Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marta Alberquilla Garcia-Velasco | -- | 2096 | 2023-01-03 11:46:54 | | | |
| 2 | Rita Xu | Meta information modification | 2096 | 2023-01-04 02:24:40 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Cañadas, P.; García-Velasco, M.A.; Verdejo, J.L.H.; Teus, M.A. Corneal Confocal Microscopy Imaging. Encyclopedia. Available online: https://encyclopedia.pub/entry/39681 (accessed on 07 February 2026).
Cañadas P, García-Velasco MA, Verdejo JLH, Teus MA. Corneal Confocal Microscopy Imaging. Encyclopedia. Available at: https://encyclopedia.pub/entry/39681. Accessed February 07, 2026.
Cañadas, Pilar, Marta Alberquilla García-Velasco, José Luis Hernández Verdejo, Miguel A. Teus. "Corneal Confocal Microscopy Imaging" Encyclopedia, https://encyclopedia.pub/entry/39681 (accessed February 07, 2026).
Cañadas, P., García-Velasco, M.A., Verdejo, J.L.H., & Teus, M.A. (2023, January 03). Corneal Confocal Microscopy Imaging. In Encyclopedia. https://encyclopedia.pub/entry/39681
Cañadas, Pilar, et al. "Corneal Confocal Microscopy Imaging." Encyclopedia. Web. 03 January, 2023.
Copy Citation
In vivo corneal confocal microscopy (IVCM) is a non-invasive ophthalmic imaging technique that provides images of the cornea at the cellular level. The observation of the corneal cells, both normal and inflammatory, and the possibility of quantification of the corneal nerve density with manual or automated tools, makes IVCM have a significant potential to improve the diagnosis and prognosis in several systemic and corneal conditions.
in vivo images
corneal confocal microscopy
neuropathy
1. Introduction
In vivo corneal confocal microscopy (IVCM) is a noninvasive imaging technique of the human corneal structure in vivo. IVCM provides wide depth and high resolution, allowing a corneal evaluation at the cellular level [1]. Thanks to the corneal confocal microscopy, all layers of the cornea may be precisely visualized and analyzed. To obtain the corneal confocal microscopy images, IVCM uses punctual illumination in such a way that it is possible to discard all light that does not come from the focal plane. The IVCM main feature is that it collects and detects the light emitted by fluorescent molecules located in the same plane of three-dimensional space [2]. Given the fact that the light source used is a laser (collimated radiation in which the beam remains lineal when propagating), the illumination of the samples is very specific and with a high and stable intensity. This allows for subcellular microscopic resolutions to be achieved [2].
Currently, three IVCM devices are commercially available, but only two use the scanning system: The Slit Scanning Confocal Microscope (SSCM): a fixed laser beam is used, and the preparation is tracked using a motorized stage on the microscope. The Laser Scanning Confocal Microscope (LSCM): the scanning is carried out by moving the laser beam, thanks to galvanometric mirrors that allow the laser beam’s point of incidence on the eye to be modified. There is only one brand that designs the LSCM, it is the Heidelberg Retina Tomograph II or III with the Rostock Corneal Module (RCM) (Heidelberg Engineering, GmBH, Germany). This is the IVCM that is usually employed due to the high contrast images it provides.
2. Normal Cornea Observed with Confocal Microscopy
2.1. Corneal Epithelium
Superficial epithelial cells are 20–30 μm long and about 5 μm wide. They are observed as polygonal cells of different sizes and reflectivity with the confocal microscopy. They show a visible nucleus surrounded by a dark band. Winged cells are also observed, and show lower reflectivity. They also show variations in size and have bright borders and nuclei but do not show the dark ring that the superficial epithelial cells’ nuclei have [3][4]. Basal epithelial cells have a 10–15 μm diameter. They form a regular mosaic with dark cell bodies and bright borders (Figure 1) [3][5].
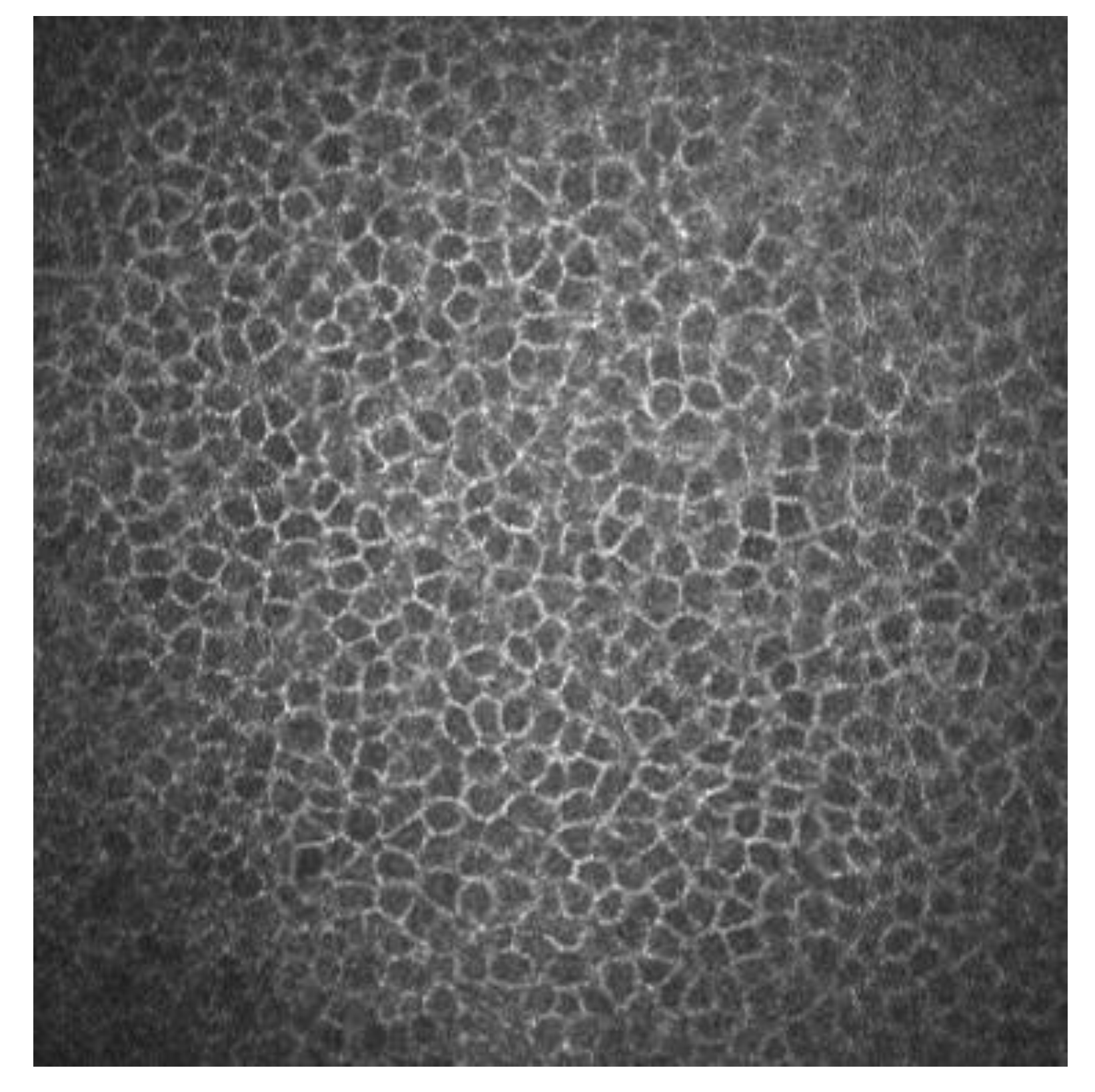
Figure 1. Basal epithelial cells of a human cornea observed with the corneal confocal microscopy HRT II.
2.2. Sub-Basal Nerves
Sub-basal corneal nerves are observed as sharp white lines showing homogeneous reflectivity (Figure 2) [6][7].
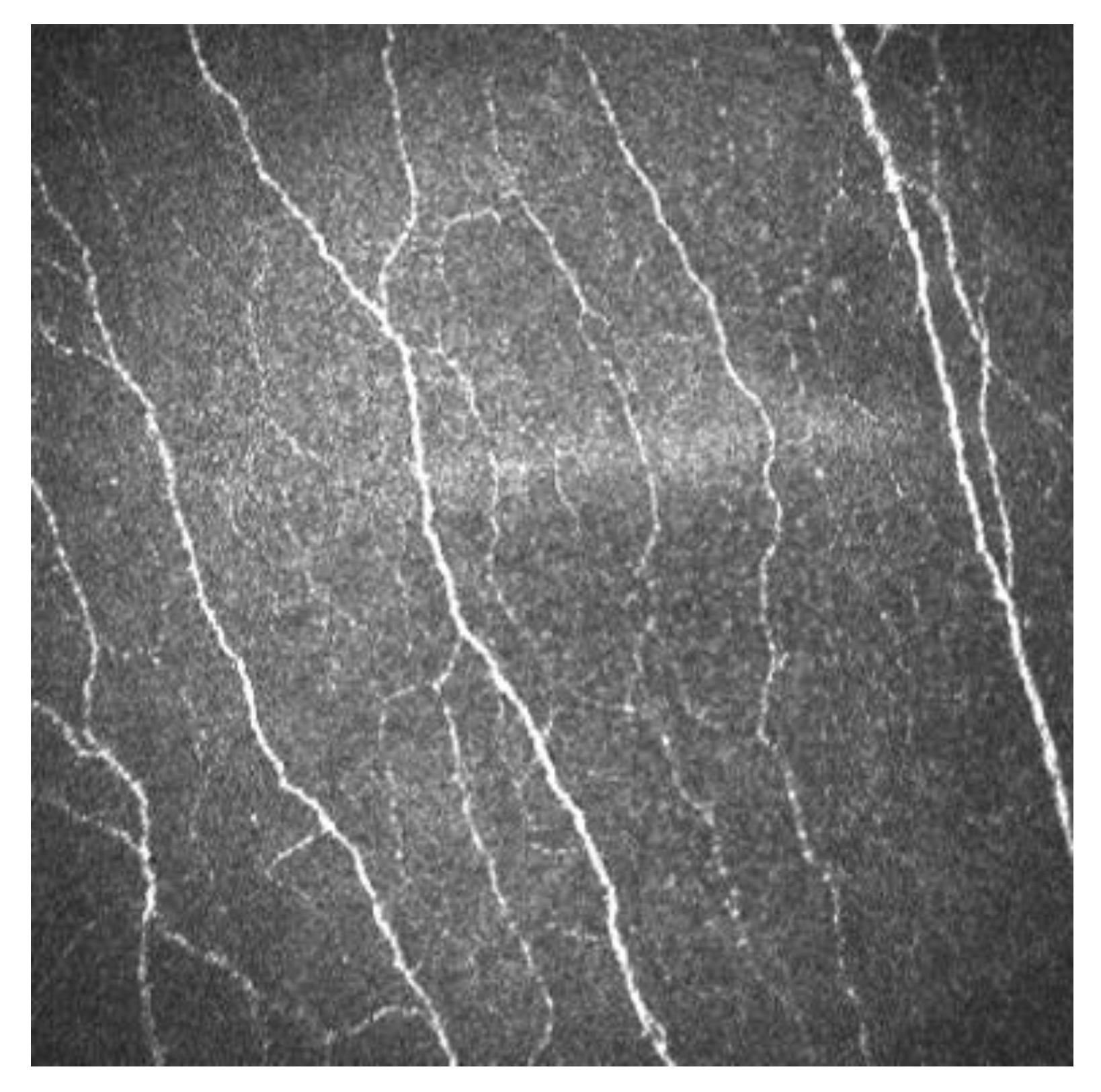
Figure 2. Sub-basal corneal nerves of a human cornea observed with corneal confocal microscopy, using the HRT II.
2.3. Bowman’s Layer
It is observed as a homogeneous and amorphous layer. It is considered an acellular layer formed by bundles of collagen fibers, but by confocal microscopy Langerhans cells have been observed at this level. These cells appear as corpuscular particles with an approximate diameter of 15 μm. Three different morphologies have been observed: individual cell bodies with processes, cells with a dendritic appearance and cells organized in a network by means of dendritic interdigitations [8].
2.4. Corneal Stroma
With corneal confocal microscopy researchers can easily observe the nucleus of keratocytes. On the other hand, the cell bodies, keratocyte processes and stromal collagen are not visible with confocal corneal microscopy. In the anterior stroma, a well-defined oval-round nucleus with different orientations is observed on a dark background. In the middle stroma, keratocytes are observed with a more regular oval shape. In the posterior stroma, they appear more elongated and axis-shaped [3].
2.5. Descemet Membrane
Descemet membrane can be observed with IVCM in aged patients. In young subjects it is not observed. It is shown as an acellular layer between the posterior stroma and the endothelium [3].
3. Corneal Laser Refractive Surgery
3.1. Corneal Wound Healing
IVCM has been used to know how refractive surgery affects the cornea wound healing and nerve regeneration. With IVCM, the corneal cells and corneal nerve plexus can be shown, and measure their changes after the ablation. After corneal refractive surgery, there is healing in corneal epithelium and stroma. Corneal wound healing is a process regulated by the interaction between epithelial and stromal cells, tear film and corneal nerve fibers [9][10]. Usually, the corneal wound healing response starts with epithelial injury. In corneal refractive surgery, the epithelial damage may be caused either by the microkeratome (MK), alcohol exposure or mechanical scraping in surface ablation procedures or the femtosecond laser. Following this damage, the epithelial cells release several cytokines that contribute to and stimulate the wound healing of the corneal process [10][11]. After the epithelial damage, cytokines are secreted by keratocytes in order to modulate the differentiation, migration and proliferation of epithelial cells to repair the stroma [12]. The keratocyte density can be measured with IVCM because they appear with an oval shape and a bright nucleus. The number of keratocytes undergoing apoptosis may be different according to the refractive procedure performed, and this fact has been demonstrated by IVCM studies [10][13][14]. In non-operated corneas, the keratocytes distribution along the corneal stroma has been studied, and there is higher keratocyte density in anterior stroma, followed by a decrease in keratocyte density in deeper layers [15]. The keratocyte depletion that occurs in the upper layers is more pronounced after surface ablation procedures than in laser in situ keratomileusis (LASIK). This may be due to how in flap procedures the corneal epithelium is preserved. Studies performed with IVCM have shown that in eyes that undergo surface ablation refractive surgeries, there is depletion of keratocytes under the ablated zone; this density decreases in a time period of 5 years, and there is an approximate loss of 5% of keratocyte density per year [16]. Corneas treated with LASIK also show a continuous decrease in the density of keratocytes. In surface ablation procedures, sub epithelial haze may occurs between 3 and 6 months postoperatively, and decreases thereafter [15][17]. Sub epithelial haze seems to be more common when there is a curvature change between the ablated area and nearby tissue, such as in high myopic errors, hyperopic corrections higher or equal to 4 diopters and in high astigmatic corrections [12][18]. The first option in corneal haze treatment is prevention with pharmacological agents that modulate wound healing response, such as Mitomycin C (MMC). MMC is topically administered intraoperatively, to avoid and minimize myofibroblast activation. MMC has an antimitotic effect and the keratocytes are the target of the MMC anti-haze mechanism, since this drug inhibits their activation, proliferation and differentiation into myofibroblasts [13][19][20]. The antimitotic effect of this drug led to the fear of a possible long-term depletion of the keratocyte population [21][22]. Keratocytes are visible with IVCM because of their hyperreflective nucleus and their oval shape (Figure 3), and there are several studies that confirm the IVCM is a useful tool to know how this drug affects corneal cells. It seems that after laser-assisted subepithelial keratectomy (LASEK) there is an initial cellular depletion in the stromal bed and a tendency towards normalization of the keratocyte density in different layers of the cornea, leading to a normal total corneal cell density 15 months and 3 years postoperatively. After a corneal injury, there is an apoptosis of keratocytes, followed by a repopulation around the wounded tissue; that theory would explain the increase in the keratocyte density found in deeper layers. However, it seems that a lower keratocyte density in the stromal bed is maintained over time, which could be caused by the extracellular matrix remodeling and the resulting fibrotic scar occurring at this level (Figure 3 and Figure 4) [13].
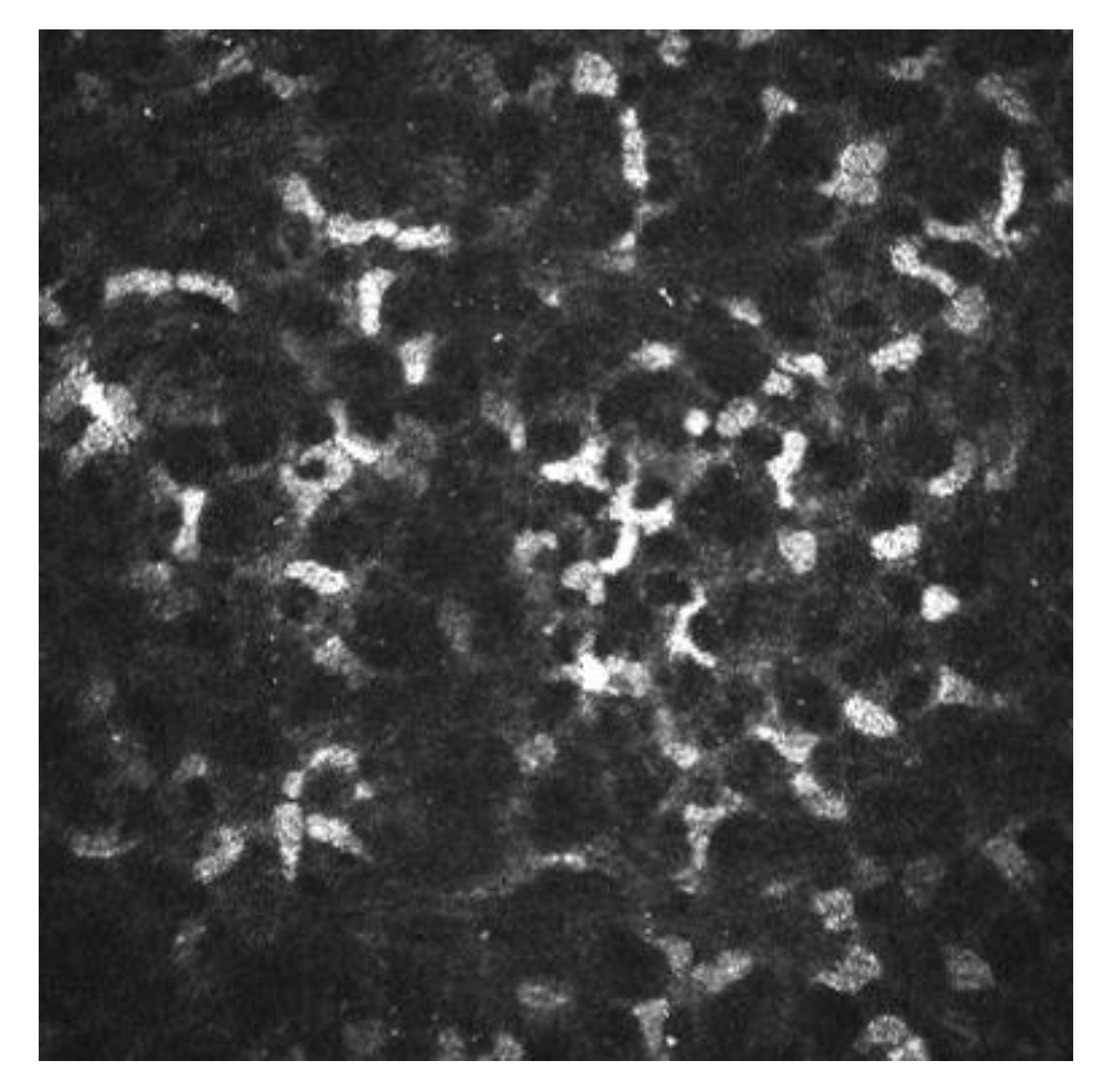
Figure 3. Corneal stroma of a human cornea observed with the confocal corneal microscopy HRTII.
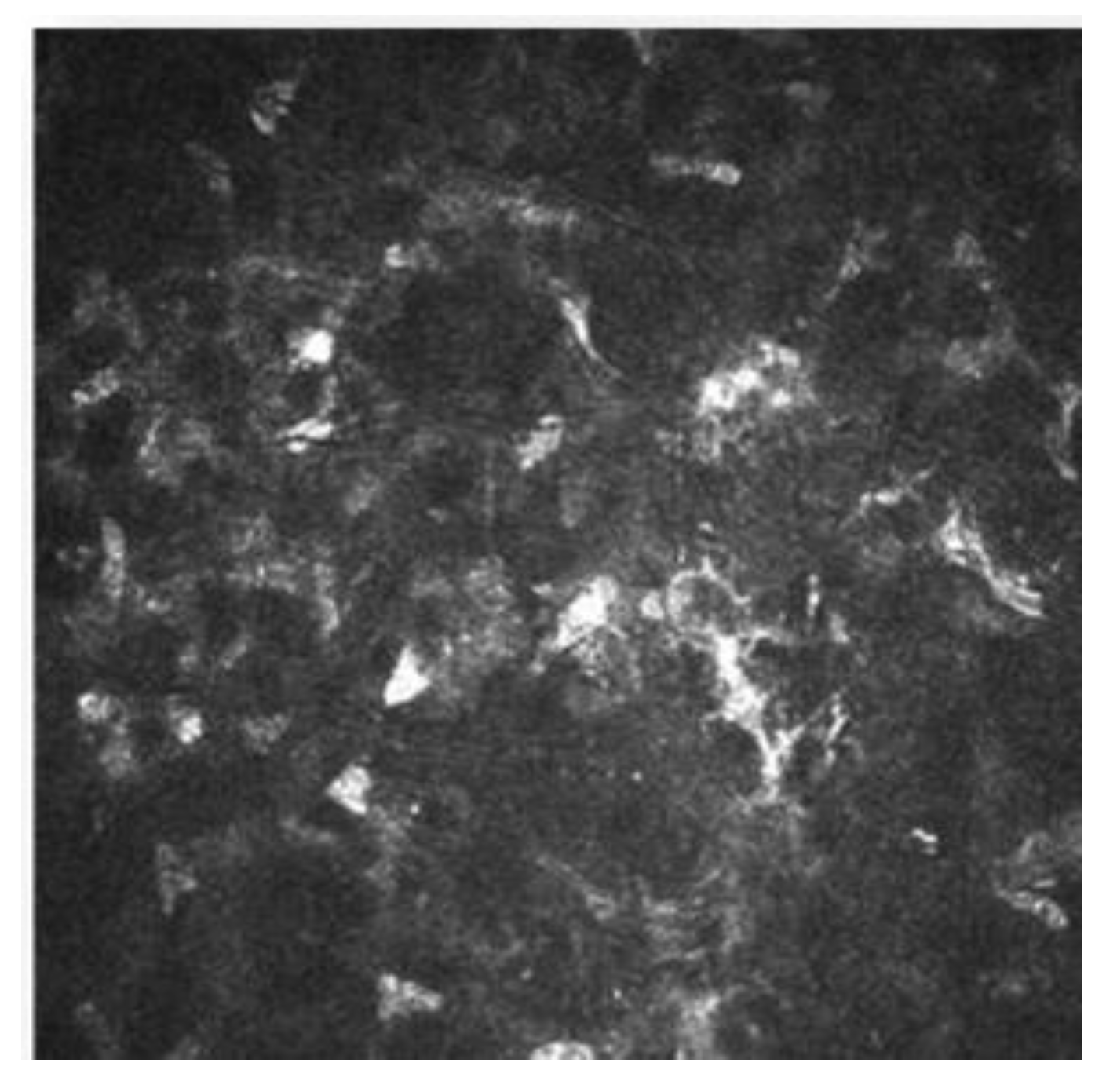
Figure 4. Postoperative haze after laser-assisted subepithelial keratectomy (LASEK).
The introduction of femtosecond lasers (FS) has increased the predictability during the creation of the stromal flap for LASIK [23]. The study of the response in vivo of the human cornea to the use of FS or MK to obtain the flap, or the interface characteristics [24][25][26] has been possible due to IVCM, and the possibility that this technique offers for the direct observation of the corneal cells [27].
3.2. Nerve Regeneration after Refractive Surgery
The cornea is the most innervated tissue in the human body. Corneal nerves, in addition to sensory function, are responsible for maintaining the functional structure of the ocular surface. They do this by releasing trophic substances that forward corneal epithelial homeostasis and activation of brainstem circuits that activate reflex tear production and blinking [28]. LASIK creates a corneal flap with an MK or FS followed by stromal ablation with an excimer laser. The IVCM allows a direct visualization of the corneal sub-basal nerve plexus in vivo, and thus the process of nerve fiber bundle regeneration after LASIK can be analyzed (Figure 5) [29][30].
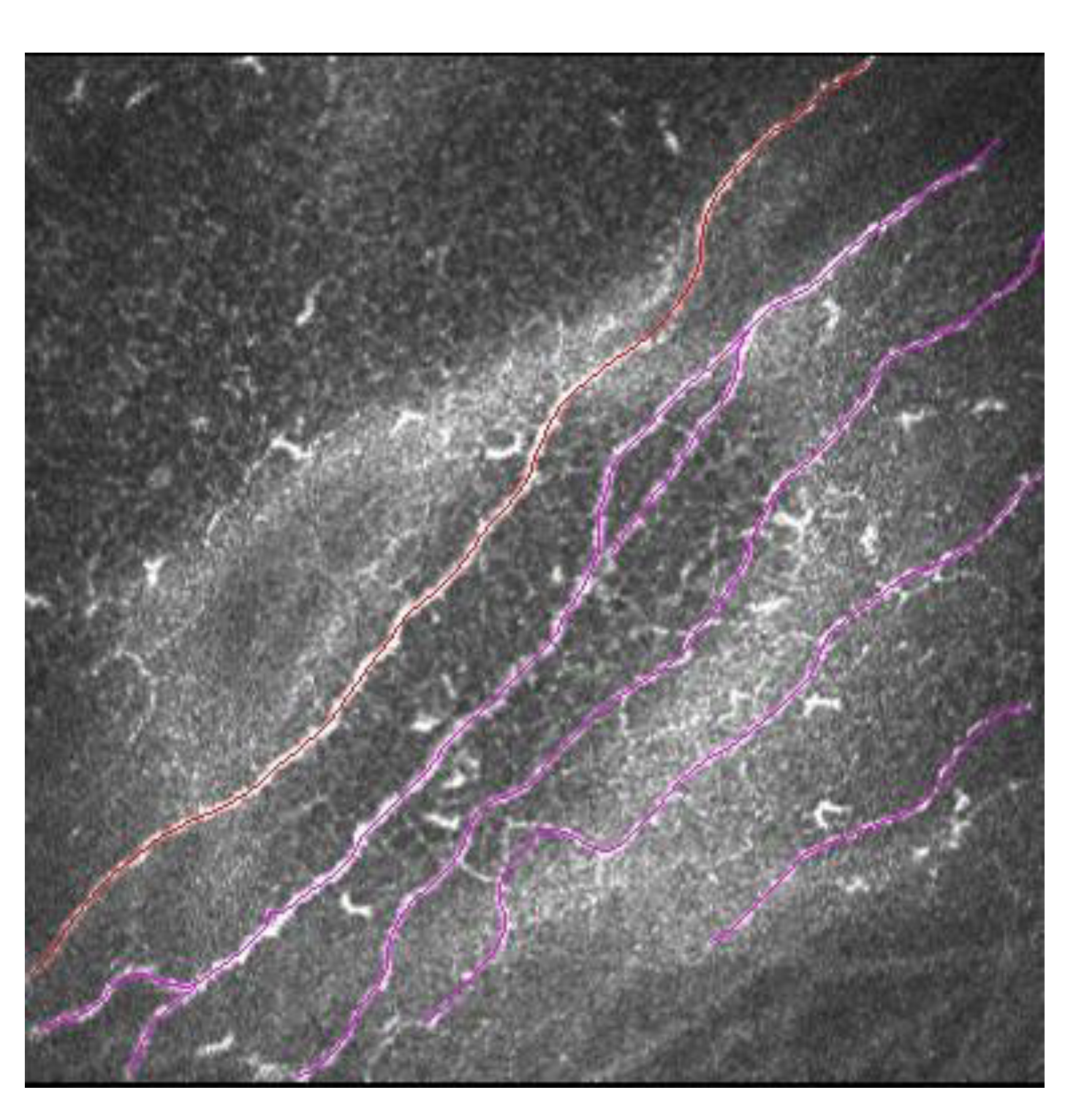
Figure 5. Sub-basal nerve plexus in LASIK patient. In red is marked a main corneal nerve, and in pink are marked secondary corneal nerves with ramifications.
Until at least 10 years after LASIK, the sub-basal nerve plexus does not fully recover its normal pattern. This has been shown with IVCM. Some sub-basal nerve morphology parameters such as nerve length, tortuosity and reflectivity returned to preoperative levels. Main nerve density and nerve branch density continued to be significantly lower compared to the control group (unoperated corneas) during a mean follow-up of 13.4 years after LASIK surgery [30].
3.3. Ocular Surface Pathologies
Development of IVCM made it feasible to investigate and quantify some of the ocular surface diseases, such as contact lens wear [31], keratitis [32], etc. In all these pathologies, with the use of the IVCM can be observed the characteristic morphology of several pathogens.
One of the most important applications of IVCM is to help in the diagnosis of a potentially severe ocular surface disease, such as Acanthamoeba keratitis (AK). AK is an infectious keratitis that represents a clinical challenge. Delays in diagnosis due to the challenging, masquerading presentation of AK are evident, and thus AK is one of the most aggressive corneal infections. The Acanthamoeba resistance to some drugs requires novel treatment approaches. The diagnosis of AK begins with the clinical suspicion [33]. IVCM can be effectively used to improve the diagnostic accuracy. The Acanthamoeba organisms have a characteristic morphology, and the use of IVCM plays an important role in the early diagnosis. The sensitivity of IVCM to help in AK diagnosis is about to 59.0 to 100%, depending mainly on the examiner expertise [34][35][36]. The Acanthamoeba organisms have specific morphological features that support the diagnosis. The most common features are: hyperreflective bodies with a round shape with double wall which can be found isolated or in clusters, and a target bright cyst with a dark center or ring-shaped signs [34][35]. The IVCM showing deeper diffusion and increased cyst density [37][38] are associated with a worse prognosis. In addition to its role in the diagnosis of AK, IVCM can also be used to assess for treatment response and examine for residual disease [39]. Figure 6 shows IVCM images of AK.
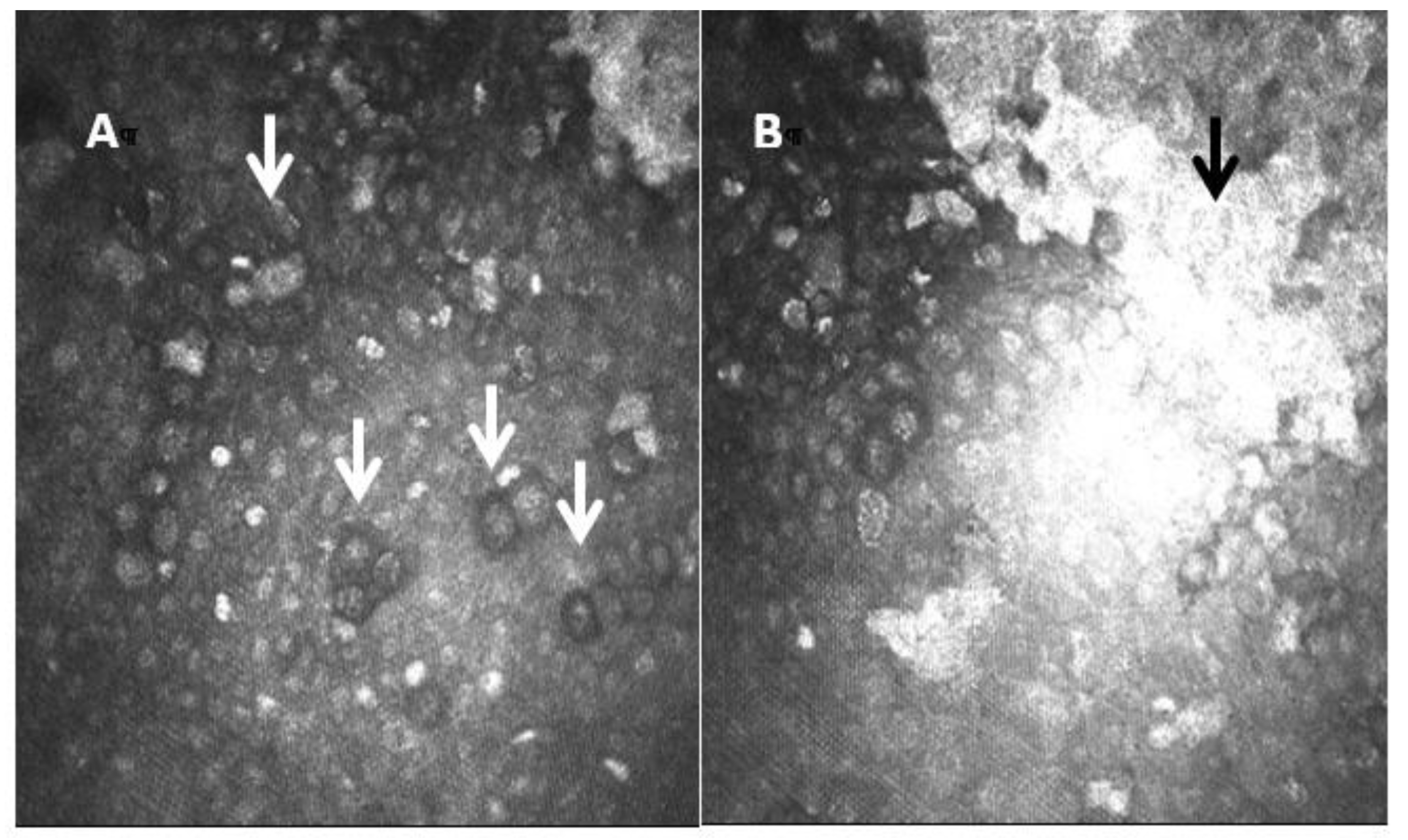
Figure 6. Laser confocal microscopic images of Acanthamoeba cysts. In image (A), the cysts show a highly reflective nucleus surrounded by a low-refractile ring wall (white arrows). The central structure is regular and round with uniform reflection. In image (B) researchers also see a hyperreflective scar (black arrow).
3.4. Dry Eye Disease (DED)
DED is characterized by tear film instability, visual disturbance, inflammation and damage of the ocular surface [40][41][42]. Recent research has shown that inflammation plays a key role in the pathogenesis of DED [41][43], particularly in DED associated with Sjogren syndrome (SS) and thus leads to a diffuse ocular surface damage [43][44].
There is increasing evidence suggesting that dendritic cells (DCs), which are equipped to induce T-cell activation and inflammatory cascade, are crucial in the DED pathogenesis [45][46][47]. With the help of IVCM, the density and morphology of DCs in DED have been identified, and thus a better insight to the pathogenesis of the clinical manifestations has been provided. [43][44]. In patients with DED and SS, it has been demonstrated there is an increased number of DCs in the central cornea [43][44]. In addition to quantity, DC morphology changes (such as size, dendrites number and length) are other biomarkers of the corneal response to inflammation and auto immunity phenomena (Figure 7) [48]. Some studies have revealed a decreased nerve density and a relatively high reflectivity, tortuosity and a substantial reduction in the corneal nerve fiber length, nerve fiber density, nerve fiber width, total nerve branch density and nerve fiber area in DED patients with ocular pain [42][43].
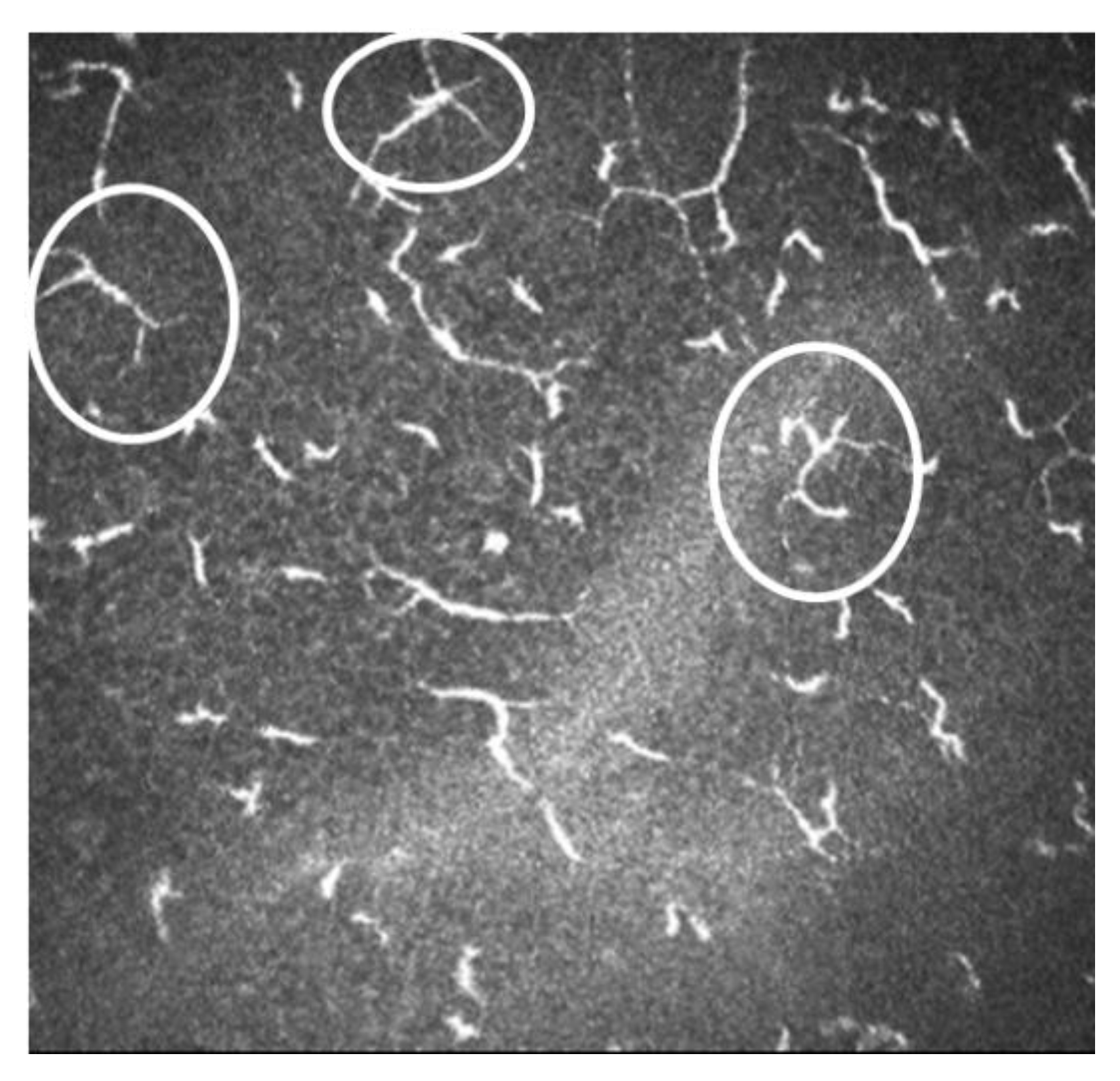
Figure 7. Image of IVCM of dendritic cells. In white circles are shown some of the active DCs.
References
- Patel, D.V.; McGhee, C.N. Mapping of the normal human corneal sub-Basal nerve plexus by in vivo laser scanning confocal microscopy. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4485–4488.
- Masters, B.R.; Böhnke, M. Confocal microscopy of the human cornea in vivo. Int. Ophthalmol. 2001, 23, 199–206.
- Mustonen, R.K.; McDonald, M.B.; Srivannaboon, S.; Tan, A.L.; Doubrava, M.W.; Kim, C.K. Normal human corneal cell populations evaluated by in vivo scanning slit confocal microscopy. Cornea 1998, 17, 485–492.
- Masters, B.R.; Thaer, A.A. In vivo human corneal confocal microscopy of identical fields of subepithelial nerve plexus, basal epithelial, and wing cells at different times. Microsc. Res. Tech. 1994, 29, 350–356.
- Popper, M.; Morgado, A.M.; Quadrado, M.J.; Van Best, J.A. Corneal cell density measurement in vivo by scanning slit confocal microscopy: Method and validation. Ophthalmic. Res. 2004, 36, 270–276.
- Oliveira-Soto, L.; Efron, N. Morphology of corneal nerves in soft contact lens wear. A comparative study using confocal microscopy. Ophthalmic. Physiol. Opt. 2003, 23, 163–174.
- Cruzat, A.; Qazi, Y.; Hamrah, P. In Vivo Confocal Microscopy of Corneal Nerves in Health and Disease. Ocul. Surf. 2017, 15, 15–47.
- Zhivov, A.; Stave, J.; Vollmar, B.; Guthoff, R. In vivo confocal microscopic evaluation of Langerhans cell density and distribution in the normal human corneal epithelium. Graefes Arch. Clin. Exp. Ophthalmol. 2005, 243, 1056–1061.
- Wilson, S.E.; Mohan, R.R.; Hong, J.W.; Lee, J.S.; Choi, R. The wound healing response after laser in situ keratomileusis and photorefractive keratectomy: Elusive control of biological variability and effect on custom laser vision correction. Arch. Ophthalmol. 2001, 119, 889–896.
- Dawson, D.G.; Kramer, T.R.; Grossniklaus, H.E.; Waring, G.O., 3rd; Edelhauser, H.F. Histologic, ultrastructural, and immunofluorescent evaluation of human laser-assisted in situ keratomileusis corneal wounds. Arch. Ophthalmol. 2005, 123, 741–756.
- Tuominen, I.S.; Tervo, T.M.; Teppo, A.M.; Valle, T.U.; Grönhagen-Riska, C.; Vesaluoma, M.H. Human tear fluid PDGF-BB, TNF-alpha and TGF-beta1 vs corneal haze and regeneration of corneal epithelium and subbasal nerve plexus after PRK. Exp. Eye Res. 2001, 72, 631–641.
- Torricelli, A.A.; Santhanam, A.; Wu, J.; Singh, V.; Wilson, S.E. The corneal fibrosis response to epithelial-stromal injury. Exp. Eye Res. 2016, 142, 110–118.
- de Benito-Llopis, L.; Canadas, P.; Drake, P.; Hernandez-Verdejo, J.L.; Teus, M.A. Keratocyte density 3 months, 15 months, and 3 years after corneal surface ablation with mitomycin C. Am. J. Ophthalmol. 2012, 153, 17–23.
- McLaren, J.W.; Bourne, W.M.; Maguire, L.J.; Patel, S.V. Changes in Keratocyte Density and Visual Function Five Years After Laser In Situ Keratomileusis: Femtosecond Laser Versus Mechanical Microkeratome. Am. J. Ophthalmol. 2015, 160, 163–170.
- de Benito Llopis, L.; Drake, P.; Cañadas, P.; Hernandez-Verdejo, J.L.; Teus, M.A. Keratocyte density after laser-assisted subepithelial keratectomy with mitomycin C. Am. J. Ophthalmol. 2010, 150, 642–649.
- Erie, J.C.; McLaren, J.W.; Hodge, D.O.; Bourne, W.M. Recovery of corneal subbasal nerve density after PRK and LASIK. Am. J. Ophthalmol. 2005, 140, 1059–1064.
- Cañadas, P.; Garcia-Gonzalez, M.; Gros-Otero, J.; Rodriguez-Perez, I.; Cañones-Zafra, R.; Teus, M.A. Effect of Laser-assisted Subepithelial Keratectomy with Mitomycin C on Corneal Optical Density Measured with Confocal Microscopy. Optom. Vis. Sci. 2021, 98, 350–354.
- Netto, M.V.; Mohan, R.R.; Sinha, S.; Sharma, A.; Dupps, W.; Wilson, S.E. Stromal haze, myofibroblasts, and surface irregularity after PRK. Exp. Eye Res. 2006, 82, 788–797.
- Chang, S.W. Corneal keratocyte apoptosis following topical intraoperative mitomycin C in rabbits. J. Refract. Surg. 2005, 21, 446–453.
- Pal-Ghosh, S.; Tadvalkar, G.; Lieberman, V.R. Transient Mitomycin C-treatment of human corneal epithelial cells and fibroblasts alters cell migration, cytokine secretion, and matrix accumulation. Sci. Rep. 2019, 9, 13905.
- Netto, M.V.; Mohan, R.R.; Sinha, S.; Sharma, A.; Gupta, P.C.; Wilson, S.E. Effect of prophylactic and therapeutic mitomycin C on corneal apoptosis, cellular proliferation, haze, and long-term keratocyte density in rabbits. J. Refract. Surg. 2006, 22, 562–574.
- Dupps, W.J., Jr.; Wilson, S.E. Biomechanics and wound healing in the cornea. Exp. Eye Res. 2006, 83, 709–720.
- Kezirian, G.M.; Stonecipher, K.G. Comparison of the IntraLase femtosecond laser and mechanical keratomes for laser in situ keratomileusis. J. Cataract Refract. Surg. 2004, 30, 804–811.
- Petroll, W.M.; Bowman, R.W.; Cavanagh, H.D.; Verity, S.M.; Mootha, V.V.; McCulley, J.P. Assessment of keratocyte activation following LASIK with flap creation using the IntraLase FS60 laser. J. Refract. Surg. 2008, 24, 847–849.
- Ortiz, D.; Alió, J.L.; Piñero, D. Measurement of corneal curvature change after mechanical laser in situ keratomileusis flap creation and femtosecond laser flap creation. J. Cataract Refract. Surg. 2008, 34, 238–242.
- Santhiago, M.R.; Wilson, S.E. Cellular effects after laser in situ keratomileusis flap formation with femtosecond lasers: A review. Cornea 2012, 31, 198–205.
- Cañadas, P.; de Benito-Llopis, L.; Hernandez-Verdejo, J.L.; Teus, M.A. Comparison of keratocyte density after femtosecond laser vs. mechanical microkeratome from 3 months up to 5 years after LASIK. Graefes Arch. Clin. Exp. Ophthalmol. 2013, 251, 2171–2179.
- Marfurt, C.F.; Cox, J.; Deek, S.; Dvorscak, L. Anatomy of the human corneal innervation. Exp. Eye Res. 2010, 90, 478–492.
- Zhang, F.; Deng, S.; Guo, N.; Wang, M.; Sun, X. Confocal comparison of corneal nerve regeneration and keratocyte reaction between FS-LASIK, OUP-SBK, and conventional LASIK. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5536–5544.
- Garcia-Gonzalez, M.; Cañadas, P.; Gros-Otero, J. Long-term corneal subbasal nerve plexus regeneration after laser in situ keratomileusis. J. Cataract Refract. Surg. 2019, 45, 966–971.
- Lopez-De La Rosa, A.; Arroyo-Del Arroyo, C.; Cañadas, P. Are Contact Lens Discomfort or Soft Contact Lens Material Properties Associated with Alterations in the Corneal Sub-Basal Nerve Plexus? Curr. Eye Res. 2018, 43, 487–492.
- Cruzat, A.; Witkin, D.; Baniasadi, N. Inflammation and the nervous system: The connection in the cornea in patients with infectious keratitis. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5136–5143.
- Maycock, N.J.; Jayaswal, R. Update on Acanthamoeba Keratitis: Diagnosis, Treatment, and Outcomes. Cornea 2016, 35, 713–720.
- Tu, E.Y.; Joslin, C.E.; Sugar, J.; Booton, G.C.; Shoff, M.E.; Fuerst, P.A. The relative value of confocal microscopy and superficial corneal scrapings in the diagnosis of Acanthamoeba keratitis. Cornea 2008, 27, 764–772.
- Goh, J.W.Y.; Harrison, R.; Hau, S.; Alexander, C.L.; Tole, D.M.; Avadhanam, V.S. Comparison of In Vivo Confocal Microscopy, PCR and Culture of Corneal Scrapes in the Diagnosis of Acanthamoeba Keratitis. Cornea 2018, 37, 480–485.
- Kheirkhah, A.; Satitpitakul, V.; Syed, Z.A. Factors Influencing the Diagnostic Accuracy of Laser-Scanning In Vivo Confocal Microscopy for Acanthamoeba Keratitis. Cornea 2018, 37, 818–823.
- Huang, P.; Tepelus, T.; Vickers, L.A. Quantitative Analysis of Depth, Distribution, and Density of Cysts in Acanthamoeba Keratitis Using Confocal Microscopy. Cornea 2017, 36, 927–932.
- Li, S.; Bian, J.; Wang, Y.; Wang, S.; Wang, X.; Shi, W. Clinical features and serial changes of Acanthamoeba keratitis: An in vivo confocal microscopy study. Eye 2020, 34, 327–334.
- Chopra, R.; Mulholland, P.J.; Hau, S.C. In Vivo Confocal Microscopy Morphologic Features and Cyst Density in Acanthamoeba Keratitis. Am. J. Ophthalmol. 2020, 217, 38–48.
- Craig, J.P.; Nichols, K.K.; Akpek, E.K. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283.
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510.
- Willcox, M.D.P.; Argüeso, P.; Georgiev, G.A.; Holopainen, J.M.; Laurie, G.W.; Millar, T.J.; Papas, E.B.; Rolland, J.P.; Schmidt, T.A.; Stahl, U.; et al. TFOS DEWS II Tear Film Report. Ocul. Surf. 2017, 15, 366–403.
- Kheirkhah, A.; Rahimi Darabad, R.; Cruzat, A. Corneal Epithelial Immune Dendritic Cell Alterations in Subtypes of Dry Eye Disease: A Pilot In Vivo Confocal Microscopic Study. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7179–7185.
- Lin, H.; Li, W.; Dong, N. Changes in corneal epithelial layer inflammatory cells in aqueous tear-deficient dry eye. Investig. Ophthalmol. Vis. Sci. 2010, 51, 122–128.
- Schaumburg, C.S.; Siemasko, K.F.; De Paiva, C.S. Ocular surface APCs are necessary for autoreactive T cell-mediated experimental autoimmune lacrimal keratoconjunctivitis. J. Immunol. 2011, 187, 3653–3662.
- Stevenson, W.; Chauhan, S.K.; Dana, R. Dry eye disease: An immune-mediated ocular surface disorder. Arch. Ophthalmol. 2012, 130, 90–100.
- Barabino, S.; Chen, Y.; Chauhan, S.; Dana, R. Ocular surface immunity: Homeostatic mechanisms and their disruption in dry eye disease. Prog. Retin. Eye Res. 2012, 31, 271–285.
- Jamali, A.; Kenyon, B.; Ortiz, G. Plasmacytoid dendritic cells in the eye. Prog. Retin. Eye Res. 2021, 80, 1008–1077.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
04 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




