Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yasuhito Shimada | -- | 1468 | 2022-12-22 13:37:18 | | | |
| 2 | Camila Xu | + 1 word(s) | 1469 | 2022-12-22 14:39:25 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ichii, S.; Matsuoka, I.; Okazaki, F.; Shimada, Y. Zebrafish Models for Skeletal Muscle Senescence. Encyclopedia. Available online: https://encyclopedia.pub/entry/39098 (accessed on 03 March 2026).
Ichii S, Matsuoka I, Okazaki F, Shimada Y. Zebrafish Models for Skeletal Muscle Senescence. Encyclopedia. Available at: https://encyclopedia.pub/entry/39098. Accessed March 03, 2026.
Ichii, Shogo, Izumi Matsuoka, Fumiyoshi Okazaki, Yasuhito Shimada. "Zebrafish Models for Skeletal Muscle Senescence" Encyclopedia, https://encyclopedia.pub/entry/39098 (accessed March 03, 2026).
Ichii, S., Matsuoka, I., Okazaki, F., & Shimada, Y. (2022, December 22). Zebrafish Models for Skeletal Muscle Senescence. In Encyclopedia. https://encyclopedia.pub/entry/39098
Ichii, Shogo, et al. "Zebrafish Models for Skeletal Muscle Senescence." Encyclopedia. Web. 22 December, 2022.
Copy Citation
Aging and sarcopenic changes in skeletal muscles not only reduce locomotor activities in elderly people but also increase the chance of trauma, such as bone fractures, and the incidence of other diseases, such as metabolic syndrome, due to reduced physical activity.
sarcopenia
drug screening
animal models
1. Introduction
In recent years, the average global life expectancy has been greater than 70 years. In fact, many developed countries have an average life expectancy of 80 years. For example, the average life expectancy at birth is 78.5, 81.4, 81.7, 82.5, 83.1, 82.2, and 84.3 years in the US, UK, Germany, France, Italy, Canada, and Japan, respectively (World Health Organization, 2020). To lead a prosperous and independent life and enjoy sports and travel after retirement, activities of daily living (ADL) and daily self-care activities, such as mobility and eating, must be maintained. With aging, the ADLs trend downward, which serves as one of the reasons for the decline in muscle mass. The skeletal muscle, the largest organ in the human body, plays an indispensable role in maintaining health as it functions as a metabolic organ and is responsible for movement and physical activity. According to epidemiological studies, people who maintain skeletal muscle mass are less likely to become sick and tend to live longer [1][2]. Therefore, the maintenance of skeletal muscles is key to a super-aging society.
Elderly people are at an increased risk for the following two skeletal muscle diseases: disuse muscular atrophy and sarcopenia. Disuse muscle atrophy is caused by prolonged rest due to severe injury, surgery, or hospitalization [3]. In atrophic muscles, the amount of DNA does not change; however, the amount of RNA is markedly reduced, suggesting that the amount of protein is decreased in atrophied muscles [4]. One of the decreases in RNA synthesis is caused by the degradation of signaling molecules in the IGF-1-mediated protein synthesis pathway [5]. Another muscle-related disease in the elderly population, sarcopenia, is defined as a loss of muscle mass [6]; however, recent definitions of sarcopenia include loss of muscle strength and muscle function [7][8][9][10][11][12][13]. Sarcopenia contributes to frailty, resulting in reduced ADL and quality of life [7][14]. Sarcopenia is classified as either primary or secondary. Primary sarcopenia is caused by muscle mass loss due to aging, while secondary sarcopenia is caused by muscle mass loss due to activity, disease, and nutrition [7]. Strictly speaking, disuse muscular atrophy differs from sarcopenia as the number of cells does not decrease, despite the simultaneous existence of the two diseases at times. Primary and/or secondary sarcopenia causes a decline in skeletal muscle performance by decreasing the number of muscle fibers and atrophy of each muscle fiber. The occurrence of falls increases by approximately three-fold in patients with sarcopenia compared with that in the same-aged population without sarcopenia [15]. Fall-induced bone fractures cause damage to skeletal muscles and cause patients to be bedridden, consequently resulting in a loss of exercise opportunities. Owing to the reduced regeneration capacity of elderly people, a single fall can accelerate sarcopenia, causing them to be bedridden. Disuse muscular atrophy often begins during hospitalization and lasts until bone fracture recovery is achieved. Disuse muscular atrophy might also be accompanied by primary sarcopenia.
Resistance training, such as squats and push-ups, is effective at preventing sarcopenia progression. However, elderly people are at risk of falling during this training. Further, the performance of a workout is difficult when bedridden. Thus, pharmacological or nutritional approaches for prevention and further recovery from sarcopenia are attractive strategies for elderly people with training difficulties.
2. Zebrafish Models for Skeletal Muscle Senescence
Rodent organs are believed to mimic human organs, as they possess a high degree of genetic homology with human genes. Therefore, rodents are typically used as animal models for drug testing. However, remarkable time, labor, space, and money are required for studies with rodents. In recent years, from the viewpoint of animal welfare, there has been an ongoing trend to reduce the number of mammals used in experiments. An ideal alternative to rodents, the zebrafish has emerged as an animal model for several types of human diseases, including skeletal muscle atrophy [16][17], aging [18], and sarcopenia [19][20], owing to several advantages (Figure 1). The skeletal muscle constitutes a large part of the zebrafish trunk and has a high degree of similarity to human muscle, both molecularly and histologically. The skeletal muscle also consists of slow muscles located directly below the body surface and fast muscles around the vertebrae [21]. Zebrafish have a set of orthologs for human MRFs that are involved in skeletal muscle myogenesis [22]. Myod and myf5 are expressed in the early phase of skeletal muscle development, followed by myogenic factor 6 (myf6). Interestingly, myod, myf5, and myf6 expression depend on the site of the muscle tissue [23], implying that even skeletal muscle tissue might have subclassifications in zebrafish.
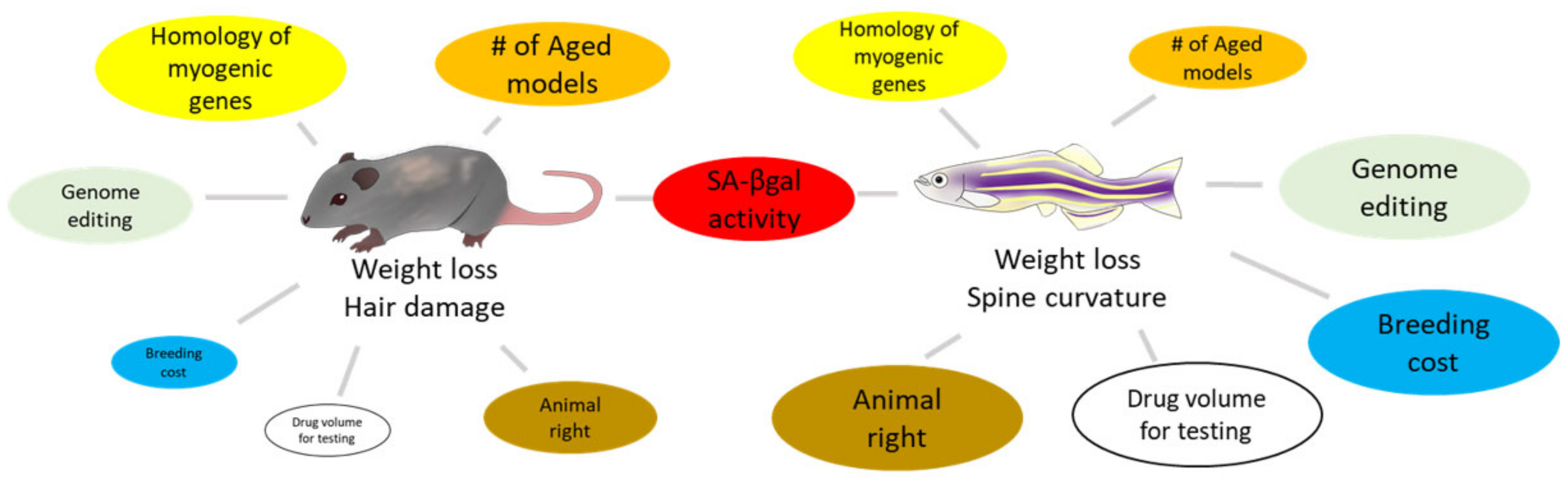
Figure 1. Comparison between rodents and zebrafish for skeletal muscle senescence. The size of each circle visualizes its advantage as a model animal.
To induce muscle atrophy or administer drugs to rodents, intravascular or oral gavage, which requires certain techniques, must be employed. However, zebrafish can absorb chemicals from their skin and gills by simply adding them to general fish water (exposure test). Protocols have also been established for oral and intraperitoneal administration in zebrafish [24][25][26].
2.1. Aged Model
Aging is the main driver of skeletal muscle atrophy and sarcopenia in fish species, like in rodents [19]. However, the lifespan of mice and zebrafish is almost identical (2–3 years in laboratory conditions) [27]. As a result, there is little advantage to using wild-type zebrafish as a model of skeletal muscle aging. Owing to the ease of mutagenesis in zebrafish, various strains have been developed in recent years using genetic manipulation (using tol2 transposase), knockdown (using antisense oligonucleotides), and knockout (using CRISPR/Cas9) techniques. For example, Kishi et al. performed an SA-β-gal activity-based mutant screen and identified 11 zebrafish mutants with high SA-β-gal activity. Of these, heterozygous mutations in telomeric repeat binding factor 2 (terf2) or spinster homolog 1 (spns1) were found to result in a shorter lifespan [28]. Further, the spns1 mutation led to an increase in lipofuscin in the skeletal muscle in the adult stage, indicating that the mutant can be used as a model for skeletal muscle aging. Previously, Da Rosa et al. reported that growth hormone (GH) overexpression accelerates spinal curvature in adult zebrafish by reducing myog and myod expression in surrounding muscles [29]. Their findings highlight the possibility of accelerated skeletal muscle aging in this transgenic fish. To the researchers' knowledge, there are no reports of the use of these aged models in studies on skeletal muscle aging; however, various studies are expected in the near future.
As described above, compared to rodent models, the use of zebrafish models certainly shortens the duration of experiments and reduces the burden of animal husbandry, though it is still labor-intensive for research on aging. Since the CRISPR/Cas9 genome editing technology was reported in 2013 [30] and used in zebrafish [31], several disease models have been reported to have been built using CRISPR-mediated targeted mutagenesis (knockout). For example, the lmna gene, whose mutation is known to accelerate aging in rodents [32], is also present in zebrafish. According to a recently published report, the deletion of five base pairs (5bpΔ) in the second exon of the lmna gene by CRISPR/Cas9 genome editing resulted in skeletal muscle damage and impaired swimming [33]. The researchers used this as a model of laminopathy, such as Emery–Dreifuss muscular dystrophy; however, it also has potential as a model of skeletal muscle senescence.
2.2. Dexamethasone Model
DEX has been used to induce skeletal muscle atrophy in zebrafish [34], like in rodents and cultured cells. Zebrafish can absorb small molecules through their skin and gills, which enables the administration of DEX by immersion in general fish water for the induction of muscle atrophy. Although limited studies have been conducted using DEX-induced zebrafish models (less than 10 papers in Web of Science), some promising studies have been reported. For example, Ryu et al. revealed that dietary supplementation with maca (Lepidium meyenii) induced preventive effects in DEX-induced muscular atrophy in zebrafish, with an increase in the distance traveled and speed of chasing food in the aquarium [34]. Similar to how it occurs in rodents, the mechanism of DEX-induced muscular atrophy in zebrafish is also thought to involve an increase in proteolysis due to the overexpression of atrogin-1 and murf1 ubiquitin ligase (as a part of aging-induced muscular atrophy); however, this notion has yet to be proven.
2.3. Chronic Alcohol Model
Prolonged and high-dose alcohol consumption induces muscle atrophy in mammals. The mechanism by which alcohol induces skeletal muscle atrophy is not fully understood; however, alcohol is known to increase the expression of ubiquitin ligase, which contributes to muscle atrophy in elderly people [35][36]. Similar to that in mammals, chronic ethanol exposure (0.5% in the general fish water for 8 weeks) can induce muscular atrophy in zebrafish [37]. Although this model has not been used for drug testing, it could serve as a useful screen for muscle atrophy in response to alcoholism in the future.
References
- Heitmann, B.L.; Frederiksen, P. Thigh circumference and risk of heart disease and premature death: Prospective cohort study. BMJ (Clin. Res. Ed.) 2009, 339, b3292.
- Srikanthan, P.; Karlamangla, A.S. Muscle mass index as a predictor of longevity in older adults. Am. J. Med. 2014, 127, 547–553.
- Bodine, S.C. Disuse-induced muscle wasting. Int. J. Biochem. Cell Biol. 2013, 45, 2200–2208.
- Steffen, J.M.; Musacchia, X.J. Effect of hypokinesia and hypodynamia on protein, RNA, and DNA in rat hindlimb muscles. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1984, 247, R728–R732.
- Nakao, R.; Hirasaka, K.; Goto, J.; Ishidoh, K.; Yamada, C.; Ohno, A.; Okumura, Y.; Nonaka, I.; Yasutomo, K.; Baldwin, K.M.; et al. Ubiquitin ligase Cbl-b is a negative regulator for insulin-like growth factor 1 signaling during muscle atrophy caused by unloading. Mol. Cell. Biol. 2009, 29, 4798–4811.
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of Sarcopenia among the Elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763.
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423.
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31.
- Muscaritoli, M.; Anker, S.D.; Argilés, J.; Aversa, Z.; Bauer, J.M.; Biolo, G.; Boirie, Y.; Bosaeus, I.; Cederholm, T.; Costelli, P.; et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin. Nutr. 2010, 29, 154–159.
- Morley, J.E.; Abbatecola, A.M.; Argiles, J.M.; Baracos, V.; Bauer, J.; Bhasin, S.; Cederholm, T.; Coats, A.J.S.; Cummings, S.R.; Evans, W.J.; et al. Sarcopenia with limited mobility: An international consensus. J. Am. Med. Dir. Assoc. 2011, 12, 403–409.
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256.
- Chen, L.-K.; Liu, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Bahyah, K.S.; Chou, M.-Y.; Chen, L.-Y.; Hsu, P.-S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus Report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101.
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69, 547–558.
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M157.
- Landi, F.; Liperoti, R.; Russo, A.; Giovannini, S.; Tosato, M.; Capoluongo, E.; Bernabei, R.; Onder, G. Sarcopenia as a risk factor for falls in elderly individuals: Results from the ilSIRENTE study. Clin. Nutr. 2012, 31, 652–658.
- Hayes, A.J.; Reynolds, S.; Nowell, M.A.; Meakin, L.B.; Habicher, J.; Ledin, J.; Bashford, A.; Caterson, B.; Hammond, C.L. Spinal deformity in aged zebrafish is accompanied by degenerative changes to their vertebrae that resemble osteoarthritis. PLoS ONE 2013, 8, e75787.
- Guyon, J.R.; Steffen, L.S.; Howell, M.H.; Pusack, T.J.; Lawrence, C.; Kunkel, L.M. Modeling human muscle disease in zebrafish. Muscular Dystrophies Mol. Basis Ther. Strateg. 2007, 1772, 205–215.
- Gerhard, G.S. Small laboratory fish as models for aging research. Ageing Res. Rev. 2007, 6, 64–72.
- Daya, A.; Donaka, R.; Karasik, D. Zebrafish models of sarcopenia. Dis. Model. Mech. 2020, 13, dmm042689.
- Christian, C.J.; Benian, G.M. Animal models of sarcopenia. Aging Cell 2020, 19, e13223.
- Luna, V.M.; Daikoku, E.; Ono, F. “Slow” skeletal muscles across vertebrate species. Cell Biosci. 2015, 5, 1–6.
- Rescan, P.Y. Regulation and functions of myogenic regulatory factors in lower vertebrates. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2001, 130, 1–12.
- Hinits, Y.; Osborn, D.P.S.; Hughes, S.M. Differential requirements for myogenic regulatory factors distinguish medial and lateral somitic, cranial and fin muscle fibre populations. Development 2009, 136, 403–414.
- Zang, L.; Morikane, D.; Shimada, Y.; Tanaka, T.; Nishimura, N. A novel protocol for the oral administration of test chemicals to adult zebrafish. Zebrafish 2011, 8, 203–210.
- Collymore, C.; Rasmussen, S.; Tolwani, R.J. Gavaging Adult Zebrafish. J. Vis. Exp. 2013, 78, e50691.
- Kinkel, M.D.; Eames, S.C.; Philipson, L.H.; Prince, V.E. Intraperitoneal injection into adult zebrafish. J. Vis. Exp. 2010, 42, e2126.
- Gerhard, G.S.; Kauffman, E.J.; Wang, X.J.; Stewart, R.; Moore, J.L.; Kasales, C.J.; Demidenko, E.; Cheng, K.C. Life spans and senescent phenotypes in two strains of Zebrafish (Danio rerio). Exp. Gerontol. 2002, 37, 1055–1068.
- Kishi, S.; Bayliss, P.E.; Uchiyama, J.; Koshimizu, E.; Qi, J.; Nanjappa, P.; Imamura, S.; Islam, A.; Neuberg, D.; Amsterdam, A.; et al. The Identification of Zebrafish Mutants Showing Alterations in Senescence-Associated Biomarkers. PLoS Genet. 2008, 4, e1000152.
- da Rosa, C.E.; Kuradomi, R.Y.; Almeida, D.V.; Lannes, C.F.C.; Figueiredo, M.D.; Dytz, A.G.; Fonseca, D.B.; Marins, L.F. GH overexpression modifies muscle expression of anti-oxidant enzymes and increases spinal curvature of old zebrafish. Exp. Gerontol. 2010, 45, 449–456.
- Adiego-Perez, B.; Randazzo, P.; Daran, J.M.; Verwaal, R.; Roubos, J.A.; Daran-Lapujade, P.; van der Oost, J. Multiplex genome editing of microorganisms using CRISPR-Cas. FEMS Microbiol. Lett. 2019, 366, fnz086.
- Hwang, W.Y.; Fu, Y.F.; Reyon, D.; Maeder, M.L.; Tsai, S.Q.; Sander, J.D.; Peterson, R.T.; Yeh, J.R.J.; Joung, J.K. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 2013, 31, 227–229.
- Mounkes, L.C.; Kozlov, S.; Hernandez, L.; Sullivan, T.; Stewart, C.L. A progeroid syndrome in mice is caused by defects in A-type lamins. Nature 2003, 423, 298–301.
- Nicolas, H.A.; Hua, K.; Quigley, H.; Ivare, J.; Tesson, F.; Akimenko, M.A. A CRISPR/Cas9 zebrafish lamin A/C mutant model of muscular laminopathy. Dev. Dyn. 2022, 251, 645–661.
- Ryu, B.; Je, J.-G.; Jeon, Y.-J.; Yang, H.-W. Zebrafish Model for Studying Dexamethasone-Induced Muscle Atrophy and Preventive Effect of Maca (Lepidium meyenii). Cells 2021, 10, 2879.
- Gumucio, J.P.; Mendias, C.L. Atrogin-1, MuRF-1, and sarcopenia. Endocrine 2013, 43, 12–21.
- Vary, T.C.; Frost, R.A.; Lang, C.H. Acute alcohol intoxication increases atrogin-1 and MuRF1 mRNA without increasing proteolysis in skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1777–R1789.
- Khayrullin, A.; Smith, L.; Mistry, D.; Dukes, A.; Pan, Y.A.; Hamrick, M.W. Chronic alcohol exposure induces muscle atrophy (myopathy) in zebrafish and alters the expression of microRNAs targeting the Notch pathway in skeletal muscle. Biochem. Biophys. Res. Commun. 2016, 479, 590–595.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
972
Revisions:
2 times
(View History)
Update Date:
22 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





