
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Despina Fotiou | -- | 3848 | 2022-12-29 07:45:59 | | | |
| 2 | Peter Tang | + 7 word(s) | 3855 | 2022-12-29 08:02:43 | | |
Video Upload Options
Multiple myeloma (MM) is associated with an increased risk of thrombotic complications, which remains substantial despite the implementation of thromboprophylaxis. The procoagulant state that characterizes the disease is multifactorial, and a greater understanding of the underlying pathophysiology is required to inform appropriate thrombosis prevention. There is a shift towards using direct oral anticoagulants (DOACs) in this setting; head-to-head comparisons in the context of controlled clinical trials between class agents are still missing. MM-specific venous thromboembolism (VTE) risk assessment scores have been developed to optimize management and minimize the associated mortality/morbidity. Their clinical utility remains to be evaluated.
1. Introduction
2. Risk Factors for Thrombosis
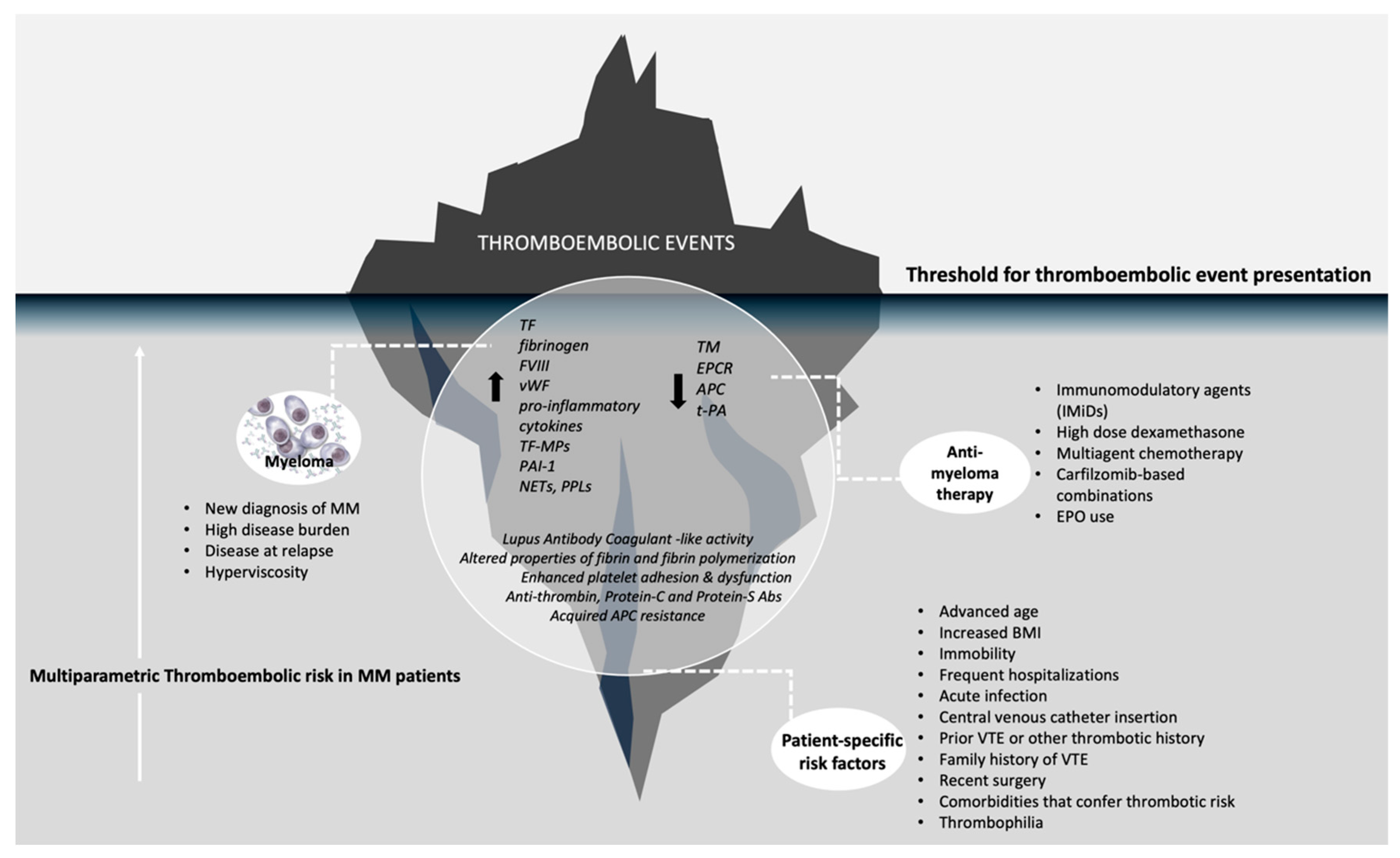
2.1. Patient-Related Risk Factors
2.2. Disease-Specific Risk Factors
2.3. Treatment-Related Risk Factors
3. Risk Assessment Models for VTE in Multiple Myeloma
Table 1a: IWMG score and algorithm for MM patient risk stratification
IWMG score and algorithm for MM patient risk stratification |
||
Patient-related risk factors1 point for each |
Disease-related riskfactors: 1 point for each |
Treatment-related risk factors:points as seen below: |
Body mass index >25, age>75, Personal or family history of VTE, Central venous catheter, Acute infection or Hospitalization, Blood clotting disorders or Thrombophilia, Immobility with a performance status of >1, Comorbidities (liver, renal impairment, Chronic obstructive pulmonary disorder, diabetes mellitus, chronic inflammatory bowel disease), Race (Caucasian) |
· Diagnosis of Multiple Myeloma· Evidence of hyperviscosity |
•IMiD in combination with low-dose dexamethasone (<480mg/month) (1 point)•IMiD plus High-dose dexamethasone (>480mg/month) or doxorubicin or multiagent chemotherapy (2 points)•IMiD alone (1 point)•Erythropoietin use (1 point) |
Risk stratification and recommended thromboprophylaxis:0 points: Low risk - None1 point: Intermediate risk - Aspirin at 100mg>1 point: High risk - Low molecular weight heparin at prophylactic dose or therapeutic dose of warfarin |
||
Table 1b: IMPEDE and SAVED risk assessment models
CLINICAL RAMs for VTE in MM |
||
IMPEDE VTE score |
SAVED* score |
|
Immunomodulatory drug (+4)BMI ≥ 25 kg/m2 (+1)Pathologic fracture pelvis/femur (+4)Erythropoiesis-stimulating agent (+1)Dexamethasone (High-dose, ≥1600mg/cycle) (+4)Dexamethasone Low-Dose (<160mg/cycle) (+2)Doxorubicin (+3)Ethnicity/Race= Asian (-3)VTE history (+5)Tunnelled line/CVC (+2)Existing use of therapeutic warfarin or low molecular weight heparin (LWMH) (-5)Existing use of prophylactic LMWH or aspirin (-3) |
Surgery (within the last 90 days) (+2)Asian Race (-3)VTE history (+3)Eight (age >=80 y) (+1)Dexamethasone doseStandard (120-160mg/cycle) (+1)High (>160mg/cycle) (+2)*for patients on IMiD-based regimens only |
|
Stratified risk groups based on a weighted scoring system |
||
Low risk (score of ≤ 3)Intermediate-risk (score of 4-7)High risk (score of ≥ 8) |
High risk (score of ≥ 2)Low risk (≤1) |
|
* for patients on IMiD-based regimens only.
4. Primary VTE Prevention in Multiple Myeloma
5. Treatment of VTE and Secondary Prevention of VTE
6. Conclusions
The main challenges in the current management of thromboembolic complications in patients with MM are (1) the suboptimal performance of the risk stratification tools that are currently available and (2) the fact that the use of thromboprophylaxis is not routinely incorporated for all patients with MM in every day clinical practice. In the clinical trial setting, despite adequate thromboprophylaxis, patients on lenalidomide-based regimens still have a substantial risk of VTE of around 6%, indicating either that risk assessment models have poor discriminatory power or that the use of thromboprophylaxis is not optimal. There is also data to support that in the real-world setting, patients often receive inappropriate thromboprophylaxis based on baseline risk stratification and that the approach is usually not guideline based.
To summarize current recommendations, all NDMM should have a baseline risk assessment concerning thrombotic complications using available tools. The baseline VTE risk should be weighed against the hemorrhagic risk in a patient-specific manner. The final choice of the agent used for thromboprophylaxis should be tailored to each patient. Finally, the baseline risk should be reevaluated throughout the disease course as it is an evolving process and pharmacological thromboprophylaxis should be adapted accordingly. Disease status (relapse or remission), performance status and patient mobilization, platelet count and renal function are a few of the factors that need to be reassessed at regular intervals. Aspirin should be reserved for very-low-risk patients. For other patients, given that there is no contraindication, a choice should be made between LMWH and DOACs. Figure 2 presents an algorithm for VTE prevention in MM patients.
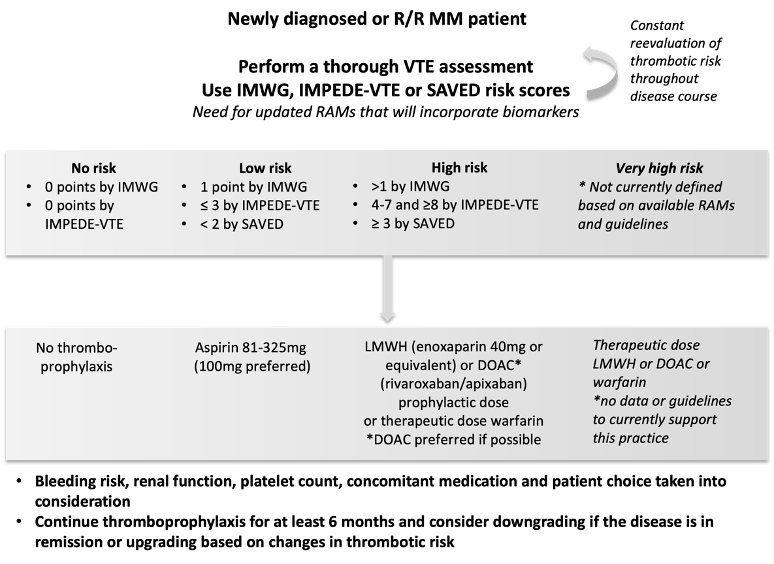
Figure 2. Algorithm for the assessment of VTE risk and choice of thromboprophylaxis. R/R: relapsed/refractory, MM: multiple myeloma, IMWG: international myeloma working group risk score, RAMs: risk assessment models, LWMH: low molecular weight heparin, DOAC: direct oral anticoagulant
Unfortunately, advances in the management of thromboembolic complications in patients with MM in recent years have not followed the evolution of the other anti-myeloma therapies, and thromboembolism remains a significant complication. To overcome the unmet need for thromboprophylaxis optimization, we need to address the gaps in existing knowledge. First, we need to understand better the procoagulant state observed in MM patients, the contribution of individual risk factors and the interplay between the disease, patient and treatment-specific prothrombotic mechanisms. Second, we need to identify biomarkers and develop tools that accurately reflect the thrombotic risk in MM patients. Incorporating coagulation biomarkers in the current risk assessment tools seems to be a potentially useful tool. Third, we need clinical trials that will provide robust data on the safety and efficacy of different modes of thromboprophylaxis, using risk-assessment tools to stratify patients. Finally, we need to increase the implementation of international guidelines in everyday clinical practice.
References
- Carrier, M.; Le Gal, G.; Tay, J.; Wu, C.; Lee, A.Y. Rates of venous thromboembolism in multiple myeloma patients undergoing immunomodulatory therapy with thalidomide or lenalidomide: A systematic review and meta-analysis. J. Thromb. Haemost. 2011, 9, 653–663.
- De Stefano, V.; Za, T.; Rossi, E. Venous thromboembolism in multiple myeloma. Semin. Thromb. Hemost. 2014, 40, 338–347.
- Zangari, M.; Barlogie, B.; Cavallo, F.; Bolejack, V.; Fink, L.; Tricot, G. Effect on survival of treatment-associated venous thromboembolism in newly diagnosed multiple myeloma patients. Blood Coagul. Fibrinolysis 2007, 18, 595–598.
- Zangari, M.; Tricot, G.; Polavaram, L.; Zhan, F.; Finlayson, A.; Knight, R.; Fu, T.; Weber, D.; Dimopoulos, M.A.; Niesvizky, R.; et al. Survival effect of venous thromboembolism in patients with multiple myeloma treated with lenalidomide and high-dose dexamethasone. J. Clin. Oncol. 2010, 28, 132–135.
- Schoen, M.W.; Carson, K.R.; Luo, S.; Gage, B.F.; Li, A.; Afzal, A.; Sanfilippo, K.M. Venous thromboembolism in multiple myeloma is associated with increased mortality. Res. Pract. Thromb. Haemost. 2020, 4, 1203–1210.
- Khorana, A.A.; Dalal, M.R.; Lin, J.; Connolly, G.C. Health care costs associated with venous thromboembolism in selected high-risk ambulatory patients with solid tumors undergoing chemotherapy in the United States. Clin. Outcomes Res. 2013, 5, 101–108.
- Palumbo, A.; Rajkumar, S.V.; San Miguel, J.F.; Larocca, A.; Niesvizky, R.; Morgan, G.; Landgren, O.; Hajek, R.; Einsele, H.; Anderson, K.C.; et al. International Myeloma Working Group consensus statement for the management, treatment, and supportive care of patients with myeloma not eligible for standard autologous stem-cell transplantation. J. Clin. Oncol. 2014, 32, 587–600.
- Streiff, M.B.; Holmstrom, B.; Angelini, D.; Ashrani, A.; Elshoury, A.; Fanikos, J.; Fertrin, K.Y.; Fogerty, A.E.; Gao, S.; Goldhaber, S.Z.; et al. Cancer-Associated Venous Thromboembolic Disease, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 1181–1201.
- De Stefano, V.; Larocca, A.; Carpenedo, M.; Cavo, M.; Di Raimondo, F.; Falanga, A.; Offidani, M.; Petrucci, M.T.; Ruggeri, M.; Santi, R.M.; et al. Thrombosis in multiple myeloma: Risk stratification, antithrombotic prophylaxis, and management of acute events. A consensus-based position paper from an ad hoc expert panel. Haematologica 2022, 107, 2536–2547.
- Sanfilippo, K.M.; Luo, S.; Wang, T.F.; Fiala, M.; Schoen, M.; Wildes, T.M.; Mikhael, J.; Kuderer, N.M.; Calverley, D.C.; Keller, J.; et al. Predicting venous thromboembolism in multiple myeloma: Development and validation of the IMPEDE VTE score. Am. J. Hematol. 2019, 94, 1176–1184.
- Kristinsson, S.Y.; Pfeiffer, R.M.; Bjorkholm, M.; Goldin, L.R.; Schulman, S.; Blimark, C.; Mellqvist, U.H.; Wahlin, A.; Turesson, I.; Landgren, O. Arterial and venous thrombosis in monoclonal gammopathy of undetermined significance and multiple myeloma: A population-based study. Blood 2010, 115, 4991–4998.
- Kristinsson, S.Y.; Fears, T.R.; Gridley, G.; Turesson, I.; Mellqvist, U.H.; Bjorkholm, M.; Landgren, O. Deep vein thrombosis after monoclonal gammopathy of undetermined significance and multiple myeloma. Blood 2008, 112, 3582–3586.
- Sallah, S.; Husain, A.; Wan, J.; Vos, P.; Nguyen, N.P. The risk of venous thromboembolic disease in patients with monoclonal gammopathy of undetermined significance. Ann. Oncol. 2004, 15, 1490–1494.
- Nielsen, T.; Kristensen, S.R.; Gregersen, H.; Teodorescu, E.M.; Pedersen, S. Prothrombotic abnormalities in patients with multiple myeloma and monoclonal gammopathy of undetermined significance. Thromb. Res. 2021, 202, 108–118.
- Palumbo, A.; Rajkumar, S.V.; Dimopoulos, M.A.; Richardson, P.G.; San Miguel, J.; Barlogie, B.; Harousseau, J.; Zonder, J.A.; Cavo, M.; Zangari, M.; et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia 2008, 22, 414–423.
- Dimopoulos, M.A.; Leleu, X.; Palumbo, A.; Moreau, P.; Delforge, M.; Cavo, M.; Ludwig, H.; Morgan, G.J.; Davies, F.E.; Sonneveld, P.; et al. Expert panel consensus statement on the optimal use of pomalidomide in relapsed and refractory multiple myeloma. Leukemia 2014, 28, 1573–1585.
- Dimopoulos, M.A.; Palumbo, A.; Attal, M.; Beksac, M.; Davies, F.E.; Delforge, M.; Einsele, H.; Hajek, R.; Harousseau, J.L.; da Costa, F.L.; et al. Optimizing the use of lenalidomide in relapsed or refractory multiple myeloma: Consensus statement. Leukemia 2011, 25, 749–760.
- Palumbo, A.; Cavo, M.; Bringhen, S.; Zamagni, E.; Romano, A.; Patriarca, F.; Rossi, D.; Gentilini, F.; Crippa, C.; Galli, M.; et al. Aspirin, warfarin, or enoxaparin thromboprophylaxis in patients with multiple myeloma treated with thalidomide: A phase III, open-label, randomized trial. J. Clin. Oncol. 2011, 29, 986–993.
- Larocca, A.; Cavallo, F.; Bringhen, S.; Di Raimondo, F.; Falanga, A.; Evangelista, A.; Cavalli, M.; Stanevsky, A.; Corradini, P.; Pezzatti, S.; et al. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma treated with lenalidomide. Blood 2012, 119, 933–939; quiz 1093.
- Cortelezzi, A.; Moia, M.; Falanga, A.; Pogliani, E.M.; Agnelli, G.; Bonizzoni, E.; Gussoni, G.; Barbui, T.; Mannucci, P.M.; Group, C.S. Incidence of thrombotic complications in patients with haematological malignancies with central venous catheters: A prospective multicentre study. Br. J. Haematol. 2005, 129, 811–817.
- Fotiou, D.; Gavriatopoulou, M.; Terpos, E. Multiple Myeloma and Thrombosis: Prophylaxis and Risk Prediction Tools. Cancers 2020, 12, 191.
- Cini, M.; Zamagni, E.; Valdre, L.; Palareti, G.; Patriarca, F.; Tacchetti, P.; Legnani, C.; Catalano, L.; Masini, L.; Tosi, P.; et al. Thalidomide-dexamethasone as up-front therapy for patients with newly diagnosed multiple myeloma: Thrombophilic alterations, thrombotic complications, and thromboprophylaxis with low-dose warfarin. Eur. J. Haematol. 2010, 84, 484–492.
- Bagratuni, T.; Kastritis, E.; Politou, M.; Roussou, M.; Kostouros, E.; Gavriatopoulou, M.; Eleutherakis-Papaiakovou, E.; Kanelias, N.; Terpos, E.; Dimopoulos, M.A. Clinical and genetic factors associated with venous thromboembolism in myeloma patients treated with lenalidomide-based regimens. Am. J. Hematol. 2013, 88, 765–770.
- Chakraborty, R.; Bin Riaz, I.; Malik, S.U.; Marneni, N.; Mejia Garcia, A.; Anwer, F.; Khorana, A.A.; Rajkumar, S.V.; Kumar, S.; Murad, M.H.; et al. Venous thromboembolism risk with contemporary lenalidomide-based regimens despite thromboprophylaxis in multiple myeloma: A systematic review and meta-analysis. Cancer 2020, 126, 1640–1650.
- Gregersen, H.; Norgaard, M.; Severinsen, M.T.; Engebjerg, M.C.; Jensen, P.; Sorensen, H.T. Monoclonal gammopathy of undetermined significance and risk of venous thromboembolism. Eur. J. Haematol. 2011, 86, 129–134.
- Yasin, Z.; Quick, D.; Thiagarajan, P.; Spoor, D.; Caraveo, J.; Palascak, J. Light-chain paraproteins with lupus anticoagulant activity. Am. J. Hematol. 1999, 62, 99–102.
- Carr, M.E., Jr.; Dent, R.M.; Carr, S.L. Abnormal fibrin structure and inhibition of fibrinolysis in patients with multiple myeloma. J. Lab. Clin. Med. 1996, 128, 83–88.
- Lackner, H.; Hunt, V.; Zucker, M.B.; Pearson, J. Abnormal fibrin ultrastructure, polymerization, and clot retraction in multiple myeloma. Br. J. Haematol. 1970, 18, 625–636.
- O’Kane, M.J.; Wisdom, G.B.; Desai, Z.R.; Archbold, G.P. Inhibition of fibrin monomer polymerisation by myeloma immunoglobulin. J. Clin. Pathol. 1994, 47, 266–268.
- van Marion, A.M.; Auwerda, J.J.; Minnema, M.C.; van Oosterom, R.; Adelmeijer, J.; de Groot, P.G.; Leebeek, F.W.; Sonneveld, P.; Lokhorst, H.M.; Lisman, T. Hypofibrinolysis during induction treatment of multiple myeloma may increase the risk of venous thrombosis. Thromb. Haemost. 2005, 94, 1341–1343.
- Martini, F.; Cecconi, N.; Paolicchi, A.; Galimberti, S.; Cervetti, G.; Buda, G.; Petrini, M. Interference of Monoclonal Gammopathy with Fibrinogen Assay Producing Spurious Dysfibrinogenemia. TH Open 2019, 3, e64–e66.
- Robak, M.; Trelinski, J.; Chojnowski, K. Hemostatic changes after 1 month of thalidomide and dexamethasone therapy in patients with multiple myeloma. Med. Oncol. 2012, 29, 3574–3580.
- Zamagni, E.; Brioli, A.; Tacchetti, P.; Zannetti, B.; Pantani, L.; Cavo, M. Multiple myeloma, venous thromboembolism, and treatment-related risk of thrombosis. Semin. Thromb. Hemost. 2011, 37, 209–219.
- Auwerda, J.J.; Yuana, Y.; Osanto, S.; de Maat, M.P.; Sonneveld, P.; Bertina, R.M.; Leebeek, F.W. Microparticle-associated tissue factor activity and venous thrombosis in multiple myeloma. Thromb. Haemost. 2011, 105, 14–20.
- Comerford, C.; Glavey, S.; Quinn, J.; O’Sullivan, J.M. The role of VWF/FVIII in thrombosis and cancer progression in multiple myeloma and other hematological malignancies. J. Thromb. Haemost. 2022, 20, 1766–1777.
- Egan, K.; Cooke, N.; Dunne, E.; Murphy, P.; Quinn, J.; Kenny, D. Platelet hyporeactivity in active myeloma. Thromb. Res. 2014, 134, 747–749.
- O’Sullivan, L.R.; Meade-Murphy, G.; Gilligan, O.M.; Mykytiv, V.; Young, P.W.; Cahill, M.R. Platelet hyperactivation in multiple myeloma is also evident in patients with premalignant monoclonal gammopathy of undetermined significance. Br. J. Haematol. 2021, 192, 322–332.
- Palumbo, A.; Palladino, C. Venous and arterial thrombotic risks with thalidomide: Evidence and practical guidance. Adv. Drug Saf. 2012, 3, 255–266.
- Fouquet, G.; Tardy, S.; Demarquette, H.; Bonnet, S.; Gay, J.; Debarri, H.; Herbaux, C.; Guidez, S.; Michel, J.; Perrot, A.; et al. Efficacy and safety profile of long-term exposure to lenalidomide in patients with recurrent multiple myeloma. Cancer 2013, 119, 3680–3686.
- Rajkumar, S.V.; Jacobus, S.; Callander, N.S.; Fonseca, R.; Vesole, D.H.; Williams, M.E.; Abonour, R.; Siegel, D.S.; Katz, M.; Greipp, P.R.; et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: An open-label randomised controlled trial. Lancet Oncol. 2010, 11, 29–37.
- Zonder, J.A.; Crowley, J.; Hussein, M.A.; Bolejack, V.; Moore, D.F., Sr.; Whittenberger, B.F.; Abidi, M.H.; Durie, B.G.; Barlogie, B. Lenalidomide and high-dose dexamethasone compared with dexamethasone as initial therapy for multiple myeloma: A randomized Southwest Oncology Group trial (S0232). Blood 2010, 116, 5838–5841.
- Bradbury, C.A.; Craig, Z.; Cook, G.; Pawlyn, C.; Cairns, D.A.; Hockaday, A.; Paterson, A.; Jenner, M.W.; Jones, J.R.; Drayson, M.T.; et al. Thrombosis in patients with myeloma treated in the Myeloma IX and Myeloma XI phase 3 randomized controlled trials. Blood 2020, 136, 1091–1104.
- Dimopoulos, M.A.; Swern, A.S.; Li, J.S.; Hussein, M.; Weiss, L.; Nagarwala, Y.; Baz, R. Efficacy and safety of long-term treatment with lenalidomide and dexamethasone in patients with relapsed/refractory multiple myeloma. Blood Cancer J. 2014, 4, e257.
- Richardson, P.G.; Siegel, D.S.; Vij, R.; Hofmeister, C.C.; Baz, R.; Jagannath, S.; Chen, C.; Lonial, S.; Jakubowiak, A.; Bahlis, N.; et al. Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: A randomized phase 2 study. Blood 2014, 123, 1826–1832.
- Miguel, J.S.; Weisel, K.; Moreau, P.; Lacy, M.; Song, K.; Delforge, M.; Karlin, L.; Goldschmidt, H.; Banos, A.; Oriol, A.; et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013, 14, 1055–1066.
- Leleu, X.; Attal, M.; Arnulf, B.; Moreau, P.; Traulle, C.; Marit, G.; Mathiot, C.; Petillon, M.O.; Macro, M.; Roussel, M.; et al. Pomalidomide plus low-dose dexamethasone is active and well tolerated in bortezomib and lenalidomide-refractory multiple myeloma: Intergroupe Francophone du Myelome 2009–2002. Blood 2013, 121, 1968–1975.
- Rupa-Matysek, J.; Gil, L.; Wojtasińska, E.; Nowicki, A.; Dytfeld, D.; Kaźmierczak, M.; Komarnicki, M. Inhibitory effects of bortezomib on platelet aggregation in patients with multiple myeloma. Thromb. Res. 2014, 134, 404–411.
- Jilma, B.; Cvitko, T.; Winter-Fabry, A.; Petroczi, K.; Quehenberger, P.; Blann, A.D. High dose dexamethasone increases circulating P-selectin and von Willebrand factor levels in healthy men. Thromb. Haemost. 2005, 94, 797–801.
- Swystun, L.L.; Shin, L.Y.Y.; Beaudin, S.; Liaw, P.C. Chemotherapeutic agents doxorubicin and epirubicin induce a procoagulant phenotype on endothelial cells and blood monocytes. J. Thromb. Haemost. 2009, 7, 619–626.
- Facon, T.; Dimopoulos, M.A.; Dispenzieri, A.; Catalano, J.V.; Belch, A.; Cavo, M.; Pinto, A.; Weisel, K.; Ludwig, H.; Bahlis, N.J.; et al. Final analysis of survival outcomes in the phase 3 FIRST trial of up-front treatment for multiple myeloma. Blood 2018, 131, 301–310.
- Hou, J.; Du, X.; Jin, J.; Cai, Z.; Chen, F.; Zhou, D.B.; Yu, L.; Ke, X.; Li, X.; Wu, D.; et al. A Multicenter, Open-Label, Phase 2 Study of Lenalidomide Plus Low-Dose Dexamethasone in Chinese Patients with Relapsed/Refractory Multiple Myeloma: The Mm-021 Trial. J. Hematol. Oncol. 2013, 6, 41.
- Bradbury, C. Thrombotic Events in Patients with Myeloma Treated with Immunomodulatory Drugs; Results of the Myeloma XI Study. Blood 2017, 130, 553.
- Hou, J.; Jin, J.; Xu, Y.; Wu, D.; Ke, X.; Zhou, D.; Lu, J.; Du, X.; Chen, X.; Li, J.; et al. Randomized, double-blind, placebo-controlled phase III study of ixazomib plus lenalidomide-dexamethasone in patients with relapsed/refractory multiple myeloma: China Continuation study. J. Hematol. Oncol. 2017, 10, 1–13.
- Lonial, S.; Dimopoulos, M.; Palumbo, A.; White, D.; Grosicki, S.; Spicka, I.; Walter-Croneck, A.; Moreau, P.; Mateos, M.V.; Magen, H.; et al. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2015, 373, 621–631.
- Moreau, P.; Masszi, T.; Grzasko, N.; Bahlis, N.J.; Hansson, M.; Pour, L.; Sandhu, I.; Ganly, P.; Baker, B.W.; Jackson, S.R.; et al. Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 374, 1621–1634.
- Stewart, A.K.; Rajkumar, S.V.; Dimopoulos, M.A.; Masszi, T.; Spicka, I.; Oriol, A.; Hajek, R.; Rosinol, L.; Siegel, D.S.; Mihaylov, G.G.; et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N. Engl. J. Med. 2015, 372, 142–152.
- Kumar, S.K.; Jacobus, S.J.; Cohen, A.D.; Weiss, M.; Callander, N.; Singh, A.K.; Parker, T.L.; Menter, A.; Yang, X.; Parsons, B.; et al. Carfilzomib or Bortezomib in Combination with Lenalidomide and Dexamethasone for Patients with Newly Diagnosed Multiple Myeloma without Intention for Immediate Autologous Stem-Cell Transplantation (Endurance): A Multicentre, Open-Label, Phase 3, Randomised, Controlled Trial. Lancet. Oncol. 2020, 21, 1317–1330.
- Piedra, K.; Peterson, T.; Tan, C.; Orozco, J.; Hultcrantz, M.; Hassoun, H.; Mailankody, S.; Lesokhin, A.; Shah, U.; Lu, S.; et al. Comparison of Venous Thromboembolism Incidence in Newly Diagnosed Multiple Myeloma Patients Receiving Bortezomib, Lenalidomide, Dexamethasone (Rvd) or Carfilzomib, Lenalidomide, Dexamethasone (Krd) with Aspirin or Rivaroxaban Thromboprophylaxis. Br. J. Haematol. 2022, 196, 105–109.
- Rosenthal, A.; Luthi, J.; Belohlavek, M.; Kortum, K.M.; Mookadam, F.; Mayo, A.; Fonseca, R.; Bergsagel, P.L.; Reeder, C.B.; Mikhael, J.R.; et al. Carfilzomib and the Cardiorenal System in Myeloma: An Endothelial Effect? Blood Cancer, J. 2016, 6, e384.
- Sayar, Z.; Gates, C.; Bristogiannis, S.; Patel, A.; Ogunbiyi, M.O.; Tailor, A.; Yong, K.; Thomas, M. Safety and efficacy of apixaban as thromboprophylaxis in myeloma patients receiving chemotherapy: A prospective cohort study. Thromb. Res. 2022, 213, 27–29.
- Dimopoulos, M.A.; Oriol, A.; Nahi, H.; San-Miguel, J.; Bahlis, N.J.; Usmani, S.Z.; Rabin, N.; Orlowski, R.Z.; Komarnicki, M.; Suzuki, K.; et al. Moreau, and Pollux Investigators. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 375, 1319–1331.
- Sborov, D.; Baljevic, M.; Reeves, B.; Laubach, J.; Efebera, Y.; Rodriguez, C.; Costa, l.; Chari, A.; Silbermann, R.; Holstein, S.; et al. Daratumumab (Dara) Plus Lenalidomide, Bortezomib, and Dexamethasone (Rvd) in Newly Diagnosed Multiple Myeloma (Ndmm): Analysis of Vascular Thrombotic Events (Vtes) in the Griffin Study . Clin. Lymphoma Myeloma 2021, 21, 135–136.
- Wang, J.; Park, C.; Arroyo-Suarez, R. Venous thromboembolism in patients with multiple myeloma receiving daratumumab-based regimens: A post hoc analysis of phase 3 clinical trials. Leuk. Lymphoma 2021, 62, 2219–2226.
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008, 111, 4902–4907.
- Sanfilippo, K.M.; Carson, K.R.; Wang, T.; Luo, S.; Edwin, N.; Kuderer, N.; Keller, J.M.; Gage, B.F. Evaluation of the Khorana score for prediction of venous thromboembolism in patients with multiple myeloma. Res. Pr. Thromb. Haemost. 2022, 6, e12634.
- Barrett, A.; Quinn, J.; Lavin, M.; Thornton, P.; O’Donnell, J.; Murphy, P.; Glavey, S. Validation of Risk-Adapted Venous Thromboembolism Prediction in Multiple Myeloma Patients. J. Clin. Med. 2021, 10, 3536.
- Li, A.; Wu, Q.; Luo, S.; Warnick, G.S.; Zakai, N.A.; Libby, E.N.; Gage, B.F.; Garcia, D.A.; Lyman, G.H.; Sanfilippo, K.M. Derivation and Validation of a Risk Assessment Model for Immunomodulatory Drug–Associated Thrombosis Among Patients With Multiple Myeloma. J. Natl. Compr. Cancer Netw. 2019, 17, 840–847.
- Baker, H.A.; Brown, A.R.; Mahnken, J.D.; Shireman, T.I.; Webb, C.E.; Lipe, B.C. Application of Risk Factors for Venous Thromboembolism in Patients with Multiple Myeloma Starting Chemotherapy, a Real-World Evaluation. Cancer Med. 2019, 8, 455–462.
- Covut, F.; Ahmed, R.; Chawla, S.; Ricaurte, F.; Samaras, C.J.; Anwer, F.; Garcia, A.V.M.; Angelini, D.E.; Mazzoni, S.; Faiman, B.; et al. Validation of the IMPEDE VTE score for prediction of venous thromboembolism in multiple myeloma: A retrospective cohort study. Br. J. Haematol. 2021, 193, 1213–1219.
- Sanfilippo, K.M. Assessing the risk of venous thromboembolism in multiple myeloma. Thromb. Res. 2020, 191, S74–S78.
- Callander, N.S.; Baljevic, M.; Adekola, K.; Anderson, L.D.; Campagnaro, E.; Castillo, J.J.; Costello, C.; Devarakonda, S.; Elsedawy, N.; Faiman, M.; et al. Nccn Guidelines(R) Insights: Multiple Myeloma, Version 3.2022. J. Natl. Compr. Canc. Netw. 2022, 20, 8–19.
- Kahale, L.A.; Matar, C.F.; Tsolakian, I.; Hakoum, M.B.; Yosuico, V.E.; Terrenato, I.; Sperati, F.; Barba, M.; Hicks, L.K.; Schunemann, H.; et al. Antithrombotic Therapy for Ambulatory Patients with Multiple Myeloma Receiving Immunomodulatory Agents. Cochrane Database Syst. Rev. 2021, 9, CD014739.
- Al-Ani, F.; Bermejo, J.M.B.; Mateos, M.-V.; Louzada, M. Thromboprophylaxis in multiple myeloma patients treated with lenalidomide – A systematic review. Thromb. Res. 2016, 141, 84–90.
- Zoppellaro, G.; Veronese, N.; Granziera, S.; Gobbi, L.; Stubbs, B.; Cohen, A.T. Primary thromboembolic prevention in multiple myeloma patients: An exploratory meta-analysis on aspirin use. Semin. Hematol. 2018, 55, 182–184.
- Sanfilippo, K.M.; Luo, S.; Carson, K.R.; Cage, B.F. Aspirin May Be Inadequate Thromboprophylaxis in Multiple Myeloma. Blood 2017, 130, 3419.
- Swan, D.; Rocci, A.; Bradbury, C.; Thachil, J. Venous Thromboembolism in Multiple Myeloma - Choice of Prophylaxis, Role of Direct Oral Anticoagulants and Special Considerations. Br. J. Haematol. 2018, 183, 538–556.
- Lim, M.S.; Enjeti, A. Safety of anticoagulation in the treatment of venous thromboembolism in patients with haematological malignancies and thrombocytopenia: Report of 5 cases and literature review. Crit. Rev. Oncol. 2016, 105, 92–99.
- Khanal, N.; Bociek, R.G.; Chen, B.; Vose, J.M.; Armitage, J.O.; Bierman, P.J.; Maness, L.J.; Lunning, M.A.; Gundabolu, K.; Bhatt, V.R. Venous thromboembolism in patients with hematologic malignancy and thrombocytopenia. Am. J. Hematol. 2016, 91, E468–E472.
- Napolitano, M.; Saccullo, G.; Marietta, M.; Carpenedo, M.; Castaman, G.; Cerchiara, E.; Chistolini, A.; Contino, L.; De Stefano, V.; Falanga, A.; et al. Platelet cut-off for anticoagulant therapy in thrombocytopenic patients with blood cancer and venous thromboembolism: An expert consensus. Blood Transfus. 2019, 17, 171–180.
- Key, N.S.; Khorana, A.A.; Kuderer, N.M.; Bohlke, K.; Lee, A.Y.; Arcelus, J.I.; Wong, S.L.; Balaban, E.P.; Flowers, C.R.; Francis, C.W.; et al. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2020, 38, 496–520.
- Khorana, A.A.; Soff, G.A.; Kakkar, A.K.; Vadhan-Raj, S.; Riess, H.; Wun, T.; Streiff, M.B.; Garcia, D.A.; Liebman, H.A.; Belani, C.P.; et al. Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer. New Engl. J. Med. 2019, 380, 720–728.
- Raskob, G.E.; van Es, N.; Verhamme, P.; Carrier, M.; Di Nisio, M.; Garcia, D.; Grosso, M.A.; Kakkar, A.K.; Kovacs, M.J.; Mercuri, M.F.; et al. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. New Engl. J. Med. 2018, 378, 615–624.
- Young, A.M.; Marshall, A.; Thirlwall, J.; Chapman, O.; Lokare, A.; Hill, C.; Hale, D.; Dunn, J.A.; Lyman, G.H.; Hutchinson, C.; et al. Comparison of an Oral Factor Xa Inhibitor With Low Molecular Weight Heparin in Patients With Cancer With Venous Thromboembolism: Results of a Randomized Trial (SELECT-D). J. Clin. Oncol. 2018, 36, 2017–2023.
- Agnelli, G. Direct Oral Anticoagulants for Thromboprophylaxis in Ambulatory Patients with Cancer. New Engl. J. Med. 2019, 380, 781–783.
- Moik, F.; Posch, F.; Zielinski, C.; Pabinger, I.; Ay, C. Direct oral anticoagulants compared to low-molecular-weight heparin for the treatment of cancer-associated thrombosis: Updated systematic review and meta-analysis of randomized controlled trials. Res. Pr. Thromb. Haemost. 2020, 4, 550–561.




