effects on humans [63]. Hence, traditional food colorants from Asia (the so-called Monascus pigments) are banned in the European Union (EU) and the United States, and there have been disputes over their safety when used in food. Strain selection and modification (via genetic manipulation) for citrinin-nonproducing M. purpureus is of importance for the commercial production of Monascus pigments in the food industry as well as that of monacolin K (viz. lovastatin, an anti-hypercholestrolemia drug) in the pharmaceutical industry.
This list of azaphilone producers is being extended to include more fungal species. These additional fungi include Phomopsis sp. (an endophytic strain producing phomopsones A-C, [64]), Pseudohalonectria adversaria (an aquatic fungus producing pseudohalonectrin A-B, [62]), Aspergillus niger ATCC 1015 (a type strain of Aspergillus producing Azanigerones B-C, [65]), Aspergillus fumigatus 14–27 (a gorgonian-derived Aspergillus producing pinophilin B, [66]), Fusarium sp. (an endophytic fungus producing fusarone, [67]), Xylariales sp. PSU-ES163 (seagrass-derived fungus producing xylariphilone [68]), Coniella fragariae (goose dung-derived fungus producing coniellins H and I [69]), Dothideomycete sp.(an endophytic strain producing austdiol [70]), Cladosporium perangustm FS62 (marine sediment-derived fungus producing perangustols A and B, [71]) and Pleosporales sp. (a marine-derived fungus producing pleosporalone A [72]).
Monascus azaphilone pigments (MonAzps) are known to be synthesized via a polyketide pathway, where polyketide synthase (PKS) and fatty acid synthase (FAS) play vital roles [73]. The sophisticated biosynthetic pathway in Monascus fungi is considered as a model system to elucidate and dissect the biosynthesis of fungal azaphilone pigments (Figure 4). M. purpureus is one of most widely used industrial strains in different areas of China for manufacturing food colorants, while in Japan it is the only authorized species for food use [74].
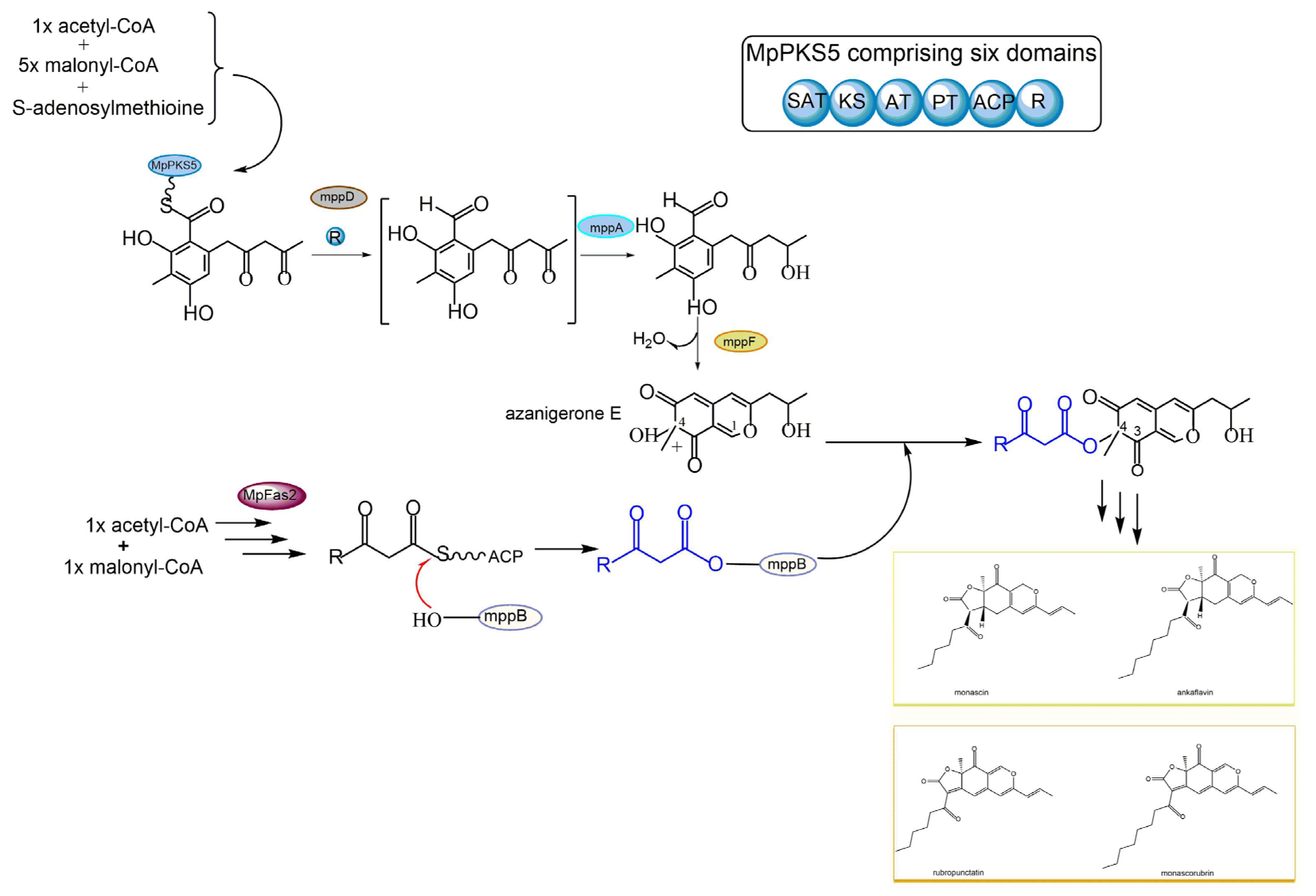
Figure 4. Schematic diagram of various azaphilone pigment biosynthesis via polyketide synthase (PKS) pathway and fatty acid synthase (FAS) pathway in Monascus purpureus.
In Monascus purpureus, the main pathway of MonAzps biosynthesis starts with a non-ribosome PKS enzyme termed MpPKS5, which consists of six essential domains: acyl carrier protein transacylase (SAT), ketoacyl synthase (KS), acyltransferase (AT), product template (PT), acyl carrier protein (ACP), and reductive release (R) domain (Figure 4). The multifunctional MpPKS5, aided by a serine hydrolase encoded by mppD, has been found to produce a highly reactive intermediate, namely hexaketide benzaldehyde. The ensuing step is catalyzed by a ketoreductase (coded by mppA), allowing the formation of the corresponding alcohol, namely FK17-P2a, the first stable intermediate in the biosynthetic pathway. FK17-P2a is subsequently oxidized by FAD-dependent monooxygenase (coded by mppF) to generate azanigerone E, giving rise to the pyranoquinone skeleton, the so-called polyketide chromophore for azaphilone biosynthesis. In parallel to the above-stated polyketide synthase pathway, MpFas2, the canonical fatty acid synthetase, is also involved in the biosynthesis of MonAzps. MpFas2 has been described to generate short-chain 3-oxo-fatty acyl thioesters, the side chain moiety for Monascus azaphilones. The M. purpureus biosynthetic gene cluster harbors an O-acyl transferase (coded by mppB), which is proposed as the catalyst for the transfer of the MpFas2 products (the side-chain fatty acyl moiety) to the C-4 hydroxyl group. Further steps result in the production of the classical yellow pigments monascin and ankaflavin, as well as orange pigments rubropunctatin and monascorubrin (Figure 4). Together, the main pathway of Monascus azaphilone biosynthesis contributes dominantly to the above-described four kinds of classical azaphilone pigments. However, the pyran rings of the classical orange pigments rubropunctatin and monascorubrin are vulnerable to spontaneous O-to-N substitution by readily reacting with endogenous amines to yield the γ-vinylogous pyridines of the red pigments termed rubropunctamine and monascorubramine [75].
3. Stress Factors and Fungal Pigment Production
Pigments are often produced to perform ecological roles in response to environmental stresses, such as ultraviolet (UV) light, ionizing radiation, oxidizing agents, nutrient deprivation, hypersaline, and host immunoreactivity. Rather than producing new compounds to build up fungal cells (primary metabolism), these fungi start to biosynthesize secondary metabolites manifested as pigmentation. The yield of the pigments might have originated from the defense demands of the fungi to fulfill the protective role, preventing their mycelia from being hydrolyzed by enzymes produced by other microbes [76]. It could also be a by-product of entering dormancy. In addition, in view of known antioxidant characteristics of these lipophilic pigments, carotenoid production in fungi has been suggested as a natural mechanism to protect against photo-oxidative damage in light-intensive habitats [77][78].
In Fusarium spp., light has been shown to stimulate the production of carotenoids. In both Fusarium aquaeductuum and F. fujikuroi, when illumination was supplied in the initially dark-grown culture, carotenoids accumulated for several hours upon light onset, exhibiting a typical orange pigmentation [79][80].
F. fujikuroi can produce carotenoids in addition to the phytohormone gibberellin [81] and the polyketide bikaverin [56][82]. Indeed, light induction of carotenoid biosynthesis has been commonly found in the genus Fusarium, being first reported in F. aquaeductuum in 1969 [83]. Investigation concerning the effects of light on the photoinduction in F. aquaeductuum [83] has unveiled that the levels of produced carotenoids depend on the logarithm of the incident light over a 100-fold range, in which the reciprocity law holds true over a broad spectrum of light intensity and time. In the case of F. aquaeductuum, the accumulation of carotenoid is induced only by light with a wavelength shorter than 520 nm [83].
Early studies with F. aquaeductuum regarding photo-regulated carotenogenesis were subsequently extended to F. fujikuroi and F. oxysporum. The major carotenoid produced by Fusarium spp. is neurosporaxanthin, an acidic apocarotenoid converted from geranylgeranyl pyrophosphate (GGPP) by the sequential activity of four enzymes, encoded by the genes carRA, carB, carT, and carD. Upon exposure to light, the mycelia rapidly displayed pigmentation due to the formation of carotenoids, which started to accumulate 1 h after illumination and reached a maximum about 16 h afterwards [80], exhibiting the pattern of photo-induction of carotenoids similar to that of N. crassa [84]. It is widely recognized that photoinduction of carotenogenesis in Fusarium species is attributed to a transcriptional elevation of most of the structural genes involved in the carotenoid synthesis [85].
Fungi often utilize light to perceive high temperature, UV radiation (genotoxic stressor), and the soil/air interface for their survival and proliferation (i.e., spore dispersal). Fungi respond to light by a sophisticated photosensory system consisting mainly of photoreceptors. Exposure to light, especially blue light, is regarded as the major triggering factor for carotenogenesis [86][87]. The blue light receptors in fungi are composed of VIVID (VVD) protein, the white collar complex (WCC), and the cryptochrome (CRY) [88]. The Vvd-null mutant of N. crassa was observed to have enhanced carotenoid accumulation as manifested by intensely pigmented mycelia under constant light. In contrast to N. crassa, a mutation of VvdA, an orthologue of N. crassa Vvd, in F. fujikuroi, might result in paler pigmentation. Further studies have unveiled that the VvdA-regulated carotenogenesis in F. fujikuroi is a biphasic photo-response, which bursts in the early phase but decays in the subsequent phase [89].
In addition, further investigation in the high-altitude lake ecosystem located in the Nahuel Huapi National Park (Bariloche, Argentina) revealed that living microorganisms have adopted such strategies as the synthesis of antioxidants and UV sunscreen compounds, including carotenoids and mycosporines, to minimize UV-induced damage [90]. Specifically, Libkind et al. reported that: (i) 24 yeast species were recovered, among which at least four represented novel species; and (ii) carotenogenic yeasts dominated in lakes with higher water transparency (more UV exposure). Taken together, the results suggest UV radiation as an important environmental factor eliciting yeast carotenoid pigment production and thereby affecting the microbial community structure in aquatic habitats.
Similarly, oxidative stress has been found to induce increased hypocrellin production in Shiraia bambusicola, a well-known pathogenic fungus of bamboo. Hypocrellins, red pigments belonging to the class of perylenequinonoids, are considered as significant photosensitizers, which can serve as potential new-generation photodynamic therapy (PDT) drugs. Hypocrellins, during illumination, display excellent anticancer [91][92][93][94] and antiviral activities [95]. Additionally, hypocrellin has been long used in Chinese folk medicine for the treatment of skin diseases and stomach aches.
Aside from oxidative agents, light can also affect hypocrellin production as well as growth and reproduction of Shiraia sp. SUPER-H168. All incubations under different light (white, red, yellow, green, blue, and purple) conditions were beneficial to aerial hyphal growth as compared to darkness. In contrast, all light conditions examined inhibited hypocrellin biosynthesis, with the strongest inhibition displayed by blue light [96].
The mycelial culture of S. bambusicola without illumination is a biotechnological alternative for hypocrellin production but accompanied by the low yield. Recent investigation using a light/dark shift (24 h/24 h) regime showed that such illumination treatment not only elevated the hypocrellin level in mycelia by 65%, but also promoted its release into the medium, with the highest total hypocrellin production (181.67 mg/L) on day 8, approximately a 73% rise in comparison to the dark controls. Moreover, light/dark shift was observed to elicit the formation of smaller and more compact fungal pellets without any growth retardation of mycelia, pinpointing a novel promising strategy for large-scale production of hypocrellin in mycelium cultures. Furthermore, the light/dark shift regime upregulated the expression of two reactive oxygen species (ROS)-related genes, including NADPH oxidase and cytochrome c peroxidase, and to induce ROS generation. It was also unveiled that ROS might be involved in the upregulation of key genes for hypocrellin biosynthesis and thereby promote hypocrellin production. Collectively, these results provide a molecular basis for deciphering the impacts of light/dark shift on fungal pigment production and for utilizing a tactical approach to increase hypocrellin production in submerged fermentation of Shiraia species [97].
Monascus pigments are a representative class of azaphilone pigments, commonly used in Asia since ancient times. Studies with Monascus pilosus IFO4520 found that compared to non-illuminated controls, the production of red pigments increased by 1.3-fold after exposure to red light for 7–10 d and displayed a 1.5-fold increase post illumination of 14 d. The above-described findings suggested that red light could stimulate the production of red pigments, especially if applied before stationary growth [98]. Meanwhile, the inhibitory effect of blue light on red pigment production in M. pilosus IFO4520 was also observed. Similarly, compared to controls, production of monacolin K (a cholesterol-lowering agent) increased by red light exposure at an early stationary phase. A further investigation with Monascus revealed that the reduced pigment accumulation under blue light was due to the increased instability under blue light exposure, even though blue light also stimulated pigment biosynthesis [99]. Interestingly, blue light can exert either inhibitory or stimulatory effects on pigment biosynthesis in Monascus, depending on light intensity and illumination time. Specifically, a study with Monascus purpureus strain M9 found that blue light at a low intensity and short exposure time (100 lux, 15 and 30 min/day; 200 lux, 15 min/day) enhanced the production of Monascus yellow pigments monascin and ankaflavin, whereas blue light with a high intensity (300 and 450 lux) and long exposure time (45 and 60 min) attenuated pigment production [100].
References
- Lin, L.; Xu, J.P. Fungal Pigments and their roles associated with human health. J. Fungi 2020, 6, 280.
- Cordero, R.J.B.; Casadevall, A. Functions of fungal melanin beyond virulence. Fungal Biol. Rev. 2017, 31, 99–112.
- Xu, J. Fungal species concepts in the genomics era. Genome 2020, 63, 459–468.
- Xu, J. Fungal DNA Barcoding. Genome 2016, 59, 913–932.
- Xu, J. Assessing global fungal threats to humans. mLife 2022, 1, 223–240.
- Boral, H.; Metinb, B.; Döğen, A.; Seyedmousavi, S.; Ilkit, M. Overview of selected virulence attributes in Aspergillus fumigatus, Candida albicans, Cryptococcus neoformans, Trichophyton rubrum and Exophiala dermatitidis. Fungal Genet. Biol. 2018, 111, 92–107.
- Chang, P.-K.; Cary, J.W.; Lebar, M.D. Biosynthesis of conidial and sclerotial pigments in Aspergillus species. Appl. Microbiol. Biotech. 2020, 104, 2277–2286.
- Liu, G.Y.; Nizet, V. Color me bad: Microbial pigments as virulence factors. Trends Microbiol. 2009, 17, 406–413.
- Venkatachalam, M.; Magalon, H.; Dufossé, L.; Fouillaud, M. Production of pigments from the tropical marine-derived fungi Talaromyces albobiverticillius: New resources for natural red-colored metabolites. J. Food Compos. Anal. 2018, 70, 35–48.
- Takahashi, J.A.; Barbosa, B.V.R.; Martins, B.D.; Guirlanda, C.P.; Moura, M.A.F. Use of the versatility of fungal metabolism to meet modern demands for healthy aging, functional foods, and sustainability. J. Fungi 2020, 6, 223.
- Oplatowska-Stachowiak, M.; Elliott, C.T. Food colors: Existing and emerging food safety concerns. Crit. Rev. Food Sci. Nutr. 2017, 57, 524–548.
- Sen, T.; Barrow, C.J.; Deshmukh, S.K. Microbial pigments in the food industry—Challenges and the way forward. Front. Nutr. 2019, 6, 7.
- Dikshit, R.; Tallapragada, P. Comparative study of natural and artificial flavoring agents and dyes. In Natural and Artificial Flavoring Agents and Food Dyes; Grumezescu, A.M., Holban, A.M., Eds.; Elsevier: London, UK, 2018; Volume 7, pp. 83–111.
- Ruiz-Sola, M.A.; Rodriguez-Conception, M. Carotenoid biosynthesis in Arabidopsis: A colorful pathway. Arab. Book 2012, 10, e0158.
- Rapoport, A.; Guzhova, I.; Bernetti, L.; Buzzini, P.; Kieliszek, M.; Kot, A.M. Carotenoids and some other pigments from fungi and yeasts. Metabolites 2021, 11, 92.
- Fraser, P.D.; Bramley, P.M. The biosynthesis and nutritional uses of carotenoids. Prog. Lipid Res. 2004, 43, 228–265.
- Mata-Gómez, L.; Montañez, J.C.; Méndez-Zavala, A.; Aguilar, C.N. Biotechnological production of carotenoids by yeasts: An overview. Microb. Cell Fact. 2014, 13, 12.
- Alija, A.J.; Bresgen, N.; Sommerburg, O.; Siems, W.; Eckl, P.M. Cytotoxic and genotoxic effects of β-carotene breakdown products on primary rat hepatocytes. Carcinogenesis 2004, 25, 827–831.
- Dufossé, L.; Galaup, P.; Yaron, A.; Arad, S.M.; Blanc, P.; Murthy, K.N.C.; Ravishanka, G.A. Microorganisms and microalgae as source of pigments for use: A scientific oddity or an industrial reality? Trends. Food Sci. Technol. 2005, 16, 389–406.
- Aksu, Z.; Eren, A.T. Carotenoids production by the yeast Rhodotorula mucilaginosa: Use of agricultural wastes as a carbon source. Process Biochem. 2005, 40, 2985–2991.
- Panesar, R.; Kaur, S.; Panesar, P.S. Production of microbial pigments utilizing agro-industrial waste: A review. Curr. Opin. Food. Sci. 2015, 1, 70–76.
- Böhme, K.; Richter, C.; Pätz, R. New insights into mechanisms of growth and β-carotene production in Blakeslea trispora. Biotechnol. J. 2006, 1, 1080–1084.
- Dufossé, L. Microbial production of food grade pigments. Food Technol. Biotechnol. 2006, 44, 313–321.
- Marcoleta, A.; Niklitschek, M.; Wozniak, A.; Lozano, C.; Alcaino, J.; Baeza, M.; Cifuentes, V. Glucose and ethanol-dependent transcriptional regulation of the astaxanthin biosynthesis pathway in Xanthophyllomyces dendrorhous. BMC Microbiol. 2011, 11, 190.
- Choudhari, S.M.; Ananthanarayan, L.; Singhal, R.S. Use of metabolic stimulators and inhibitors for enhanced production of β-carotene and lycopene by Blakeslea trispora NRRL 2895 and 2896. Bioresour. Technol. 2008, 99, 3166–3173.
- Lee, P.C.; Schmidt-Dannert, C. Metabolic engineering towards biotechnological production of carotenoids in microorganisms. Appl. Microbiol. Biotechnol. 2002, 60, 1–11.
- Srivastava, S.; Srivastava, A.K. Lycopene; chemistry, biosynthesis, metabolism and degradation under various abiotic parameters. J. Food Sci. Technol. 2015, 52, 41–53.
- Hernandez-Almanza, A.; Montañez, J.; Martínez, G.; Aguilar-Jimenez, A.; Contreras-Esquivel, J.C.; Aguilar, C.N. Lycopene: Progress in microbial production. Trends Food Sci. Technol. 2016, 56, 142–148.
- Wang, L.; Liu, Z.; Jiang, H.; Mao, X. Biotechnology advances in β-carotene production by microorganisms. Trends Food Sci. Technol. 2021, 111, 322–332.
- Eisenman, H.C.; Casadevall, A. Synthesis and assembly of fungal melanin. Appl. Microbiol. Biotechnol. 2012, 93, 931–940.
- Nosanchuk, J.D.; Casadevall, A. The contribution of melanin to microbial pathogenesis. Cell Microbiol 2003, 5, 203–223.
- Plonka, P.M.; Grabacka, M. Melanin synthesis in microorganisms—Biotechnological and medical aspects. Acta Biochim. Pol. 2006, 53, 429–443.
- Pais, P.; Costa, C.; Cavalheiro, M.; Romao, D.; Teixeira, M.C. Transcriptional control of drug resistance, virulence and immune system evasion in pathogenic fungi: A cross-species comparison. Front. Cell. Infect. Microbiol. 2016, 6, 131.
- Feng, B.; Wang, X.; Hauser, M.; Kaufmann, S.; Jentsch, S.; Haase, G.; Becker, J.M.; Szaniszlo, P.J. Molecular cloning and characterization of WdPKS1, a gene involved in dihydroxynaphthalene melanin biosynthesis and virulence in Wangiella (Exophiala) dermatitidis. Infect. Immun. 2001, 69, 1781–1794.
- McCallum, N.C.; Son, F.A.; Clemons, T.D.; Weigand, S.J.; Gnanasekaran, K.; Battistella, C.; Barnes, B.E.; Abeyratne-Perera, H.; Siwicka, Z.E.; Forman, C.J.; et al. Allomelanin: A biopolymer of intrinsic microporosity. J. Am. Chem. Soc. 2021, 143, 4005–4016.
- Kogej, T.; Wheeler, M.H.; Lanišnik RiŽner, T.; Gunde-Cimerman, N. Evidence for 1,8-dihydroxynaphthalene melanin in three halophilic black yeasts grown under saline and non-saline conditions. FEMS Microbiol. Lett. 2004, 232, 203–209.
- Pacelli, C.; Bryan, R.A.; Onofri, S.; Selbmann, L.; Shuryak, I.; Dadachova, E. Melanin is effective in protecting fast and slow growing fungi from various types of ionizing radiation. Environ. Microbiol. 2017, 19, 1612–1624.
- Perez-Cuesta, U.; Aparicio-Fernandez, L.; Guruceaga, X.; Martin-Souto, L.; Abad-Diaz-De-Cerio, A.; Antoran, A.; Buldain, I.; Hernando, F.L.; Ramirez-Garcia, A.; Rementeria, A. Melanin and pyomelanin in Aspergillus fumigatus: From its genetics to host interaction. Intl. Microbiol. 2020, 23, 55–63.
- Polacheck, I.; Kwon-Chung, K.J. Melanogenesis in Cryptococcus neoformans. J. Gen. Microbiol. 1988, 134, 1037–1041.
- Tsai, H.F.; Wheeler, M.H.; Chang, Y.C.; Kwon-Chung, K.J. A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J. Bacteriol. 1999, 181, 6469–6477.
- Bayry, J.; Beaussart, A.; Dufrêne, Y.F.; Sharma, M.; Bansal, K.; Kniemeyer, O.; Aimanianda, V.; Brakhage, A.A.; Kaveri, S.V.; Kwon-Chung, K.J.; et al. Surface structure characterization of Aspergillus fumigatus conidia mutated in the melanin synthesis pathway and their human cellular immune response. Infect. Immun. 2014, 82, 3141–3153.
- Watanabe, A.; Fujii, I.; Sankawa, U.; Mayorga, M.E.; Timberlake, W.E.; Ebizuka, Y. Re-identification of Aspergillus nidulans wA gene to code for a polyketide synthase of naphthopyrone. Tetrahedron Lett. 1999, 40, 91–94.
- Prados-Rosales, R.; Toriola, S.; Nakouzi, A.; Chatterjee, S.; Stark, R.; Gerfen, G.; Tumpowsky, P.; Dadachova, E.; Casadevall, A. Structural characterization of melanin pigments from commercial preparations of the edible mushroom Auricularia auricula. J. Agric. Food Chem. 2015, 63, 7326–7332.
- Li, J.; Li, Z.; Zhao, T.; Yan, X.; Pang, Q. Proteomic analysis of Auricularia auricula-judae under freezing treatment revealed proteins and pathways associated with melanin reduction. Front. Microbiol. 2021, 11, 610173.
- Fogarty, R.V.; Tobin, J.M. Fungal melanins and their interaction with metals. Enzyme Microb. Technol. 1996, 19, 311–317.
- Liu, X.; Hou, R.; Wang, D.; Mai, M.; Wu, X.; Zheng, M.; Fu, J. Comprehensive utilization of edible mushroom Auricularia auricula waste residue—Extraction, physicochemical properties of melanin and its antioxidant activity. Food Nutr. Sci. 2019, 7, 3774–3783.
- Schweitzer, A.; Howell, R.C.; Jiang, Z.; Bryan, R.A.; Gerfen, G.; Chen, C.-C.; Mah, D.; Cahill, S.; Casadevall, A.; Dadachova, E. Physico-chemical evaluation of rationally designed melanins as novel natureInspired radioprotectors. PLoS ONE 2009, 4, e7229.
- Dighton, J.; Tugay, T.; Zhdanova, N. Fungi and ionizing radiation from radionuclides. FEMS Microbiol. Lett. 2008, 281, 109–120.
- Gesslera, N.N.; Egorovaa, A.S.; Belozerskaya, T.A. Fungal anthraquinones. Appl. Biochem. Microbiol. 2013, 49, 85–99.
- Sardaryan, E. Food Supplement. US 2004/0105864 A1 3 June 2004.
- Babula, P.; Adam, V.; Havel, L.; Kizek, R. Noteworthy secondary metabolites naphthoquinones—Their occurrence, pharmacological properties and analysis. Curr. Pharm. Anal. 2009, 5, 47–68.
- Medentsev, A.G.; Arinbasarova, A.Y.; Akimenko, V.K. Biosynthesis of naphthoquinone pigments by fungi of the genus Fusarium. Appl. Biochem. Microbiol. 2005, 41, 503–507.
- Medentsev, A.G.; Akimenko, V.K. Naphthoquinone metabolites of the fungi. Phytochemistry 1998, 47, 935–959.
- Venil, C.K.; Velmurugan, P.; Dufossé, L.; Devi, P.R.; Ravi, A.V. Fungal pigments: Potential coloring compounds for wide ranging applications in textile dyeing. J. Fungi 2020, 6, 68.
- Giordano, W.; Avalos, J.; Cerda-Olmedo, E.; Domenech, C.E. Nitrogen availability and production of bikaverin and gibberellins in Gibberella fujikuroi. FEMS Microbiol. Lett. 1999, 173, 389–393.
- Wiemann, P.; Willmann, A.; Straeten, M.; Kleigrewe, K.; Beyer, M.; Humpf, H.U.; Tudzynski, B. Biosynthesis of the red pigment bikaverin in Fusarium fujikuroi: Genes, their function and regulation. Mol. Microbiol. 2009, 72, 931–946.
- Janevska, S.; Tudzynski, B. Secondary metabolism in Fusarium fujikuroi: Strategies to unravel the function of biosynthetic pathways. Appl. Microbiol. Biotechnol. 2018, 102, 615–630.
- Zhao, M.; Zhao, Y.; Yao, M.; Iqbal, H.; Hu, Q.; Liu, H.; Bin Qiao, B.; Li, C.; Skovbjerg, C.A.S.; Nielsen, J.C.; et al. Pathway engineering in yeast for synthesizing the complex polyketide bikaverin. Nat. Commun. 2020, 11, 6197.
- dos Santos, M.C.; Bicas, J.L. Natural blue pigments and bikaverin. Microbiol. Res. 2021, 244, 126653.
- Sturdikova, M.; Slugen, D.; Lesova, K.; Rosenberg, M. Microbial production of coloured azaphiolone metabolites. Chem. Listy 2000, 94, 105–110.
- Zhu, J.; Nicholas, P.; Grigoriadis, N.P.; Lee, J.P.; Porco, J.A. Synthesis of the azaphilones using copper-mediated enantioselective oxidative dearomatization. J. Am. Chem. Soc. 2005, 127, 9342–9343.
- Dong, J.; Zhou, Y.; Li, R.; Zhou, W.; Li, L.; Zhu, Y.; Huang, R.; Zhang, K. New nematicidal azaphilonesfrom the aquatic fungus Pseudohalonectria adversaria YMF1.01019. FEMS Microbiol. Lett. 2006, 264, 65–69.
- Shimizu, T.; Kinoshita, H.; Ishihara, S.; Sakai, K.; Nagai, S.; Nihira, T. Polyketide synthase gene responsible for citrinin biosynthesis in Monascus purpureus. Appl. Environ. Microbiol. 2005, 71, 3453–3457.
- Yang, Z.-J.; Zhang, Y.-F.; Wu, K.; Xu, Y.-X.; Meng, X.-G.; Jiang, Z.-T.; Ge, M.; Shao, L. New azaphilones, phomopsones A-C with biological activities from an endophytic fungus Phomopsis sp. CGMCC No.5416. Fitoterapia 2020, 145, 104573.
- Zabala, A.O.; Xu, W.; Chooi, Y.-H.; Tang, Y. Discovery and characterization of a silent gene cluster that produces azaphilones from Aspergillus niger ATCC 1015 reveal a hydroxylation-mediated pyran-ring formation. Chem. Biol. 2012, 19, 1049–1059.
- Zhang, Y.-H.; Peng, X.-Y.; Feng, L.-X.; Zhu, H.-J.; Cao, F.; Wang, C. A new epimer of azaphilone derivative pinophilin B from the gorgonian-derived fungus Aspergillus fumigatus 14–27. Nat. Prod. Res. 2021, 35, 2232–2238.
- Yang, S.; Gao, J.; Laatsch, H.; Tian, J.; Pescitelli, G. Absolute configuration of fusarone, a new azaphilone from the endophytic fungus Fusarium sp. isolated from Melia azedarach, and of related azaphilones. Chirality 2012, 24, 621–627.
- Arunpanichlert, J.; Rukachaisirikul, V.; Phongpaichit, S.; Supaphon, O.; Sakayaroj, J. Xylariphilone: A new azaphilone derivative from the seagrass-derived fungus Xylariales sp. PSU-ES163. Nat. Prod. Res. 2016, 30, 46–51.
- Yu, H.; Sperlich, J.; Höfert, S.-P.; Janiak, C.; Teusch, N.; Stuhldreier, F.; Wesselborg, S.; Wang, C.; Kassack, M.U.; Dai, H.; et al. Azaphilone pigments and macrodiolides from the coprophilous fungus Coniella fragariae. Fitoterapia 2019, 137, 104249.
- Senadeera, S.P.D.; Wiyakrutt, S.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. A novel tricyclic polyketide and its biosynthetic precursor azaphilone derivatives from the endophytic fungus Dothideomycete sp. Org. Biomol. Chem. 2012, 10, 7220–7226.
- Fan, Z.; Sun, Z.H.; Liu, H.X.; Chen, Y.C.; Li, H.H.; Zhang, W.M. Perangustols A and B, a pair of new azaphilone epimers from a marine sediment-derived fungus Cladosporium perangustm FS62. J. Asian Nat. Prod. Res. 2016, 18, 1024–1029.
- Cao, F.; Yang, J.-K.; Liu, Y.-F.; Zhu, H.-J.; Wang, C.-Y. Pleosporalone A, the first azaphilone characterized with aromatic A-ring from a marine-derived Pleosporales sp. fungus. Nat. Prod. Res. 2016, 30, 2448–2452.
- Yang, Y.; Liu, B.; Du, X.; Li, P.; Liang, B.; Cheng, X.; Du, L.; Huang, D.; Wang, L.; Wang, S. Complete genome sequence and transcriptomics analyses reveal pigment biosynthesis and regulatory mechanisms in an industrial strain, Monascus purpureus YY-1. Sci. Rep. 2015, 5, 8331.
- Blanc, P.J.; Loret, M.O.; Santerre, A.L.; Pareilleux, A.; Prome, D.; Prome, J.C.; Prome, J.P.; Laussac, G. Pigments of Monascus. J. Food Sci. 1994, 59, 862–865.
- Chen, W.; Feng, Y.; Molnár, I.; Chen, F. Nature and nurture: Confluence of pathway determinism with metabolic and chemical serendipity diversifies Monascus azaphilone pigments. Nat. Prod. Rep. 2019, 36, 561–572.
- Isaac, S. Many fungi are brightly colored; Does pigmentation provide any advantage to those species? Mycologist 1994, 8, 178–179.
- Avalos, J.; Limon, M.C. Biological roles of fungal carotenoids. Curr. Genet. 2015, 61, 309–324.
- Echavarri-Erasun, C.; Johnson, E.A. Stimulation of astaxanthin formation in the yeast Xanthophyllomyces dendrorhous by the fungus Epicoccum nigrum. FEMS Yeast Res. 2004, 4, 511–519.
- Bindl, E.; Lang, W.; Rau, W. Light dependent carotenoid synthesis. 6. Time course of synthesis of various carotenoids in Fusarium aquaeductuum after various inductive treatments. Planta 1970, 94, 156–174.
- Avalos, J.; Schrott, E.L. Photoinduction of carotenoid biosynthesis in Gibberella fujikuroi. FEMS Microbiol. Lett. 1990, 66, 295–298.
- Bömke, C.; Tudzynski, B. Diversity, regulation, and evolution of the gibberellin biosynthetic pathway in fungi compared to plants and bacteria. Phytochemistry 2009, 70, 1876–1893.
- Rodriguez-Ortiz, R.; Limon, M.C.; Avalos, J. Regulation of carotenogenesis and secondary metabolism by nitrogen in wild-type Fusarium fujikuroi and carotenoid-overproducing mutants. Appl. Environ. Microbiol. 2009, 75, 405–413.
- Rau, W. Light-dependent carotenoid synthesis. I. Action spectrum of photoinduction in Fusarium aquaeductuum. Planta 1967, 72, 14–28.
- Avalos, J.; Corrochano, L.M. Carotenoid biosynthesis in Neurospora. In Neurospora: Genomics and Molecular Biology; Kasbekar, D.P., McCluskey, K., Eds.; Caister Academic Press: Norfolk, UK, 2013; pp. 227–241.
- Avalos, J.; Pardo-Medina, J.; Parra-Rivero, O.; Ruger-Herreros, M.; Rodríguez-Ortiz, R.; Hornero-Méndez, D.; Limón, M.C. Carotenoid biosynthesis in Fusarium. J. Fungi 2017, 3, 39.
- Corrochano, L.M.; Garre, V. Photobiology in the Zygomycota: Multiple photoreceptor genes for complex responses to light. Fungal Genet. Biol. 2010, 47, 893–899.
- Dzurendova, S.; Losada, C.B.; Dupuy-Galet, B.X.; Fjær, K.; Shapaval, V. Mucoromycota fungi as powerful cell factories for modern biorefinery. Appl. Microbiol. Biotechnol. 2022, 106, 101–115.
- Dasgupta, A.; Fuller, K.K.; Dunlap, J.C.; Loros, J.J. Seeing the world differently: Variability in the photosensory mechanisms of two model fungi. Environ. Microbiol. 2016, 18, 5–20.
- Ádám, A.L.; García-Martíne, J.; Szűcs, E.P.; Avalos, J.; Hornok, L. The MAT1-2-1 mating-type gene upregulates photo-inducible carotenoid biosynthesis in Fusarium verticillioides. FEMS Microbiol. Lett. 2011, 318, 76–83.
- Libkind, D.; Moliné, M.; Sampaio, J.P.; van Broock, M. Yeasts from high-altitude lakes: Influence of UV radiation. FEMS Microbiol. Ecol. 2009, 69, 353–362.
- Deng, H.; Li, T.; Xie, J.; Huang, N.; Gu, Y.; Zhao, J. Synthesis and bioevaluation of novel hypocrellin derivatives: Potential photosensitizers for photodynamic therapy of age-related macular degeneration. Dyes Pigment. 2013, 99, 930–939.
- Deininger, M.H.; Weinschenk, T.; Morgalla, M.H.; Meyermann, R.; Schluesener, H.J. Release of regulators of angiogenesis following hypocrellin-A and -B photodynamic therapy of human brain tumor cells. Biochem. Biophys. Res. Commun. 2002, 298, 520–530.
- Xu, S.; Chen, S.; Zhang, M.; Shen, T.; Zhao, Y.; Liu, Z.; Wu, Y. Butylamino-demethoxy-hypocrellins and photodynamic therapy decreases human cancer in vitro and in vivo. Biochim. Biophys. Acta 2001, 1537, 222–232.
- Yang, H.-Y.; Zhang, W.-G.; Ma, L.-P.; Wang, S.-W.; Zhang, Z.-Y. An approach to enhancing the phototoxicity of a novel hypocrellin congener to MGC803 cells. Dyes Pigment. 2001, 51, 103–110.
- Hudson, J.B.; Zhou, J.; Chen, J.; Harris, L.; Yip, L.; Towers, G.H.N. Hypocrellin, from Hypocrella bambuase, is phototoxic to human immunodeficiency virus. Photochem. Photobiol. 1994, 60, 253–255.
- Gao, R.J.; Xu, Z.C.; Deng, H.X.; Guan, Z.B.; Liao, X.R.; Zhao, Y.; Zheng, X.H. Influences of light on growth, reproduction and hypocrellin production by Shiraia sp. SUPER-H168. Arch. Microbiol. 2018, 200, 1217–1225.
- Sun, C.X.; Ma, Y.J.; Wang, J.W. Improved hypocrellin A production in Shiraia bambusicola by light–dark shift. J. Photochem. Photobiol. B. 2018, 182, 100.
- Miyake, T.; Mori, A.; Kii, T.; Okuno, T.; Usui, Y.; Sato, F. Light effects on cell development and secondary metabolism in Monascus. J. Ind. Microbiol. Biotechnol. 2005, 32, 103–108.
- Wang, C.; Fu, Z.; Chen, M.; Ban, Z.; Wang, Y.; Zhang, X. Blue light effects on pigment and citrinin production in Monascus. In Proceedings of the 2009 3rd International Conference on Bioinformatics and Biomedical Engineering, Beijing, China, 11–13 June 2009; pp. 4397–4400.
- Wang, C.; Chen, D.; Chen, M.; Wang, Y.; Li, Z.; Li, F. Stimulatory effects of blue light on the growth, monascin and ankaflavin production in Monascus. Biotechnol. Lett. 2015, 37, 1043–1048.
More
Information
Subjects:
Mycology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
10 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




