Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Veronica Manescu (Paltanea) | -- | 8127 | 2022-12-22 13:12:58 | | | |
| 2 | Jason Zhu | -4 word(s) | 8123 | 2022-12-23 04:15:42 | | | | |
| 3 | Veronica Manescu (Paltanea) | + 48 word(s) | 8171 | 2023-01-04 20:28:11 | | | | |
| 4 | Veronica Manescu (Paltanea) | Meta information modification | 8171 | 2023-01-04 20:29:15 | | | | |
| 5 | Jason Zhu | + 2 word(s) | 8173 | 2023-01-05 03:14:12 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Iulian, A.; (Paltanea), V.M.; Paltanea, G.; Aurora, A.; Nemoianu, I.V.; Petrescu, M.I.; Dura, H.; Bodog, A. Additive Manufactured Magnesium-Based Scaffolds for Tissue Engineering. Encyclopedia. Available online: https://encyclopedia.pub/entry/39096 (accessed on 07 February 2026).
Iulian A, (Paltanea) VM, Paltanea G, Aurora A, Nemoianu IV, Petrescu MI, et al. Additive Manufactured Magnesium-Based Scaffolds for Tissue Engineering. Encyclopedia. Available at: https://encyclopedia.pub/entry/39096. Accessed February 07, 2026.
Iulian, Antoniac, Veronica Manescu (Paltanea), Gheorghe Paltanea, Antoniac Aurora, Iosif Vasile Nemoianu, Mircea Ionut Petrescu, Horatiu Dura, Alin Bodog. "Additive Manufactured Magnesium-Based Scaffolds for Tissue Engineering" Encyclopedia, https://encyclopedia.pub/entry/39096 (accessed February 07, 2026).
Iulian, A., (Paltanea), V.M., Paltanea, G., Aurora, A., Nemoianu, I.V., Petrescu, M.I., Dura, H., & Bodog, A. (2022, December 22). Additive Manufactured Magnesium-Based Scaffolds for Tissue Engineering. In Encyclopedia. https://encyclopedia.pub/entry/39096
Iulian, Antoniac, et al. "Additive Manufactured Magnesium-Based Scaffolds for Tissue Engineering." Encyclopedia. Web. 22 December, 2022.
Copy Citation
Additive manufacturing (AM) is an important technology that led to a high evolution in the manufacture of personalized implants adapted to the anatomical requirements of patients. Due to a worldwide graft shortage, synthetic scaffolds must be developed. Regarding this aspect, biodegradable materials such as magnesium and its alloys are a possible solution because the second surgery for implant removal is eliminated. Magnesium (Mg) exhibits mechanical properties, which are similar to human bone, biodegradability in human fluids, high biocompatibility, and increased ability to stimulate new bone formation.
Mg-based scaffolds
tissue engineering
additive manufacturing
bone defect treatment
regenerative medicine
bioresorbable implants
1. Magnesium-Based Scaffold Production by Additive Manufacturing
Additive manufacturing (AM) is a suitable method to produce scaffolds, which have complicated geometrical shapes and mimic the ECM structures very well. This technology involves a layer-by-layer manufacturing process using computer-aided design (CAD) structures [1][2]. The AM methods are classified based on the feedstock material type (wire or powder) and the heat sources (arc, laser, or electron beam). According to American Society for Testing and Materials (ASTM) Standard F2792, there are two important types of AM technologies: directed energy deposition (DED) and powder bed fusion (PBF) [3][4].
PBF is one of the most used methods for metallic scaffold manufacture. Selective laser melting (SLM) and electron-beam melting (EBM) are the main thermal energy sources, which melt and fuse the metal powders in layers placed on a powder bed to generate a solid pattern [5][6]. Firstly, one layer of metal powder is put on a building platform, and then it is melted to a computer-designed shape [7]. The building platform is placed at a pre-defined distance, and a piston is involved in spreading and melting the next layer of powder on the previous layers [8]. In this way, layer by layer, the desired shape is constructed [9]. The thickness of one individual layer is between 20 and 100 μm. Rapid cooling of the build chamber is ensured by the circulation of argon or nitrogen, which creates conditions for a high-purity material of the scaffold by minimizing the oxygen and hydrogen in the chamber atmosphere [6][7].
Conventional manufacturing technologies such as powder metallurgy, foaming, casting, sintering electrodeposition, or chemical vapor deposition cannot provide a scaffold structure with a uniform shape and homogenous placement of the pores. AM gives the advantage of personalized implant production, made after the patient’s anatomy and with a regular pore shape and dimensions. This way, high control of pore architecture is directly linked to good porosity, permeability, mechanical strength, and stiffness [10][11]. Interconnected porous Mg scaffolds are obtained through AM with personalized shapes and internal architecture [10][12]. Ng et al. [13] have reported the successful melting of a single Mg layer. Recently, many studies have investigated scaffold manufacture with the SLM process, selective laser sintering (SLS), and binder jetting. It can be concluded that AM is the best way to produce metallic scaffolds with desired geometrical shape, controlled pore architecture, good mechanical properties, and high biocompatibility.
The PBF process provides control over material distribution and phase composition. Marangoni convection occurs due to high-temperature gradients in melting points, leading to a homogenous alloying material. It is well known that the reduced precipitated phase of the amalgam matrix solution could decrease the deterioration process by diminishing the electrochemical cell coupling between the return leg and the matrix. Magnesium exhibits a heat capacity of 650 °C and a boiling point of 1091 °C, making laser shaping a difficult procedure. During this step, Mg is burned off and oxidized, at the same time losing its binding efficiency. An important disadvantage of AM consists of the surface quality, and post-processing steps must be applied. In addition, custom alloying of materials is impossible through this technology, and almost all of the AM procedures are mainly linked to a given limit of the fabricated sample sizes [14].
1.1. Selective Laser Melting
SLM is included in PBF class methods. Its principle consists of melting and fusing the pre-spread powders layer by layer in a selected place based on a strong laser source and a CAD model, as mentioned above. The employed system comprises a laser, a construction station, a powder particle supply system that is automatized, dedicated software, and some important accessories [15]. A laser diffraction device made of a galvanometer and a flatter sector lens gives direction to the laser beam on the building table. SLM selectively melts the powder particles layer by layer to finish the printing of the desired component, which has 99.9% relative density [16]. The entire process is controlled through a dedicated program, which considers the powder input, the layering process, the scanning, the cooling and the heating steps, and the component construction. The main SLM phases can be synthesized as follows: firstly, a CAD model is created, and then it is divided into layers with a thickness between 20 and 100 μm; a substrate is leveled and secured on the build platform to prevent surface damage, a protective layer of inert gas is supplied. After that, a coating with powder material, whose thickness is equal to that of the thin designed layer, is applied. Scanning and handling of powder beds are carried out to build the part layer by layer. In the SLM process, a contour outline of the part geometry is generated, and the powder is melted inside this outline [17].
The SLM technique is characterized by inhibiting grain growth through rapid solidification that is obtained by involving fast heating and cooling cycles higher than 105 K/s [18][19]. The segregation of the composition is reduced, having, as a result, a homogeneous microstructural architecture of the scaffold. Through this method are produced implants with high density, good mechanical properties, and degradation resistance [20]. During SLM, there are a high number of heating and cooling cycles that may have an unwanted effect consisting of a small heat-affected zone (HAZ), which grows around a melting pool. This phenomenon changes the material’s chemical composition and can have an important effect on physical properties.
Zumdick et al. [21] have analyzed the properties of Mg-4Y-3RE-0.5Zr (WE43) magnesium-based alloys produced through the SLM technique. A very fine grain structure with an average grain size of around 1 μm was observed. The samples had an ultimate tensile strength of 308 MPa and 12% elongation to failure. Bar et al. [22] have given evidence of an improved biodegradation property in the case of SLM-manufactured samples by comparing these with those made through the casting method. Li et al. [23] have fabricated topologically ordered porous scaffolds made from WE43 alloy with a diamond unit cell through the SLM process. The implant strut size was about 400 μm, the pore diameter was equal to 600 μm, and the material porosity was 67%. The mechanical properties of porous Mg-based alloys were in the range of those for the trabecular bone, with a Young’s modulus between 0.5 and 20 GPa. AM-produced scaffolds exhibited a biodegradation behavior characterized by 20% volume loss after 4 weeks. These scaffolds had a low toxicity level. The sample fatigue resistance of the AM-manufactured WE43 scaffold was reduced to about 0.2 σy [24]. It was noticed that the optimization of the topological design and laser processing parameters have a great influence on the scaffold microstructure.
Chen et al. [20] have made a binary Mg-Zn alloy through the SLM technique and found a homogenous grain structure with an average size of 15 μm. The precipitation of the MgZn phase and the rapid solidification process inhibited grain growth. Wei et al. [25] have studied the influence of Zn content in the Mg-Zn binary compound. They have found that 1 wt% of Zn has a beneficial effect on the mechanical properties, which were in the range of those measured for as-cast samples. The corrosion resistance of the binary Mg-Zn alloys is improved by adding aluminum (Al). In [25], the manufacture of parts from ternary Mg-6Al-1Zn alloy (AZ61), using the SLM process, was analyzed. At a laser input energy of 80 W, an equiaxed grain structure and a maximum microhardness of 93 HV were reported. Another study [26] investigated the effect of yttrium (Y) addition on the degradation behavior of magnesium and an increase in this parameter was found. Another modality to improve the Mg-Zn binary alloy corrosion resistance is hydroxyapatite (HAp) incorporation. Shuai et al. [27] have made Mg-3Zn/xHAp composite materials using the SLM method. The rapid solidification process prevented the HAp particles’ agglomeration, and a homogenous dispersion process was observed. When the hydroxyapatite percent increased, a structure with finer grains was obtained, leading to an increased corrosion resistance due to apatite coating. The material hardness becomes higher due to fine grain structure and second-phase strengthening.
Zhang et al. [28] have investigated the addition of Zr to binary Mg-Zn alloys, and they have found that by increasing the Zr content, the grain size is reduced, and the material exhibits a low degradation rate. Additionally, by modifying the laser intensity, a very good surface quality was noticed. By adding dysprosium (Dy), ternary alloys of Mg-Zn-Dy are obtained. They are characterized by low degradation and hydrogen evolution rate, small grain size, and homogenous microstructure [18]. The selected studies evidence that the SLM procedure leads to Mg-based scaffolds with high-quality microstructure, good mechanical properties, and high biodegradation performance.
1.2. Binder Jetting
This method is a two-step process. During the first stage, a metallic powder layer is deposited on the powder bed. The particles are bonded in a specified region through a chemical reaction or adhesion process [19]. In the case of chemical reactions, a solution, which is dropped on the specific bonding points, reacts with the powder particles [29][30]. For the adhesion process, a solid or liquid polymer is used to glue the particles in the desired shape layer [31][32]. Some technologies require a curing process to increase the green compound’s mechanical strength. After this step is finished, a self-supported structure results and must be extracted from the powder bed. The second step consists of a debinding process to remove the binder and unbonded powder and a post-treatment process, such as infiltration with different materials [33], sintering [34], and hot isostatic pressing [35].
Farag and Yun [36] have investigated the effect of gelatin addition, in an amount lower than 6 wt%, on the fabrication of magnesium phosphate-based scaffolds (MgP). The formation of a dense strut and an enhancement of the mechanical properties were observed. Furthermore, the MgP/gelatin scaffolds exhibit a hydrophilic behavior and a very good cell affinity. Meininger et al. [37] have manufactured, using the binder jetting method, strontium (Sr)-substituted Mg3(PO4)2 scaffolds. Good mechanical properties such as compression (36.7 MPa), bending (24.2 MPa), and tension (10.7 MPa) strength were experimentally determined. Water was used as a binder, and sintering and hardening processes were applied as post-treatments. In vitro tests showed a reduced release of magnesium ions and improved corrosion resistance. The microstructural analysis gives evidence of an interconnected topology characterized by a 20 μm average pore size. Salehi et al. [38] have shown how the capillarity-driven bridging phenomenon can be used for assembling powder particles in 3D structures. The method is based on magnesium oxide (MgO) film conversion on the outermost layer of Mg powder into an interparticle bridge. In this way, the capillary-mediated assembly of particles makes the use of the polymeric binder unnecessary. A scaffold with a constant composition was obtained.
The main drawbacks of the above-described method are sacrificial binder use and additional processes necessary to remove the binder. As the powder particles are glued together, the resulting green compounds exhibit low mechanical properties. Post-processing operations are mandatory for scaffold densification.
1.3. Selective Laser Sintering
This method is based on a bed of compacted powder particles, which are heated at a temperature close to the melting transition point. The powder particles are bound together with the help of a laser beam that is traced over the bed surface. The laser draws different patterns onto the powder surface during the printing process. When the first layer is synthesized, the incorporated platform is lowered by 100–200 μm, and new fresh powder particles are spread using a roller. After each powder layer is finished, new layers must be heated over their crystallization temperature to be completely melted to ensure an adequate bonding between particles and to avoid the cooling of the previous layers. In this way, the deformation of the sintered layer is hindered. The 3D objects are printed layer by layer, and they can be collected from the powder bed. Local thermal sintering of the particles is given by a high-power carbon dioxide (CO2) laser that melts the powder after scanning it in a given way. The fabrication chamber is sealed, and its temperature is kept under the melting point value [39].
Tsai et al. [40] have manufactured and analyzed 3D composite scaffolds made from magnesium–calcium silicate/poly-ε-caprolactone (Mg-CS/PCL). The composite powder of Mg-CS was incorporated into PCL, and the scaffolds were made based on the selective laser sintering method. The developed implants exhibited a high porosity grade and an interconnected design macropore structure. Good hydrophilic properties and degradation rates were noticed. In vitro analysis has shown high biocompatibility of the scaffolds, which enhanced human mesenchymal stem cells’ multiplication and adhesion with the help of released Mg2+ ions.
Selective laser sintering-manufactured scaffolds have a low value of density and poor mechanical properties because the partial melting process of the powder particles is linked to pore formation and struts inside the metallic material.
1.4. Indirect Additive Manufacturing
Magnesium scaffolds can also be manufactured through infiltration technology. A polymeric template, whose model is designed in CAD software, is made using the AM method. The template is infiltrated with a NaCl paste, and the polymer is removed later by heating at a specific temperature. The result consists of a negative NaCl template formation, in which liquid Mg is cast using applied pressure. Finally, the NaCl is dissolved, and only the Mg structure remains [41].
Based on this technology, Nguyen et al. [11] have developed an indirect solid free-form fabrication (SFF) process to manufacture topologically ordered Mg structures. It was shown, using different characterization devices, that the developed Mg-based structures were made with a high accuracy grade. Minor differences between CAD models in the interval of 2.5% and 8.33% were determined. A maximum of 6.1% reduction in the porosity of Mg implants was detected in the case of some structures by comparing them with the initial design. An average increase of 70% in surface area was seen, and it was concluded that the technology is reliable, simple, and safe for implant manufacture.
Lin et al. [42] have fabricated metallic hybrid composites by printing 3D CoCr scaffolds, in which they have infiltrated Mg-3Al-1Zn (AZ31) magnesium alloy based on a pressure-less infiltration technique. The degradation behavior of the scaffolds was investigated through the immersion method in Hanks’ solution. It was concluded that the degradation rate of the scaffolds is higher than in the case of AZ31 alloy due to galvanic corrosion effects. It was noticed that this phenomenon is strongly dependent on the surface area and composite interface. A reduced value for stiffness and strength is observed regarding the mechanical properties. The authors have concluded that parameters such as scanning speed and part geometries are essential and can significantly influence indirect AM process failure.
In [43] is reported a novel technique, which combines the salt-leaching method with AM. They have successfully modified the solvent and surfactant composition, so the salt-based paste is engineered from a rheological point of view and directly printed into grid-like structures. This way, porous scaffolds with controlled pore size and ordered geometry were made. It was concluded that the implant’s mechanical properties are a function of the material porosity and can be modified until a specific performance is obtained.
The infiltrating technology eliminates the use of Mg powder, and the explosion danger of the previously discussed methods is hindered. Unfortunately, using indirect additive manufacturing, the geometrical characteristics of struts and pores are related only to the macroscale, which in some cases is a drawback in medical applications, where an open porous structure is necessary.
2. Specific Properties of the Magnesium-Based Scaffolds
The design geometry and the scaffold microstructure have an important influence on the biological and mechanical properties of the magnesium-based scaffolds. Some important aspects, such as biodegradation, densification, mechanical properties, and biocompatibility, should be considered.
2.1. Biodegradation
The scaffold biodegradation depends on the design, material, and manufacturing process [44][45]. In [46], the corrosion behavior of pure Mg fabricated through the SLM procedure was investigated. It was concluded that the process parameters significantly influence the material porosity and corrosion rate. This last parameter was computed as a function of the material mass before and after the corrosion and of the immersion time.
It is well known that the Mg alloy corrosion can be diminished through grain refining treatments. Li et al. [23] manufactured scaffolds made from WE43 using the SLM technique. They obtained an enhanced biodegradation resistance of 0.17 mL/cm2 day by comparing this value with as-cast or as-extruded samples. He et al. [47] studied the degradation resistance of AZ61 alloy and observed that this factor was improved by rapid solidification through SLM. The laser power was increased, and enhanced microhardness and degradation rate were evidenced. It was concluded that this fact is due to the coarsened equiaxed grains and a reduced solution of aluminum in the magnesium matrix. Shuai et al. [48] fabricated Mg-6Zn-0.6Zr (ZK60) based on the SLM method. They increased the laser’s energy density, leading to grain refinement, homogenized microstructure, and rapid solidification during the SLM procedure. The material corrosion resistance was highly improved.
Magnesium-based alloys reinforced in bioceramic materials can exhibit good mechanical strength and corrosion resistance. Deng et al. [49] have prepared β-tricalcium phosphate (β-TCP) combined with ZK60 using the SLM method. Due to the rapid solidification process, a homogenous distribution of β-TCP placed along the grain boundaries of α-Mg was noticed. If rare-earth metal such as Nd is incorporated into Mg-based alloys via SLM technology, fine α-Mg grains and intermetallic phases are obtained. A surface layer promoted by Nd2O3 formation inhibited the degradation resistance of the composite material, and the neodymium (Nd)-induced honeycomb structure was evidenced [50].
Intermetallic phase apparition is considered an important factor that influences biodegradation property. Different grain sizes and intermetallic phase volume fractions were created by varying the Al concentration in Mg-3Zn-0.6Zr (ZK30) alloy using SLM. It was noticed that the fraction of the intermetallic phase and grain size refinement are enhanced by increasing the Al content. However, when the Al content is below 3 wt% due to the apparition of numerous grain boundaries, it is possible to passivate the material surface faster, and thus an increased corrosion resistance is obtained.
2.2. Densification
The formation quality is described in the literature with the term densification, which also considers process parameter optimization. During the manufacturing procedure, processing pores can appear, which can be located inside the scaffold struts. They have a detrimental influence on the implant’s mechanical and biological properties. A relative density higher than 99.5% was obtained through SLM technology in the case of bulk Mg alloys [51]. Liu et al. [52] made porous Mg-Ca alloys through a laser additive manufacturing process. They noticed that the porosity and surface morphology are directly linked to the laser energy input. This parameter was varied between 875 J/mm3 and 1000 J/mm3, and the obtained porosity was between 18.48% and 24.60%. The microhardness of the porous Mg-Ca was superior to that of as-cast pure magnesium and was between 60HV and 68HV. The formation quality of the developed porous scaffold was about 81%. Shuai et al. [48] found that by increasing the laser energy density, the crystalline structure of Mg-Zn-Zr alloys successively changed from clustered finer dendrites to uniform equiaxed grains and coarsened equiaxed grains. An ideal structure without open pores and a relative density of 97.4% was obtained at a laser energy of 600 J/mm3. By increasing this factor to 750 J/mm3, the apparition of microcracks on the surface was observed. Li et al. [23] have manufactured from WE43 porous scaffolds exhibiting a diamond lattice. They have reported some geometrical discrepancies regarding the as-built strut size of 420 μm by comparing it with the designed value of 400 μm and an as-built porosity of 64%, slightly smaller than the design value of 67%. Qin et al. [53] have made a diamond lattice porous scaffold using Zn + WE43 powder. The relative density was about 99.7. Unfortunately, geometrical discrepancies were also reported in this case. The as-built strut size was 562 μm, much larger than the design value of 400 μm. The as-built geometrical porosity was about 45% to 67%, characteristic of the initial design.
Usually, the properties of the PBF components depend on the input laser power, spot diameter, hatch spacing, scanning speed, and layer thickness [54]. By selecting the correct values for these parameters, high-density parts can be manufactured. The ideal energy density dependence on the parameters mentioned above is given in [51]. Lower values of the scanning rate increase the density of the component due to a longer interaction time between the laser beam and powder that determines a higher energy delivery rate to the powder layer. In the case of high scanning rates, the laser energy transferred to the powder layer is reduced, so a partial melting between powder particles appears, which leads to pore creation within the struts. For a proper energy value, the particles are fully melted and penetrate the spaces, and a thick part is obtained.
2.3. Mechanical Properties
Mechanical properties of the AM Mg-based implants include tensile and compressive strength, stiffness, durability, ductility, and hardness flexibility. The elastic modulus of the scaffold must have a similar value to that of cortical bone to avoid the stress shielding effect. The relative density of the implant is the most important factor, which affects Young’s modulus and failure strength considerably, according to Gibson and Ashby’s model [55].
σpl = 0.3 (ρ/ρs)1/2 ρys,
E = ρ(ρs)2Es,
Microhardness of Mg-Ca alloys depends on different parameters during the PBF process according to the Hall–Petch formula H = H0 + kd−1/2, in which H0 and k are constants that depend on the material crystalline lattice, d represents the grain size, and H depicts the sample hardness. The average value of the microhardness is between 60 HV and 68 HV [14]. For the PBF-produced Mg-Ca, a solid-dissolution of calcium (Ca) into the α-magnesium matrix is noticed. This solid solution strengthening can improve the hardness and strength of the alloy and is directly linked to the alloy’s higher value of microhardness. The dislocation density and lattice deformation are due to the rapid cooling rate and impact the microhardness value. For PBF Mg-Ca alloys, the grain size is between 5 and 30 μm, and in the case of pure magnesium, the same quantity has higher values between 300 and 500 μm. It is expected that the hardness of Mg-Ca materials is higher.
In the case of Mg-Zn-Zr alloys, the microhardness exponentially rises with the Zn content. An average value between 57.67 HV and 58.28 HV in the longitudinal section of Mg-5.2%Zn-0.3%Zr alloy was found, and regarding the Mg-15%Zn-0.3%Zr material, this value was between 75.51 HV and 80.23 HV. In the case of Mg-30%Zn-0.3%Zr, an interval of 106.75–109.36 HV was obtained [28]. According to the Hall–Petch equation H = H0 + kd−1/2, in which H is the sample hardness, H0 and k are material constants, and d is the grain size, it is expected that the grain size will decrease directly proportional to the Zn content.
The alloying elements influence the material grain size. In the case of Mg-Zn-Dy alloys, the grain size decreases directly proportional to the Dy content, so as a consequence, the microhardness increases [18]. The Dy content was varied from 0 to 5 wt%, and for the maximum wt%, a microhardness of 121.28 HV was obtained. This value is 1.38 times higher than that obtained for Mg-3Zn alloy.
By considering the solid solution strengthening theory, better mechanical strength results from a higher solid solubility. The atomic radii of Al and Zn are of the order of 0.11 nm, a value lower than the Mg radius of 0.13 nm. A lattice distortion occurs when these alloying elements are dissolved into the Mg matrix. In the case of binary alloys, Mg-Al, the variation of microhardness is directly proportional to the Al content in the α-Mg matrix [56]. The intermetallic compound in Mg-Al alloys is β-Mg17Al12, whose hardness can be reduced by applying SLM at the value of 150 ± 60 Hv since the hardness of α-Mg alloy is maintained at 126 ± 3 Hv. The SLM technique reduces the dispersion of the β-Mg17Al12 phase, but it leaves the hardness value of the alloy unchanged.
AM techniques that use the laser beam as a heat source are characterized through different thermal histories in the same sample (e.g., for SLM-processed materials, the microhardness measured at the center of the molten pool and the microhardness at the edge zone have different values) [57]. The presence of defects also has an essential influence on the material’s microhardness. A reduced value is obtained when the scaffolds exhibit a high porosity or cracks in the material microstructure. In the case of SLM technology, the rapid solidification process has an important effect on the alloy microstructure and the solid solution of the elements. The solute trapping effect in the AM process implies the existence of the alloying elements in the matrix, and different strengthening effects characterize the resulting solid solution. When the dislocation motion between the grains is suppressed, the hardness of secondary phases is higher than that of magnesium.
Qin et al. [53] have produced AM Zn + xWE43 alloys with a maximum tensile strength of 335.4 MPa and an elongation of 1%. In the Zn + 5WE43 porous substrate, Young’s modulus was found to be 2480 MPa, and the compressive strength was equal to 73.2 MPa. Li et al. [23] developed, using SLM technology, porous WE43 scaffolds with diamond cells. The Young’s modulus was 0.7–0.8 GPa, very close to the value of trabecular bone after 4 weeks of biodegradation. The implant pore size was about 600 μm, and the strut size was 400 μm.
There is little information in the literature regarding SLM-produced Mg alloys and their mechanical properties, which are better than in the case of as-cast magnesium [57][58]. At a laser energy density between 104 and 167 J/mm3, the UTS and YS of SLM-produced AZ91 alloy are 30% and 50% higher than values obtained for as-cast AZ91. The elongation is 40% lower compared to values obtained for as-cast materials. For AZ61 alloy at a laser energy density of 156 J/mm3, the UTS and YS are equal to 287 MPa and 233 MPa, respectively, which are about 93% and 135% higher than in the case of as-cast material [59]. For this alloy, the elongation rate has an average value of 2.71%, which is lower than the values obtained for as-cast AZ61. A physical explanation of these observations is that the SLM process is directly linked to smaller grain sizes and a uniform microstructure. It was noticed that the UCS and Young’s modulus increase is directly proportional to the laser power energy density [52]. A possible cause can be considered by the material’s different porosities obtained at variable energy inputs. When the alloy porosity decreases, an increase in UCS, plasticity, and elastic modulus is seen.
In addition, an important experimental observation is the fact that the mechanical properties of magnesium exhibit an important anisotropy, and the longitudinal mechanical properties are better than the transverse mechanical properties. Stress concentrations are due to pores, and reduced mechanical properties are expected. It is important to control the pore size in Mg-Ca alloys by modifying the laser energy [52].
2.4. Microstructure
The scaffold microstructure is important regarding the physical properties such as toughness, strength, ductility, wear resistance, hardness, and corrosion rate. The AM implant microstructure depends on processing conditions and the chemical composition of the powder material. Due to high cooling rates, a microstructure characterized by much finer grains is obtained using the SLM technique. The Mg-based parts fabricated through AM have an average grain size of 30 μm. They are characterized by a homogenized microstructure that appears during the rapid solidification process, which is characteristic of the SLM method. An improved microstructure is directly connected to better mechanical and biological properties.
Xie et al. [60] investigated the microstructure of AM-manufactured scaffolds of Mg-Nd-Zn-Zr (JDBM). In the case of the as-fabricated scaffold, fish-scale-shaped melt pools were observed. These structures are composed of equiaxed and columnar grains. The latter grew along the molten pool, and the equiaxed grains were formed on the boundary of the molten pool. White dot particles were located in the grain interiors. The authors attributed this to the Mg12Nd phase induced during the solidification process. After a heat treatment was applied, the columnar grains disappeared. The material contained only equiaxed grains with an average size of about 22.5 μm and white particles. The white dot chemical composition was investigated through X-ray diffraction analysis (XRD), and it was found that some dots represent a rare earth hydride NdH2 and Mg12Nd eutectic phase.
Dong et al. [61] analyzed the microstructure of Mg-Zn alloy scaffolds produced through extrusion-based additive manufacturing. The implants had a porosity of 50.3% and strut density of 93.1% and were composed of a Mg matrix and MgZn2 second-phase particles. It was concluded that the etched Mg-Zn samples exhibited a grain size of 26.5 ± 3.5 μm with second-phase particles dispersing at grain boundaries. For the pure Mg specimen, an average grain size of 28.3 ± 1.2 μm with clean grain boundaries was found. The XRD analysis gives evidence in the case of Mg-Zn for the presence of α-Mg phase and MgZn2 second phase.
The influence of the Mg content on the microstructure was investigated in [53] for laser powder bed fusion (L-PBF) Zn-Mg alloy porous scaffolds. The porosity of L-PBF samples was found to be in the range of 50–53%. Secondary phases such as Mg2Zn11 and MgZn2 were detected based on X-ray diffraction (XRD) and energy dispersive X-ray (EDX) investigations. Scanning electron microscopy (SEM) gave evidence, in the case of Zn-1Mg, of a polygonal α-Zn phase, and fine α-Zn + Mg2Zn11 eutectic phases. When the Mg concentration increases, a reduction of the primary α-Zn phase, an increase in the eutectic phase, and the apparition of MgZn2 phase dots can be noticed. It was concluded that the increased Mg content is directly linked to a refined grain structure.
L-PBF-manufactured WE43 porous scaffolds composed of 4.26% Y, 2.46% Nd, 1.28% gadolinium (Gd), 0.43%Zr, and residual Mg were investigated in [62]. XRD analysis proves the presence of α-Mg, Y2O3, and β phase. The flake phases were supposed to be oxides, which were crushed into flakes during the L-PBF process. This structure could not be melted due to experimental conditions, and it was assumed that the dissolved element Y could react with the residual oxygen in the L-PBF chamber. Mg3X eutectic compounds precipitated from the liquid WE43, where X represents rare earth (Y, Gd, or Nd). STEM showed the presence of a hybrid oxide of (Y,Zr)2O3. Secondary phases such as Mg14(Nd,Gd)2Y along grain boundaries were observed. Li et al. [63] manufactured WE43 Mg alloy scaffolds using the laser powder bed fusion method. The presence of oxide particles and intermetallic precipitates of Mg and rare earth (RE) was noticed in the Mg matrix. The latter are smaller and can be observed in the vicinity of the melt pool boundaries. Electron backscatter diffraction (EBSD) analysis gave evidence of a bi-modal grain size distribution with small grains with random texture located near the melt pool and large grains oriented parallel to the building direction.
2.5. Biocompatibility
Biocompatibility is a fundamental property of medical implants. The powder composition that is used in the additive manufacturing procedure must be properly designed, and information regarding bulk material could represent a starting point in this process. In order to obtain increased biocompatibility, an improvement of the mechanical properties, and maintain the material integrity for magnesium-based alloys through surface biofunctionalization, microstructure modification and surface treatments have to be considered.
Li et al. [63] investigated open porous scaffolds made from WE43 Mg alloy designed using the laser powder bed fusion technology. A body-center cubic pattern with different strut diameters was produced. The scaffolds’ microstructure was improved with a thermal solution and heat aging treatments. It was noticed that the increase in the strut diameter to up 800 μm generated an increase in the elastic modulus from 0.2 to 0.8 GPa. Additionally, through plasma electrolytic oxidation (PEO) treatments, the material corrosion rate was decreased to approximately 0.1 mm/year, and good biocompatibility was achieved. Wu et al. [64] prepared Mg-6Zn-0.6Zr (ZK60) scaffolds using the selective laser melting (SLM) method. A laser power of 50 W and a scanning velocity of 500–800 mm/s led to minimal defects and high dimensional accuracy samples. It was noticed that SLM ZK60 has a reduced grain size of 7.3 μm in comparison with the 56.4 μm obtained in the case of cast ZK60. On the same consideration, it exhibited a higher hardness of 0.78 GPa and similar values of the elastic modulus. Higher corrosion resistance was identified for SLM ZK60 in Hanks’ solution with a decrease of 30% in hydrogen evolution rate and 50% in the corrosion current density. Yang et al. [65] made bioglass-reinforced Mg-based composite via laser additive manufacturing. The samples were characterized by a refined and homogenized structure, which improves the material corrosion rate. The composite material has good biocompatibility because it promotes cell growth and differentiation, and rapid bone healing was reported. Yao et al. [66] have prepared binary and ternary Mg-based alloys using SLM technology. An improvement of the microhardness was noticed in the case of Mg-0.6Ca and Mg-0.5Zn-0.3Ca, with measured values being approximately equal to 55 HV. These laser-processed magnesium alloys with an improved corrosion rate showed excellent biocompatibility. Xu et al. [67] improved the biodegradation resistance through grain refinement methods in the case of ZK30 + Cu alloys produced via SLM. These types of materials exhibit antibacterial properties due to the copper alloying procedure. When the Cu percent is increased, the Vickers hardness can be up to 98 HV for 0.3 wt% Cu, and the corrosion current is equal to 47.8 μA/cm2. These materials exhibited good cytocompatibility and high antibacterial properties against colonies of S. aureus. It was observed that an increase in the Cu content is directly linked to a faster decrease in the colonies’ number. Yin et al. [68] studied in vitro degradation behavior and cytocompatibility of ZK30/bioactive glass composites produced through SLM. They have concluded that the integration of bioactive glass (BG) into Mg-based alloys leads to increased corrosion resistance, microhardness, and biocompatibility and that the alloy ZK30/10BG is a promising material for orthopedic applications. Shuai et al. [69] developed an antibacterial Mg-based ZK60-xCu alloy, with x between 0.2 and 0.8 wt%, prepared by SLM. Antibacterial properties of the material were analyzed through the bacterial counting method using Escherichia coli as a bacterium model. It was observed that the colonies decreased with the increase in the immersion time on ZK60 + Cu alloys. In the case of ZK60-0.6Cu and ZK60-0.8Cu, the colonies disappeared after a specific time. This fact was due to a combination of copper (Cu) ions’ release and an alkaline environment that is beneficial for cellular membrane structure deterioration and bacterial annihilation. Copper can modify enzyme activity and can inhibit deoxyribonucleic acid (DNA) replication. In order to test the alloy’s biocompatibility, human osteosarcoma MG63 cells were used. The reported results showed very good cytocompatibility. Regarding the material microstructure, the alloying process of ZK60 with Cu produced a grain refinement that improved mechanical properties.
3. Biological Properties of Magnesium-Based Scaffolds
The standards regulating the interaction between cells and new scaffold materials are ISO 10993-5 and 10993:12. The standardized tests include direct and indirect contact between cells and materials and cytotoxicity tests such as extraction-based assays [70][71]. Unfortunately, the latter were developed for non-degradable implants, and in the case of Mg-based alloys, this method cannot be applied according to the International Organization for Standardization (ISO). A gas release is observed when a Mg scaffold is immersed in the extraction medium. This phenomenon is followed by an increase in the pH value and a strong degradation effect. Sometimes an osmotic shock that kills the living cells can be seen. In order to assess this drawback, in vitro bioreactors that simulate the human body through a dynamic flow system can be involved. Many researchers have undertaken cytotoxicity tests consisting of cell culture medium [72] analyzed under physiological conditions (CO2 level of 5%, O2 level of 20%, relative medium humidity of 95%, and temperature of 37 °C) such as 10% fetal bovine serum. Dilution of pure extracts can lead to experimental mistakes due to the percentage of Mg ion reduction. The EN ISO standards 10993:5 and 10993:12 recommend that the sample weight to extraction medium ratio be 0.2 g/mL. Fischer et al. [73] conducted experiments to analyze the influence of magnesium extracts on osteosarcoma and human osteoblast cell lines. The specimen samples were prepared from pure magnesium (99.95%), magnesium with 0.6 wt% Ca, and magnesium with 1 wt% Ca using permanent mold direct chill casting. These samples were incubated in Dulbecco’s modified Eagle medium (DMEM) Glutamax-I supplemented with 10% fetal bovine serum. The osmolality of the extract was measured with a Gonotec 030-D cryoscopic osmometer. An increase in osmolality was noticed, and it was concluded that this phenomenon is directly linked to increased Mg concentration. All cytotoxicity tests proved a higher tolerance of the osteoblasts towards Mg extracts compared with the human osteosarcoma cell line. The alloys that contain Ca showed better cell proliferation qualities.
Some authors consider that MTT and XTT assays, which involve tetrazolium salt use, can also provide altered results because degraded Mg reacts with tetrazolium salts, resulting in formazan formation that has a negative impact on in vitro tests [74]. These assays are fast, facile, non-radioactive, and work with metabolically active and living cells. An important disadvantage of this technique is that the tetrazolium-based analysis does not distinguish between cell death and cell reduced growth rate. The tests can be influenced by different substances such as human serum albumin or vitamins C and D that reduce the MTT and XTT tetrazolium salt percent. Other chemicals that influence these assays are flavonoids, thiol-containing antioxidants, D-glucose, nanoparticles, or glutathione S-transferase. Fischer et al. [74] compared the efficiency of MTT and XTT assays with luminescence-based assay (BrdU). Using the permanent mold casting method and dry pressing and sintering technology, pure magnesium, Mg-Y (4 wt% yttrium), and Mg-Ca-1eu (1 wt% calcium, eutectic) samples were prepared. All the samples were sonicated for 20 min in dry isopropanol, and after that, they were gamma sterilized. Regarding the cell culture, they used human osteosarcoma MG63 cultured in Dulbecco’s modified Eagle medium combined with 10% fetal bovine serum. It was concluded that sometimes MTT or XTT assays could not identify the cell viability in the right manner, and the influence of different factors must be considered. An adequate method for Mg-based alloys in the case of cytotoxicity tests is luminescence-based assay (BrdU), which does not interfere with the Mg corrosion process.
For in vitro tests, different cell lines such as fibroblastic, human osteoblast, mouse pre-osteoblastic, or human osteosarcoma cells are used. Unfortunately, in all the investigated cases the osmosis phenomenon can lead to cell apoptosis. Osmotic swelling or shrinkage can have an important influence on the cell proliferation process [75][76]. The interactions between Mg ions and different cell types must be separately analyzed because different cytotoxicity responses can be obtained. Bobe et al. [75] manufactured open porous scaffolds made of sintered Mg-Y (W4) short fibers. The material’s biocompatibility was tested based on material extracts and the mouse fibroblast (L929) cellular line. Ten open porous samples were prepared, and then they were incubated in physiological conditions. Another 10 W4 samples were made following the same protocol; the only difference was that human osteoblast cells (HOB) were used. Weight loss and corrosion rate estimation was performed, and similar viability and proliferation testing for L929 and HOB was carried out. The authors have concluded that the cytotoxicity tests depend on many environmental conditions. The study proved that different cell lines, such as fibroblastic cell lines (L929) and human osteoblast cells (HOB), have different responses to ionic and osmotic modifications. HOB survival rate is higher than L929 in high-concentration osmotic solutions, but cellular proliferation in the case of human lines is much more reduced than that obtained for mouse cell lines when highly osmotic extracts are used. Wu et al. [77] prepared degradable Zn-0.04Mg-2Ag alloy scaffolds for large-scale bone defect treatment. Regarding the cytocompatibility of the scaffold, the authors used MC3T3-E1 mouse pre-osteoblast cells cultured in an α-minimum essential medium (MEM) that contains 10% fetal bovine serum (FBS). The cell suspension had a 50,000 cells/mL density, and 5000 MC3T3-E1 were added to wells in 96-cell plates. The cytoskeleton staining of cultured cells with Zn-0.04Mg-2Ag exhibited an evident outline of cells and nuclei, and the cells infiltrated into the scaffold structure. The proposed implants showed a slight antibacterial effect on Escherichia coli and a highly antibacterial effect against Staphylococcus aureus and Staphylococcus epidermis. The investigated Mg-based alloys proved to up-regulate the mRNA expression for osteoblast-specific transcription factors such as osteopontin and osteocalcin. Wang et al. [78] proposed novel porous Mg-Nd-Zn-Zr coated with brushite and proved that these implants are adequate for cell adhesion, osteogenic differentiation, and bone regeneration of a critical defect surgically induced in the femoral condylar of rats and radius segmental bone defects of rabbits. For in vitro tests, they used rat bone marrow mesenchymal stem cells (rBMSCs) collected from the femoral zone of Sprague Dawley (SD) rats. An α-MEM medium with 10% fetal bovine serum, 100 units/mL penicillin, and 100 units/mL streptomycin was prepared and then incubated for 72 h. Ninety-six-well cell culture plates with a cell density of 5 × 104 cells/mL were cultured with 200μL extract in each well for a time interval between 1 and 7 days. The scaffolds promoted osteogenic differentiation, and enhanced the mineralization process, angiogenesis, and osteogenesis. Xie et al. [60] prepared 3D-printed JDBM scaffolds and analyzed the implant cytocompatibility in clonal murine cell line of immature osteoblasts derived from mice (MC3T3-E1) and murine macrophage (RAW267.4) cells. They cultured the cells in the presence of different extract concentrations. It was concluded that 50, 25, and 12.5% of sample extracts did not inhibit the cell viability, did not increase the number of dead cells, and did not change the cell morphology. Dong et al. [61] manufactured an Mg-Zn alloy scaffold using an extrusion-based manufacturing process. The indirect culture of MC3T3-E1 mouse pre-osteoblasts in Mg-Zn extract proved good cytocompatibility. The MC3T3-E1 cells were pre-cultured for 7 days in α-MEM solution without ascorbic acid and supplemented with 10% FBS and 1% penicillin/streptomycin under physiological conditions. It was observed that the pH values of extracts of Mg-Zn and pure Mg were lower than the pH tolerance threshold of MC3T3-E1 cells. The Mg2+ ion release limit for MC3T3-E1 cells is less than 360 mg/L, and it was noticed that in the case of 50% and 100% extracts of Mg-Zn and pure Mg, there were some cytotoxic reactions. High Zn2+ concentration had an inhibitory effect on the pre-osteoblasts’ growth. It was concluded that a low Zn2+ concentration promotes migration, viability, and proliferation. A safe value for the concentration of Zn2+ was reported to be 3 mg/L, and only the 10% Mg-Zn extract was cytocompatible. Qin et al. [4] used the same cell line to test the cytocompatibility of Mg-Zn scaffolds manufactured through laser powder bed fusion (L-PBF). Pure Zn and Zn-1Mg 100% extracts exhibited a high toxicity grade to MC3T3-E1 cells. In the case of Zn-5Mg alloy extracts, the best cell viability was found, and after 5 days, the differences in cell viability between samples decreased.
It is well known that the Mg ions regulate the calcium (Ca) ion level, which has a beneficial effect on cell metabolism and shape. Zhang et al. [79] gave evidence of the importance of Mg ions (Mg2+) and the antagonistic effects of Ca2+ ions and Mg2+ in vascular smooth muscle cells to control vascular reactivity. Mg2+ affects the Ca2+ flux across vascular muscle membranes and its release from the human body. Lang et al. [80] investigated the cell volume regulatory ion channels in cell proliferation and death. An increase in cell volume characterizes cell proliferation, and apoptosis is directly linked to cell shrinkage. In order to modify the volume of the cell, one must involve ion transport across the cell membrane and an adequate activity of Ca2+, Cl−, and K+ channels. K+ exit decreases the cytosolic K+ concentration, which can induce the cells’ apoptosis. Ca2+ enters through Ca2+-permeable cation channels. A hyperosmotic shock activates this process, and an increase in cytosolic Ca2+ activates both cell proliferation and apoptosis. Cl− channels have an influence on cytosolic Cl− activity, and it can mediate the osmolyte flux.
4. In Vivo Behavior of the Magnesium-Based Scaffolds
When a scaffold is designed for a given medical application, the interaction between host tissue and scaffold materials, the corrosion process of the biodegradable implants, the ingrowth and ongrowth of newly generated tissue combined with the apparition of new blood vessels, and foreign body responses must be taken into consideration. It is well known that scaffold integration in the human body is made up of three steps. The first one appears 2 weeks after surgery and consists of initiating and developing an inflammatory response. This phenomenon is characterized by the apparition of plasma cells, polymorphonuclear leukocytes, lymphocytes, and monocytes. During the second phase, the monocytes become predominant and differentiate into macrophages. New blood vessels and implant encapsulation with fibrous tissue are present. A direct link was observed between the scaffold’s degradation and the time interval of this second step. In addition, foreign body giant cells are seen at the scaffold surface due to the fusion or joining of the macrophage cells. In the third phase, the implant is degraded, losing its mechanical integrity. The scaffold is slowly replaced with new fibrous tissues, which change its structure during a certain time. Finally, the scaffold breaks down into particles, which are eliminated through phagocytosis by the macrophage cells (Figure 1).
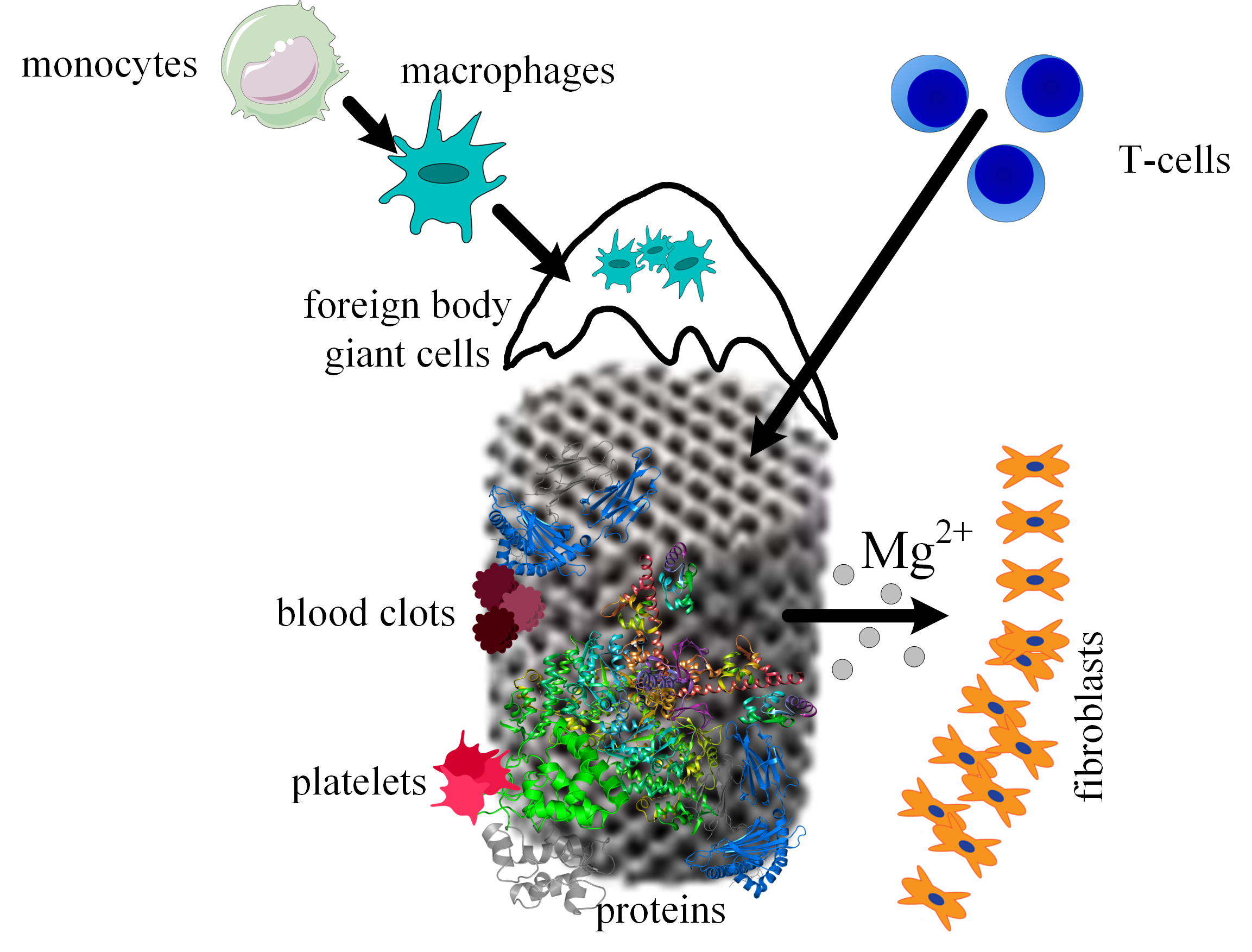
Figure 1. Osseointegration process of the scaffold. Firstly, proteins adhere to the scaffold material, then platelets begin the blood clotting formation, immune system cells migrate to the wounded tissues due to the inflammation process, Mg2+ ions diffuse away as a consequence of the metal corrosion, and fibrous tissue encapsulation appears.
Xie et al. [60] manufactured biodegradable porous Mg-Nd-Zn-Zr implants using the SLM technique for scaffold-related infections. The in vivo test consisted of scaffold implantation in a rabbit model. They were proved to have high antibacterial properties against methicillin-resistant S. aureus and Escherichia coli. The implant biocompatibility was investigated based on blood tests, histological evaluation, and Mg2+ deposition measurements. During the first stage of the implantation, inflammatory response and TNF-α secretion were seen at the boundary between the scaffold and rabbit tissue. It was noticed that the high concentration of Mg2+ ions promotes the M1 phenotype of macrophages, enhancing their phagocytic ability. In conclusion, it was stated that 3D-printed porous JDBM scaffolds have great potential in the orthopedic field, especially for patients that have a high risk of infections.
Qin et al. [4] developed Zn-Mg alloy porous scaffolds for enhanced osseointegration produced through laser powder bed fusion, using pre-alloyed Zn-xMg powder, with x between 1 wt% and 5 wt%. The in vivo investigation took into account histological analysis after 6-week and 12-week implantation in rabbit femurs. Enhanced bone formation for the Zn-xMg scaffold compared with pure Zn implants was found. It was concluded that this material is promising for large bone defect treatment because it has a high osteogenic effect. The biocompatibility and osteogenesis increase directly proportional to the Mg percent. Excessive addition of Mg produces a decrease in mechanical properties.
WE43 porous scaffolds were fabricated through laser powder bed fusion by Liu et al. [81]. For in vivo tests, they used 45 6-month-old male New Zealand white rabbits with weights between 3 and 3.5 kg. In the left knee of the animal models, a surgical defect with a diameter of 5 × 6 mm2 was created. The animals were divided into three groups: for the first one, the defect was left empty; for the second one, WE43 scaffold treatment was applied; and in the case of the third group, calcium sulfate bone cement was used to fill the defect. At 4, 8, and 12 weeks, the rabbits were euthanized, and femur samples were analyzed. The in vivo compatibility was evaluated as a function of the presence or absence of rejection reaction, inflammation, infection, and fester. The animal blood was used to determine alanine transaminase (ALT) levels, UREA, and Mg2+ concentrations. It was concluded that the WE43 scaffolds lost mechanical integrity at 4 weeks after implantation. The healing time of the defect was found to be different, and it depended on the clinical conditions. It is well known that clinical studies reported a time between 6 and 12 months for the complete degradation of Mg screws. The gas bubbles resulting due to the Mg corrosion process were visible at 4 weeks, and they completely disappeared after 8 and 12 weeks. Regarding the osseointegration and osteoinduction properties of the WE43 scaffolds, it was noticed that at 12 weeks after the surgery, the defect was filled with new trabecula. Empty cavities were seen in the case of the untreated group and cement group. It was concluded that if the degradation rate of WE43 is improved, the developed scaffolds show high biocompatibility and good osteoinduction properties and can be used in the orthopedic field.
References
- Wang, Z.; Wu, W.; Qian, G.; Sun, L.; Li, X.; Correia, J.A.F.O. In-Situ SEM Investigation on Fatigue Behaviors of Additive Manufactured Al-Si10-Mg Alloy at Elevated Temperature. Eng. Fract. Mech. 2019, 214, 149–163.
- Yue, X.; Shang, J.; Zhang, M.; Hur, B.; Ma, X. Additive Manufacturing of High Porosity Magnesium Scaffolds with Lattice Structure and Random Structure. Mater. Sci. Eng. A 2022, 859, 144167.
- DebRoy, T.; Wei, H.L.; Zuback, J.S.; Mukherjee, T.; Elmer, J.W.; Milewski, J.O.; Beese, A.M.; Wilson-Heid, A.; De, A.; Zhang, W. Additive Manufacturing of Metallic Components—Process, Structure and Properties. Prog. Mater. Sci. 2018, 92, 112–224.
- Qin, Y.; Liu, A.; Guo, H.; Shen, Y.; Wen, P.; Lin, H.; Xia, D.; Voshage, M.; Tian, Y.; Zheng, Y. Additive Manufacturing of Zn-Mg Alloy Porous Scaffolds with Enhanced Osseointegration: In Vitro and in Vivo Studies. Acta Biomater. 2022, 145, 403–415.
- Tan, X.P.; Tan, Y.J.; Chow, C.S.L.; Tor, S.B.; Yeong, W.Y. Metallic Powder-Bed Based 3D Printing of Cellular Scaffolds for Orthopaedic Implants: A State-of-the-Art Review on Manufacturing, Topological Design, Mechanical Properties and Biocompatibility. Mater. Sci. Eng. C 2017, 76, 1328–1343.
- Sing, S.L.; An, J.; Yeong, W.Y.; Wiria, F.E. Laser and Electron-Beam Powder-Bed Additive Manufacturing of Metallic Implants: A Review on Processes, Materials and Designs. J. Orthop. Res. 2016, 34, 369–385.
- Salehi, M.; Maleksaeedi, S.; Farnoush, H.; Nai, M.L.S.; Meenashisundaram, G.K.; Gupta, M. An Investigation into Interaction between Magnesium Powder and Ar Gas: Implications for Selective Laser Melting of Magnesium. Powder Technol. 2018, 333, 252–261.
- Wang, P.; Gammer, C.; Brenne, F.; Prashanth, K.G.; Mendes, R.G.; Rümmeli, M.H.; Gemming, T.; Eckert, J.; Scudino, S. Microstructure and Mechanical Properties of a Heat-Treatable Al-3.5Cu-1.5Mg-1Si Alloy Produced by Selective Laser Melting. Mater. Sci. Eng. A 2018, 711, 562–570.
- Qin, Y.; Wen, P.; Guo, H.; Xia, D.; Zheng, Y.; Jauer, L.; Poprawe, R.; Voshage, M.; Schleifenbaum, J.H. Additive Manufacturing of Biodegradable Metals: Current Research Status and Future Perspectives. Acta Biomater. 2019, 98, 3–22.
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological Design and Additive Manufacturing of Porous Metals for Bone Scaffolds and Orthopaedic Implants: A Review. Biomaterials 2016, 83, 127–141.
- Nguyen, T.L.; Staiger, M.P.; Dias, G.J.; Woodfield, T.B.F. A Novel Manufacturing Route for Fabrication of Topologically-Ordered Porous Magnesium Scaffolds. Adv. Eng. Mater. 2011, 13, 872–881.
- Hollister, S.J. Porous Scaffold Design for Tissue Engineering. Nat. Mater. 2005, 4, 518–524.
- Ng, C.C.; Savalani, M.M.; Man, H.C.; Gibson, I. Layer Manufacturing of Magnesium and Its Alloy Structures for Future Applications. Virtual Phys. Prototyp. 2010, 5, 13–19.
- Kaushik, V.; Nithish Kumar, B.; Sakthi Kumar, S.; Vignesh, M. Magnesium Role in Additive Manufacturing of Biomedical Implants—Challenges and Opportunities. Addit. Manuf. 2022, 55, 102802.
- Kruth, J.; Mercelis, P.; Van Vaerenbergh, J.; Froyen, L.; Rombouts, M. Binding Mechanisms in Selective Laser Sintering and Selective Laser Melting. Rapid Prototyp. J. 2005, 11, 26–36.
- Huang, S.; Narayan, R.L.; Tan, J.H.K.; Sing, S.L.; Yeong, W.Y. Resolving the Porosity-Unmelted Inclusion Dilemma during in-Situ Alloying of Ti34Nb via Laser Powder Bed Fusion. Acta Mater. 2021, 204, 116522.
- Manakari, V.; Parande, G.; Gupta, M. Selective Laser Melting of Magnesium and Magnesium Alloy Powders: A Review. Metals 2017, 7, 2.
- Long, T.; Zhang, X.; Huang, Q.; Liu, L.; Liu, Y.; Ren, J.; Yin, Y.; Wu, D.; Wu, H. Novel Mg-Based Alloys by Selective Laser Melting for Biomedical Applications: Microstructure Evolution, Microhardness and in Vitro Degradation Behaviour. Virtual Phys. Prototyp. 2018, 13, 71–81.
- Salehi, M.; Maleksaeedi, S.; Nai, M.L.S.; Gupta, M. Towards Additive Manufacturing of Magnesium Alloys through Integration of Binderless 3D Printing and Rapid Microwave Sintering. Addit. Manuf. 2019, 29, 100790.
- Chen, J.; Wu, P.; Wang, Q.; Yang, Y.; Peng, S.; Zhou, Y.; Shuai, C.; Deng, Y. Influence of Alloying Treatment and Rapid Solidification on the Degradation Behavior and Mechanical Properties of Mg. Metals 2016, 6, 259.
- Zumdick, N.A.; Jauer, L.; Kersting, L.C.; Kutz, T.N.; Schleifenbaum, J.H.; Zander, D. Additive Manufactured WE43 Magnesium: A Comparative Study of the Microstructure and Mechanical Properties with Those of Powder Extruded and as-Cast WE43. Mater. Charact. 2019, 147, 384–397.
- Bär, F.; Berger, L.; Jauer, L.; Kurtuldu, G.; Schäublin, R.; Schleifenbaum, J.H.; Löffler, J.F. Laser Additive Manufacturing of Biodegradable Magnesium Alloy WE43: A Detailed Microstructure Analysis. Acta Biomater. 2019, 98, 36–49.
- Li, Y.; Zhou, J.; Pavanram, P.; Leeflang, M.A.; Fockaert, L.I.; Pouran, B.; Tümer, N.; Schröder, K.-U.; Mol, J.M.C.; Weinans, H.; et al. Additively Manufactured Biodegradable Porous Magnesium. Acta Biomater. 2018, 67, 378–392.
- Li, Y.; Jahr, H.; Zhang, X.-Y.; Leeflang, M.A.; Li, W.; Pouran, B.; Tichelaar, F.D.; Weinans, H.; Zhou, J.; Zadpoor, A.A. Biodegradation-Affected Fatigue Behavior of Additively Manufactured Porous Magnesium. Addit. Manuf. 2019, 28, 299–311.
- Wei, K.; Zeng, X.; Wang, Z.; Deng, J.; Liu, M.; Huang, G.; Yuan, X. Selective Laser Melting of Mg-Zn Binary Alloys: Effects of Zn Content on Densification Behavior, Microstructure, and Mechanical Property. Mater. Sci. Eng. A 2019, 756, 226–236.
- Liu, L.; Yuan, F.; Zhao, M.; Gao, C.; Feng, P.; Yang, Y.; Yang, S.; Shuai, C. Rare Earth Element Yttrium Modified Mg-Al-Zn Alloy: Microstructure, Degradation Properties and Hardness. Materials 2017, 10, 477.
- Shuai, C.; Zhou, Y.; Yang, Y.; Feng, P.; Liu, L.; He, C.; Zhao, M.; Yang, S.; Gao, C.; Wu, P. Biodegradation Resistance and Bioactivity of Hydroxyapatite Enhanced Mg-Zn Composites via Selective Laser Melting. Materials 2017, 10, 307.
- Zhang, M.; Chen, C.; Liu, C.; Wang, S. Study on Porous Mg-Zn-Zr ZK61 Alloys Produced by Laser Additive Manufacturing. Metals 2018, 8, 635.
- Wilts, E.M.; Ma, D.; Bai, Y.; Williams, C.B.; Long, T.E. Comparison of Linear and 4-Arm Star Poly(Vinyl Pyrrolidone) for Aqueous Binder Jetting Additive Manufacturing of Personalized Dosage Tablets. ACS Appl. Mater. Interfaces 2019, 11, 23938–23947.
- Huang, S.-J.; Ye, C.-S.; Zhao, H.-P.; Fan, Z.-T. Parameters Optimization of Binder Jetting Process Using Modified Silicate as a Binder. Mater. Manuf. Process. 2020, 35, 214–220.
- Karlsson, D.; Lindwall, G.; Lundbäck, A.; Amnebrink, M.; Boström, M.; Riekehr, L.; Schuisky, M.; Sahlberg, M.; Jansson, U. Binder Jetting of the AlCoCrFeNi Alloy. Addit. Manuf. 2019, 27, 72–79.
- Huang, S.; Ye, C.; Zhao, H.; Fan, Z.; Wei, Q. Binder Jetting Yttria Stabilised Zirconia Ceramic with Inorganic Colloid as a Binder. Adv. Appl. Ceram. 2019, 118, 458–465.
- Cramer, C.L.; Nandwana, P.; Lowden, R.A.; Elliott, A.M. Infiltration Studies of Additive Manufacture of WC with Co Using Binder Jetting and Pressureless Melt Method. Addit. Manuf. 2019, 28, 333–343.
- Lv, X.; Ye, F.; Cheng, L.; Fan, S.; Liu, Y. Binder Jetting of Ceramics: Powders, Binders, Printing Parameters, Equipment, and Post-Treatment. Ceram. Int. 2019, 45, 12609–12624.
- Kimes, K.; Myers, K.; Klein, A.; Ahlfors, M.; Stevens, E.; Chmielus, M. Binder Jet 3D Printing of 316L Stainless Steel: Effects of HIP on Fatigue. Microsc. Microanal. 2019, 25, 2600–2601.
- Farag, M.M.; Yun, H. Effect of Gelatin Addition on Fabrication of Magnesium Phosphate-Based Scaffolds Prepared by Additive Manufacturing System. Mater. Lett. 2014, 132, 111–115.
- Meininger, S.; Mandal, S.; Kumar, A.; Groll, J.; Basu, B.; Gbureck, U. Strength Reliability and in Vitro Degradation of Three-Dimensional Powder Printed Strontium-Substituted Magnesium Phosphate Scaffolds. Acta Biomater. 2016, 31, 401–411.
- Salehi, M.; Maleksaeedi, S.; Nai, S.M.L.; Meenashisundaram, G.K.; Goh, M.H.; Gupta, M. A Paradigm Shift towards Compositionally Zero-Sum Binderless 3D Printing of Magnesium Alloys via Capillary-Mediated Bridging. Acta Mater. 2019, 165, 294–306.
- 3D and 4D Printing in Biomedical Applications: Process Engineering and Additive Manufacturing|Wiley. Available online: https://www.wiley.com/en-us/3D+and+4D+Printing+in+Biomedical+Applications%3A+Process+Engineering+and+Additive+Manufacturing-p-9783527344437 (accessed on 29 March 2022).
- Tsai, K.-Y.; Lin, H.-Y.; Chen, Y.-W.; Lin, C.-Y.; Hsu, T.-T.; Kao, C.-T. Laser Sintered Magnesium-Calcium Silicate/Poly-ε-Caprolactone Scaffold for Bone Tissue Engineering. Materials 2017, 10, 65.
- Yusop, A.H.; Bakir, A.A.; Shaharom, N.A.; Abdul Kadir, M.R.; Hermawan, H. Porous Biodegradable Metals for Hard Tissue Scaffolds: A Review. Int. J. Biomater. 2012, 2012, e641430.
- Lin, W.; Franco, B.E.; Karaman, I.; Elwany, A.; Ma, J. Evolution of Mechanical Behavior of Magnesium Alloy Infiltrated 3D-Printed CoCr Scaffolds under Corrosion in Simulated Body Fluid. Mater. Sci. Eng. C 2019, 105, 109747.
- Kleger, N.; Cihova, M.; Masania, K.; Studart, A.R.; Löffler, J.F. 3D Printing of Salt as a Template for Magnesium with Structured Porosity. Adv. Mater. 2019, 31, 1903783.
- Rau, J.V.; Antoniac, I.; Fosca, M.; De Bonis, A.; Blajan, A.I.; Cotrut, C.; Graziani, V.; Curcio, M.; Cricenti, A.; Niculescu, M.; et al. Glass-Ceramic Coated Mg-Ca Alloys for Biomedical Implant Applications. Mater. Sci. Eng. C 2016, 64, 362–369.
- Rau, J.V.; Antoniac, I.; Cama, G.; Komlev, V.S.; Ravaglioli, A. Bioactive Materials for Bone Tissue Engineering. BioMed Res. Int. 2016, 2016, 3741428.
- Niu, X.; Shen, H.; Fu, J.; Yan, J.; Wang, Y. Corrosion Behaviour of Laser Powder Bed Fused Bulk Pure Magnesium in Hank’s Solution. Corros. Sci. 2019, 157, 284–294.
- He, C.; Bin, S.; Wu, P.; Gao, C.; Feng, P.; Yang, Y.; Liu, L.; Zhou, Y.; Zhao, M.; Yang, S.; et al. Microstructure Evolution and Biodegradation Behavior of Laser Rapid Solidified Mg–Al–Zn Alloy. Metals 2017, 7, 105.
- Shuai, C.; Yang, Y.; Wu, P.; Lin, X.; Liu, Y.; Zhou, Y.; Feng, P.; Liu, X.; Peng, S. Laser Rapid Solidification Improves Corrosion Behavior of Mg-Zn-Zr Alloy. J. Alloys Compd. 2017, 691, 961–969.
- Deng, Y.; Yang, Y.; Gao, C.; Feng, P.; Guo, W.; He, C.; Chen, J.; Shuai, C. Mechanism for Corrosion Protection of β-TCP Reinforced ZK60 via Laser Rapid Solidification. Int. J. Bioprint. 2017, 4, 124.
- Shuai, C.; Yang, Y.; Peng, S.; Gao, C.; Feng, P.; Chen, J.; Liu, Y.; Lin, X.; Yang, S.; Yuan, F. Nd-Induced Honeycomb Structure of Intermetallic Phase Enhances the Corrosion Resistance of Mg Alloys for Bone Implants. J. Mater. Sci. Mater. Med. 2017, 28, 130.
- Sezer, N.; Evis, Z.; Koç, M. Additive Manufacturing of Biodegradable Magnesium Implants and Scaffolds: Review of the Recent Advances and Research Trends. J. Magnes. Alloy. 2021, 9, 392–415.
- Liu, C.; Zhang, M.; Chen, C. Effect of Laser Processing Parameters on Porosity, Microstructure and Mechanical Properties of Porous Mg-Ca Alloys Produced by Laser Additive Manufacturing. Mater. Sci. Eng. A 2017, 703, 359–371.
- Qin, Y.; Wen, P.; Voshage, M.; Chen, Y.; Schückler, P.G.; Jauer, L.; Xia, D.; Guo, H.; Zheng, Y.; Schleifenbaum, J.H. Additive Manufacturing of Biodegradable Zn-XWE43 Porous Scaffolds: Formation Quality, Microstructure and Mechanical Properties. Mater. Des. 2019, 181, 107937.
- Sing, S.L.; Yeong, W.Y. Laser Powder Bed Fusion for Metal Additive Manufacturing: Perspectives on Recent Developments. Virtual Phys. Prototyp. 2020, 15, 359–370.
- Gibson, L.J. Biomechanics of Cellular Solids. J. Biomech. 2005, 38, 377–399.
- Cáceres, C.H.; Rovera, D.M. Solid Solution Strengthening in Concentrated Mg–Al Alloys. J. Light Met. 2001, 1, 151–156.
- Wei, K.; Gao, M.; Wang, Z.; Zeng, X. Effect of Energy Input on Formability, Microstructure and Mechanical Properties of Selective Laser Melted AZ91D Magnesium Alloy. Mater. Sci. Eng. A 2014, 611, 212–222.
- Liu, S.; Yang, W.; Shi, X.; Li, B.; Duan, S.; Guo, H.; Guo, J. Influence of Laser Process Parameters on the Densification, Microstructure, and Mechanical Properties of a Selective Laser Melted AZ61 Magnesium Alloy. J. Alloys Compd. 2019, 808, 151160.
- Teng, H.; Li, T.; Zhang, X.; Zhang, Z. Influence of Sub-Rapid Solidification on Microstructure and Mechanical Properties of AZ61A Magnesium Alloy. Trans. Nonferr. Met. Soc. China 2008, 18, s86–s90.
- Xie, K.; Wang, N.; Guo, Y.; Zhao, S.; Tan, J.; Wang, L.; Li, G.; Wu, J.; Yang, Y.; Xu, W.; et al. Additively Manufactured Biodegradable Porous Magnesium Implants for Elimination of Implant-Related Infections: An in Vitro and in Vivo Study. Bioact. Mater. 2021, 8, 140–152.
- Dong, J.; Tümer, N.; Leeflang, M.A.; Taheri, P.; Fratila-Apachitei, L.E.; Mol, J.M.C.; Zadpoor, A.A.; Zhou, J. Extrusion-Based Additive Manufacturing of Mg-Zn Alloy Scaffolds. J. Magnes. Alloy. 2022, 10, 2491–2509.
- Hyer, H.; Zhou, L.; Benson, G.; McWilliams, B.; Cho, K.; Sohn, Y. Additive Manufacturing of Dense WE43 Mg Alloy by Laser Powder Bed Fusion. Addit. Manuf. 2020, 33, 101123.
- Li, M.; Benn, F.; Derra, T.; Kröger, N.; Zinser, M.; Smeets, R.; Molina-Aldareguia, J.M.; Kopp, A.; LLorca, J. Microstructure, Mechanical Properties, Corrosion Resistance and Cytocompatibility of WE43 Mg Alloy Scaffolds Fabricated by Laser Powder Bed Fusion for Biomedical Applications. Mater. Sci. Eng. C 2021, 119, 111623.
- Wu, C.L.; Zai, W.; Man, H.C. Additive Manufacturing of ZK60 Magnesium Alloy by Selective Laser Melting: Parameter Optimization, Microstructure and Biodegradability. Mater. Today Commun. 2021, 26, 101922.
- Yang, Y.; Lu, C.; Peng, S.; Shen, L.; Wang, D.; Qi, F.; Shuai, C. Laser Additive Manufacturing of Mg-Based Composite with Improved Degradation Behaviour. Virtual Phys. Prototyp. 2020, 15, 278–293.
- Yao, X.; Tang, J.; Zhou, Y.; Atrens, A.; Dargusch, M.S.; Wiese, B.; Ebel, T.; Yan, M. Surface Modification of Biomedical Mg-Ca and Mg-Zn-Ca Alloys Using Selective Laser Melting: Corrosion Behaviour, Microhardness and Biocompatibility. J. Magnes. Alloy. 2021, 9, 2155–2168.
- Xu, R.; Zhao, M.-C.; Zhao, Y.-C.; Liu, L.; Liu, C.; Gao, C.; Shuai, C.; Atrens, A. Improved Biodegradation Resistance by Grain Refinement of Novel Antibacterial ZK30-Cu Alloys Produced via Selective Laser Melting. Mater. Lett. 2019, 237, 253–257.
- Yin, Y.; Huang, Q.; Liang, L.; Hu, X.; Liu, T.; Weng, Y.; Long, T.; Liu, Y.; Li, Q.; Zhou, S.; et al. In Vitro Degradation Behavior and Cytocompatibility of ZK30/Bioactive Glass Composites Fabricated by Selective Laser Melting for Biomedical Applications. J. Alloys Compd. 2019, 785, 38–45.
- Shuai, C.; Liu, L.; Zhao, M.; Feng, P.; Yang, Y.; Guo, W.; Gao, C.; Yuan, F. Microstructure, Biodegradation, Antibacterial and Mechanical Properties of ZK60-Cu Alloys Prepared by Selective Laser Melting Technique. J. Mater. Sci. Technol. 2018, 34, 1944–1952.
- Antoniac, I.; Adam, R.; Biță, A.; Miculescu, M.; Trante, O.; Petrescu, I.M.; Pogărășteanu, M. Comparative Assessment of In Vitro and In Vivo Biodegradation of Mg-1Ca Magnesium Alloys for Orthopedic Applications. Materials 2020, 14, 84.
- Antoniac, I.V.; Antoniac, A.; Vasile, E.; Tecu, C.; Fosca, M.; Yankova, V.G.; Rau, J.V. In Vitro Characterization of Novel Nanostructured Collagen-Hydroxyapatite Composite Scaffolds Doped with Magnesium with Improved Biodegradation Rate for Hard Tissue Regeneration. Bioact. Mater. 2021, 6, 3383–3395.
- Huniadi, A.; Sorian, A.; Iuhas, C.; Bodog, A.; Sandor, M.I. The Effect of Cannabis in the Treatment of Hodgkin’s Lymphoma in a Pregnant Patient—Extensive Case Report and Literature Review. J. BUON 2021, 26, 11–16.
- Fischer, J.; Proefrock, D.; Hort, N.; Willumeit, R.; Feyerabend, F. Reprint of: Improved Cytotoxicity Testing of Magnesium Materials. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2011, 176, 1773–1777.
- Fischer, J.; Prosenc, M.H.; Wolff, M.; Hort, N.; Willumeit, R.; Feyerabend, F. Interference of Magnesium Corrosion with Tetrazolium-Based Cytotoxicity Assays. Acta Biomater. 2010, 6, 1813–1823.
- Bobe, K.; Willbold, E.; Morgenthal, I.; Andersen, O.; Studnitzky, T.; Nellesen, J.; Tillmann, W.; Vogt, C.; Vano, K.; Witte, F. In Vitro and in Vivo Evaluation of Biodegradable, Open-Porous Scaffolds Made of Sintered Magnesium W4 Short Fibres. Acta Biomater. 2013, 9, 8611–8623.
- Domocos, D.; Popovici, R.; Bei, M.; Anchidin, O.; Todor, L.; Bodog, F.D.; Marcu, O.A.; Bodog, A.; Ciavoi, G.; Pogan, M.D. The Effect of Antioxidants on the Evolution of Precancerous Oral Lesions. Int. J. Med. Dent. 2021, 25, 154–158.
- Wu, H.; Xie, X.; Wang, J.; Ke, G.; Huang, H.; Liao, Y.; Kong, Q. Biological Properties of Zn–0.04Mg–2Ag: A New Degradable Zinc Alloy Scaffold for Repairing Large-Scale Bone Defects. J. Mater. Res. Technol. 2021, 13, 1779–1789.
- Wang, W.; Jia, G.; Wang, Q.; Huang, H.; Li, X.; Zeng, H.; Ding, W.; Witte, F.; Zhang, C.; Jia, W.; et al. The in Vitro and in Vivo Biological Effects and Osteogenic Activity of Novel Biodegradable Porous Mg Alloy Scaffolds. Mater. Des. 2020, 189, 108514.
- Zhang, A.; Cheng, T.P.O.; Altura, B.M. Magnesium Regulates Intracellular Free Ionized Calcium Concentration and Cell Geometry in Vascular Smooth Muscle Cells. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 1992, 1134, 25–29.
- Lang, F.; Föller, M.; Lang, K.; Lang, P.; Ritter, M.; Vereninov, A.; Szabo, I.; Huber, S.M.; Gulbins, E. Cell Volume Regulatory Ion Channels in Cell Proliferation and Cell Death. Methods Enzymol. 2007, 428, 209–225.
- Liu, J.; Liu, B.; Min, S.; Yin, B.; Peng, B.; Yu, Z.; Wang, C.; Ma, X.; Wen, P.; Tian, Y.; et al. Biodegradable Magnesium Alloy WE43 Porous Scaffolds Fabricated by Laser Powder Bed Fusion for Orthopedic Applications: Process Optimization, in Vitro and in Vivo Investigation. Bioact. Mater. 2022, 16, 301–319.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
969
Revisions:
5 times
(View History)
Update Date:
05 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




