Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andrea Corsi | -- | 2744 | 2022-12-22 11:37:35 | | | |
| 2 | Amina Yu | + 3 word(s) | 2747 | 2022-12-23 02:06:03 | | | | |
| 3 | Amina Yu | Meta information modification | 2747 | 2022-12-23 02:07:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Corsi, A.; Bombieri, C.; Valenti, M.T.; Romanelli, M.G. Tau Isoforms. Encyclopedia. Available online: https://encyclopedia.pub/entry/39084 (accessed on 07 February 2026).
Corsi A, Bombieri C, Valenti MT, Romanelli MG. Tau Isoforms. Encyclopedia. Available at: https://encyclopedia.pub/entry/39084. Accessed February 07, 2026.
Corsi, Andrea, Cristina Bombieri, Maria Teresa Valenti, Maria Grazia Romanelli. "Tau Isoforms" Encyclopedia, https://encyclopedia.pub/entry/39084 (accessed February 07, 2026).
Corsi, A., Bombieri, C., Valenti, M.T., & Romanelli, M.G. (2022, December 22). Tau Isoforms. In Encyclopedia. https://encyclopedia.pub/entry/39084
Corsi, Andrea, et al. "Tau Isoforms." Encyclopedia. Web. 22 December, 2022.
Copy Citation
Tau was first described as a natively unfolded microtubule-associated protein. In fact, its main function is to promote the assembly of microtubules and stabilize their structure. However, Tau proteins have a variety of other functions, which include maintaining the structural integrity of neurons, contributing to signal transmission between neurons, and axonal transport. Tau also plays a role in regulating myelination, iron homeostasis, and neurogenesis and may also support synaptic plasticity. Other roles attributed to Tau are gene expression regulation, DNA protection, genome stability, microRNA activity, RNA protection, RNA metabolism, and protein synthesis.
Tau
tauopathies
isoform
alternative splicing
microtubule binding domain
1.Introduction
Tau knock-out mice developed deterioration of cardiovascular function, glucose intolerance, pancreatic disorders, anxiety, and impairment of contextual and cued fear memory, implying a wide range of undiscovered functions of Tau [1][2][3]. The regional distribution of mRNA expression and total Tau protein expression levels were largely in agreement, appearing to be highly correlated [4].
Tau is widely distributed in the nervous system. Its main localizations are peripheral nerves and the brain, but this protein has also been found in other organs or tissues, such as the salivary glands, pancreas, breast, kidney, testes, myocardium, and skeletal muscle (https://www.genecards.org, accessed on 2 October 2022), suggesting other functions for Tau that are not limited to the central nervous system (CNS) [5]. The subcellular distribution of Tau is regulated during development, becoming enriched in axons as neurons establish their polarity via mechanisms that may include isoform specificity, local synthesis of Tau in axons, preferential binding of Tau to axonal microtubules, and/or preferential axonal transport of the protein [6].
In the brain, under physiological conditions, Tau is mainly present in the axons of neurons and at low levels in glia (both astrocytes and oligodendrocytes) [7][8], but it has also been detected outside the cells [9]. Tau is continuously secreted both enclosed within extracellular vesicles, such as exosomes or autophagosomes, and using non-vesicle-mediated pathways and direct secretion through the plasma membrane. This could indicate a functional role of extracellular Tau in neuronal activities that has not yet been physiologically clarified. Conversely, under pathological conditions, this mechanism contributes to the spread of altered forms of Tau across different brain areas and thus to the spatio-temporal disease progression in tauopathies [10][11].
2. Functional Domains Characterizing Tau Protein
Tau protein is organized into four main functional domains: the N-terminal region (NTR), the proline-rich region (PRR), the microtubule-binding domain (MTBD), and the C-terminal assembly region [9][12]. The organization of the functional domains of the main Tau isoforms is described in Figure 1.
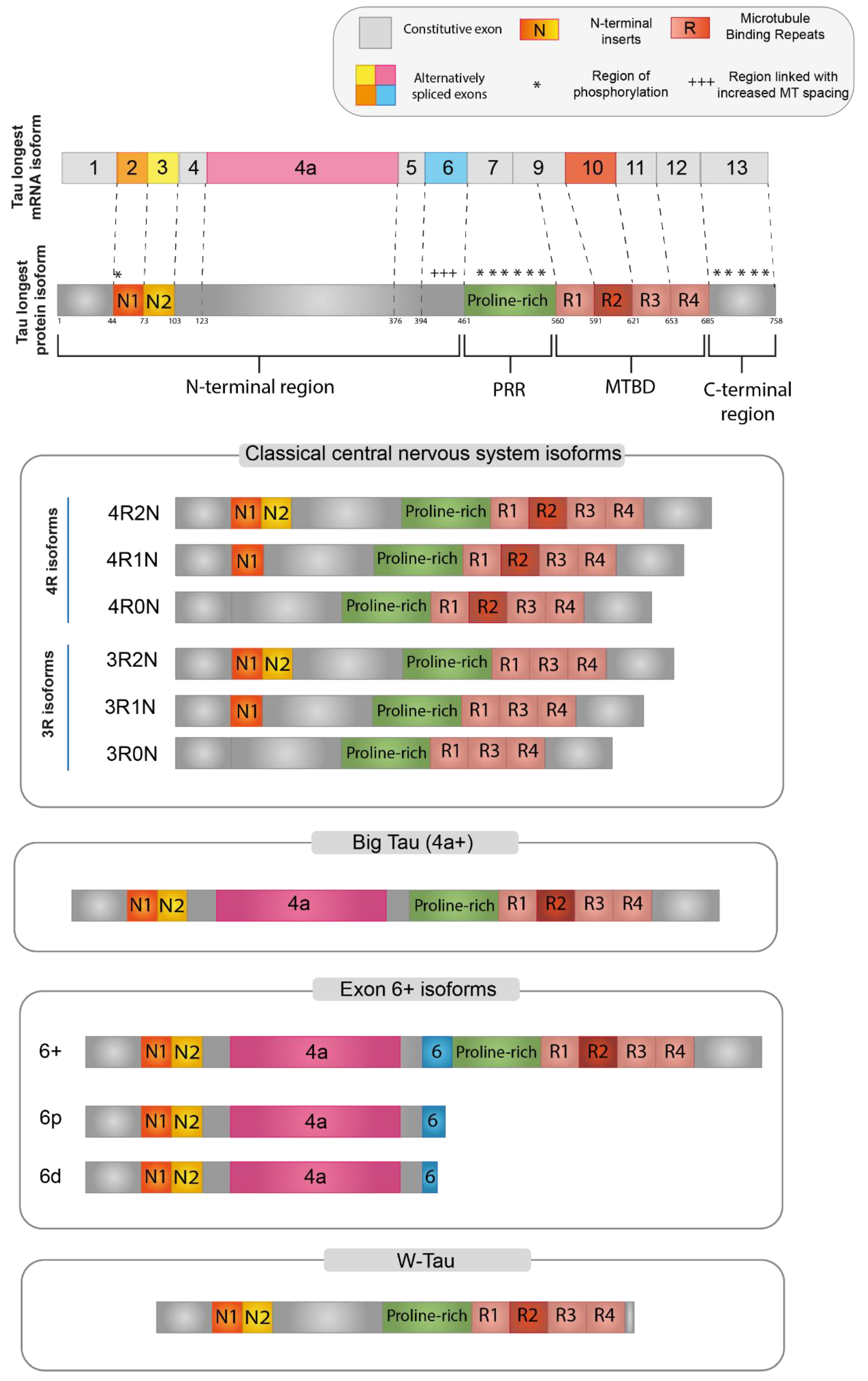
Figure 1. Tau protein isoforms. Schematic representation of the main Tau protein isoforms generated via alternative splicing. At the top, the longest Tau isoform found in humans, containing all the alternatively spliced exons. Red boxes represent the microtubule binding domain (MTBD), while the N-terminal projection domain is represented by exon 2 (orange) and exon 3 (yellow). For Big Tau (4+), Exon 6+ isoforms and W-Tau only the 2N4R isoforms are depicted, but all combinations are possible.
Each different region present in the final protein has been demonstrated to be related to specific functions, while there is a considerable gap in the literature regarding the specific function of each Tau isoform. However, Tau-specific functions can be potentially derived based on the specific domains that are included in each isoform [12][13]. It is important to note that these domains are not functionally independent, but many of their functions overlap and are also dependent on the intramolecular interactions between them [14].
The N-terminal region (NTR) encompasses the beginning of the Tau protein until the first residue of exon 7 (aa 1–150; numbering refers to the 441aa CNS isoform). The structure of this domain is shown in Figure 1, where N1 represents the segment translated by exon 2 and N2 the segment translated by exon 3. NTR is also called “Projection domain”, as it projects away from the microtubule to interact with other cytoskeletal and cytoplasmic proteins or organelles, such as annexins, synaptic vesicle-associated proteins, or mitochondria [14][15][16]. NTR may regulate the subcellular distribution of Tau into axons and may also have a role in signaling and Tau aggregation [6][17][18]. Tau has been demonstrated to bind to DNA and RNA, as well as interact with chromatin and within the inner side of the nuclear lamina. Since the alternatively spliced isoforms including exon 2 are the only ones specifically targeted towards the nucleus, it was suggested that a portion of NTR encoded by exon 2 is responsible for these nuclear interactions of Tau [12]. Furthermore, N-terminally truncated Tau shows an increased association with microtubules. In vitro experiments revealed that two eight amino acid-long motifs within exon 1, which are conserved in mammals, are needed for the interaction with annexins A2 and A6. The lack of these amino acid segments moderately increased the Tau association rate to microtubules, consistent with the supposition that the presence of the Tau-annexin interaction reduces the availability of Tau to interact with microtubules [17]. Finally, when Tau is attached to microtubules, the projection domain regulates the spacing between microtubules in the axon and, therefore, the axonal diameter. A larger diameter reduces the resistance of the axoplasm, helping axonal transport, particularly in peripheral neurons [6][16].
The proline-rich region (PRR) is codified by exon 7 and part of exon 9 (aa 151–243). The high proportion of proline residues (more than 20% higher than the average of human proteins) confers to this region an increased rigidity and basicity [12][14]. This region interacts with many kinases and phosphatases and seems to be involved in the regulation of Tau phosphorylation and in promoting microtubule assembly and stability with MTBD. The PRR is also involved in interactions with proteins containing a Src homology-3 (SH3) domain, playing an important role in protein folding [14][18][19][20]. Gene-ontology analysis revealed that the group of proteins that specifically interact with the PRR showed a remarkable enrichment of proteins involved in cell signaling mechanisms [14].
Regulation of affinity between Tau and microtubules mainly depends on phosphorylation of Tau, which mostly targets the proline-rich region and C-terminus [2][21]. PRR indeed has the highest relative content of serine and threonine, making it Tau’s prime region for phosphorylation. Both the interactions of the NTR and the MTBD can be affected by phosphorylation within the PRR, suggesting that the PRR can act as a signaling module for the functions of both the NTR and the MTBD [14].
The microtubule-binding domain (MTBD) ranges from the rest of exon 9 to exon 12 (aa 244–368). Each exon encodes one of the four highly conserved repeats of 18 aa, each separated by 13 or 14 residues (R1-R4 in Figure 1) [14][16]. The size of the MTBD in different Tau isoforms depends on the alternative splicing of exon 10, which is translated into the second repeat (R2 segment). The domain with the longest size, which contains all four repeats, shows a higher affinity for microtubule binding, in contrast to that lacking an R2 segment [9][16][22].
MTBD is responsible for Tau binding with microtubules through the interaction with microtubular tubulin, thus promoting microtubule assembly and stability [2]. Being positively charged, the MTBD can also easily make electrostatic interactions with the negatively charged C-terminal region, which seems to be essential for the extremely dynamic “kiss-and-hop” interaction (rapid binding and detaching from the microtubule surface, with a mean dwell time of single Tau molecules in the millisecond range) between microtubules and Tau. This mechanism was described for axonal microtubules in cultured neurons and seems to be essential for axonal transport [14][18][23]. Moreover, this region also interacts with other proteins, such as actin or heat shock proteins [24][25]. Due to its interaction with microtubules, Tau is a modulator in a number of cellular processes involving cytoskeletal structure, such as morphogenesis, cellular division, and intracellular trafficking of organelles and vesicles [14][16]. MTBD is also responsible for Tau self-aggregation and polymerization into filaments and neurofibrillary tangles, the typical lesions found in tauopathies [16][26][27].
Several proteins associated with neurodegenerative disorders interact with the MTBD and PRR domains of Tau, suggesting that the structural composition and protein–protein interactions of Tau play an important role in pathological processes [28].
The C-terminal region, translated by exon 13 (aa 369–441) contributes to microtubule binding and interacts with the N-terminus in the paperclip conformation crucial for Tau functions. The Tau molecule shows a preference for changing its global conformations to form a “paperclip” shape, in which the N-terminal, C-terminal, and repeat domains all approach each other [29]. The paperclip conformation is proposed to be a usual conformation of soluble Tau and crucial for Tau physiological functions, to protect Tau from aggregation. Modifications, such as phosphorylation or truncation on either end, can greatly disturb or prevent the formation of this structure and might thereby promote Tau aggregation [9][12].
Alternative splicing events lead to the extension, reduction, or deletion of Tau domains, as described in the following sections.
3. Central Nervous System (CNS) Isoforms
In the adult CNS, six major Tau isoforms of 352–441 aa and 37–46 kDa molecular mass are expressed, deriving from the alternative splicing of exons 2, 3, and 10 (Figure 1) [15][22]. According to the inclusion or exclusion of exon 10, which encodes for the R2 segment, the isoforms are labeled “4R-” or “3R-”, respectively. The inclusion or exclusion of exons 2 and 3, which encode for the N1 and N2 segments, is indicated in the second half of the isoform name as: “0N” (absence of both exons), “1N” (inclusion of exon 2 only), or “2N” (inclusion of both exons 2 and 3) [9].
In the adult human brain, the expression pattern of the MAPT gene is consistent with the production of an equimolar ratio of 4R and 3R CNS isoforms, while 0N, 1N, and 2N isoforms are differently expressed as 37%, 54%, and 9% of total Tau, respectively [9]. The balance between 4R and 3R isoforms is relevant for human brain function, as the splicing dysregulations of exon 10 were associated with tauopathies [22][30]. Differently from humans, adult mouse brain almost exclusively expresses the 4R isoforms, while the 3R isoforms are only transiently expressed in mouse newborn neurons [9]. Human MAPT gene expression is under temporal regulation. The above described landscape with all six CNS isoforms is found in the adult brain, whereas the 3R0N hyperphosphorylated isoform is predominant in the fetal brain, with a marked shift in exons 2 and/or 10 expression in the perinatal period [12][22][30][31].
4. Big-Tau
A high-molecular-weight isoform of about 110 kDa named “Big-Tau” was discovered in the 1990s. This isoform is predominantly expressed in adult PNS and can also be found in the optic nerve, in neurons of the CNS extending their processes into the periphery, and in PC12, a rat cell line derived from neural crest [6]. Big-Tau arises from a MAPT transcript of about 8–9 kb. This mRNA includes a large additional exon (encoding 254 aa in rats and 237 aa in mice) between exons 4 and 5, which was called 4a [32][33], resulting in a protein doubled in size with a great extension of the N-terminal projection domain (from 198 to 510 aa). Moreover, in some of the neural rodent cell lines that have been analyzed, the sequence of Big-Tau also includes exon 6 [6].
Most of the available information about Big-Tau derives from studies on mouse and rat models as well as from cell lines derived from them. Data on Big-Tau isoforms from humans and other species have been derived mostly from genomic analysis based on transcript alignments [6]. Big-Tau expression in rodents begins late in embryonic development and gradually increases postnatally [33][34]. The functional reason for the switch from a low-molecular-weight isoform to Big-Tau in specific neuronal populations is still unknown. Oblinger’s group speculated that Big-Tau can play a role in stabilizing the mature axonal cytoskeleton, while the other smaller isoforms might be more associated with axonal growth [12][34]. Big-Tau isoform is, in fact, mainly expressed in neurons projected to the periphery, which, having long or high-caliber axons, need robust and efficient axonal transport. The inclusion of the additional large amino acid segment, specific to the Big-Tau isoform, may determine a significant alteration of the Tau effect on microtubule spacing [6].
Unlike the rest of the Tau protein, which is highly phosphorylated (>80 sites), the insert specific to Big-Tau contains only two phosphorylated sites. This can result in a lower propensity to form toxic aggregates and fibrils than the low-molecular-weight isoforms. Moreover, there is no evidence of homology of the Big-Tau insert with known proteins or functional domains. Taken together, these considerations led some authors to speculate that the big insert of this isoform could be a functionally inert zone that only provides length to the projection domain of Tau and that the evolutionary origin of the insert was from an event of exonization, in which an intronic sequence from a different protein became a de novo exon [6].
5. Isoforms including Exon 6
Tau isoforms, including exon 6, are preferentially expressed in peripheral tissues, such as skeletal muscle and the spinal cord, but they can also be found at a minor level in some regions of the adult brain and in the fetal brain [35]. Three possible isoforms, 6c, 6p, and 6d, can be generated through the alternative of a different 3′ splice site for E6. Isoform 6c includes the whole sequence of the exon 6 and expands the PRR, increasing the area susceptible to phosphorylation and therefore the possibility of Tau function regulation, albeit a clear consensus on exon 6 function in Tau protein is still missing [15][36][37].
The use of two other alternative 3′ splice sites, 6p and 6d (proximal and distal to the beginning of exon 6, respectively), determines a frameshift that introduces a premature stop codon, thus originating truncated forms of Tau protein represented only by the N-terminal region [35][36][38].
The biological function of these short-Tau isoforms remained completely elusive until recent times [12]. In 2006, Leroy and colleagues identified a brain-specific decrease of the 6c Tau isoform and an increase of the 6d isoform in patients bearing type 1 myotonic dystrophy and described a similar dysregulation of the 6c isoforms in in vitro differentiating neurons treated with retinoic acid [39]. Moreover, Lapointe and colleagues demonstrated that short truncated Tau isoforms (2N6P and 2N6D) were able to block the full-length Tau polymerization in vitro [36]. As fatty acids have been proven to induce Tau aggregate formation [40], the authors used arachidonic acid to induce protein aggregation of various Tau isoforms and demonstrated that not only were these isoforms less prone to form aggregates, but they also inhibited the aggregation of the normal-length Tau protein [36]. The lack of MBTD, which is needed for self-aggregation, can explain why 6p and 6d isoforms do not aggregate and possess anti-aggregative properties [36][41]. The expression pattern of exon 6 is spatially and temporally regulated. The 6p isoforms are the predominant ones, with similar levels in fetal and adult brains, while the 6d level is higher in the fetal brain. Both 6p and 6d can be found in different CNS areas, with the highest levels in the spinal cord and cerebellum [4][36][38]. Particularly, in the cerebellum, the 6d isoforms show levels comparable to those of the full-length isoforms, but in this anatomic region, neurofibrillary tangles are absent, even in patients with neurodegenerative disorders [12][36].
6. W-Tau
A novel Tau isoform was recently described by Garcia-Escudero et al. [41]. This isoform derives from the TIR-MAPT transcript, which is generated by the retention of intron 12. The TIR-MAPT translation gives rise to a protein with a unique 18-aa sequence, close to the MTBD, and lacking the C-terminal region due to the presence of a premature stop codon in the retained intron. The name “W-Tau” was proposed to indicate the presence of two tryptophan residues, an amino acid that is not present in any other known Tau molecule. This 18-aa sequence specific to W-Tau has also been proposed to trigger a different Tau conformation that elicits the paper-clip state [41].
While other Tau isoforms show a high degree of interspecies homology, W-Tau is human-specific as it contains a sequence translated from an intronic region, which is usually not phylogenetically conserved [12]. The W-Tau expression differs based on brain areas and in the presence of pathological conditions; diminished levels of this new isoform have been found in the brains of Alzheimer’s patients, suggesting a possible role in the pathology. This new Tau isoform exhibits similar post-transcriptional modifications by phosphorylation and affinity for microtubule binding compared to the main Tau isoforms, but more interestingly, it is less prone to aggregate than other Tau isoforms [41]. The reasons behind this decreased aggregation capacity but conservation of microtubule assembly capacity remain unclear and purely speculative. It was suggested that the particular W-Tau domain composition might explain this different behavior. The unique 18-aa extra-peptide includes the sequence GVGWVG, which could be similar in nature to that of some recently described inhibitors of Tau and amyloid β aggregation. In addition, the lack of the C-terminal region implies the loss of a 12-aa sequence after the R4 segment of the MTBD (exon 13), which is found in the core of Tau filaments isolated from the brain of patients affected by some tauopathies, including AD [41].
References
- Gonçalves, R.A.; Wijesekara, N.; Fraser, P.E.; De Felice, F.G. Behavioral Abnormalities in Knockout and Humanized Tau Mice. Front. Endocrinol. 2020, 11, 124.
- Zhang, Y.; Wu, K.-M.; Yang, L.; Dong, Q.; Yu, J.-T. Tauopathies: New Perspectives and Challenges. Mol. Neurodegener. 2022, 17, 28.
- Betrie, A.H.; Ayton, S.; Bush, A.I.; Angus, J.A.; Lei, P.; Wright, C.E. Evidence of a Cardiovascular Function for Microtubule-Associated Protein Tau. J. Alzheimers Dis. JAD 2017, 56, 849–860.
- Trabzuni, D.; Wray, S.; Vandrovcova, J.; Ramasamy, A.; Walker, R.; Smith, C.; Luk, C.; Gibbs, J.R.; Dillman, A.; Hernandez, D.G.; et al. MAPT Expression and Splicing Is Differentially Regulated by Brain Region: Relation to Genotype and Implication for Tauopathies. Hum. Mol. Genet. 2012, 21, 4094–4103.
- Liang, S.-Y.; Wang, Z.-T.; Tan, L.; Yu, J.-T. Tau Toxicity in Neurodegeneration. Mol. Neurobiol. 2022, 59, 3617–3634.
- Fischer, I.; Baas, P.W. Resurrecting the Mysteries of Big Tau. Trends Neurosci. 2020, 43, 493–504.
- Gentile, A.; Mori, F.; Bernardini, S.; Centonze, D. Role of Amyloid-β CSF Levels in Cognitive Deficit in MS. Clin. Chim. Acta Int. J. Clin. Chem. 2015, 449, 23–30.
- LoPresti, P.; Szuchet, S.; Papasozomenos, S.C.; Zinkowski, R.P.; Binder, L.I. Functional Implications for the Microtubule-Associated Protein Tau: Localization in Oligodendrocytes. Proc. Natl. Acad. Sci. USA 1995, 92, 10369–10373.
- Wang, Y.; Mandelkow, E. Tau in Physiology and Pathology. Nat. Rev. Neurosci. 2016, 17, 5–21.
- Brunello, C.A.; Merezhko, M.; Uronen, R.-L.; Huttunen, H.J. Mechanisms of Secretion and Spreading of Pathological Tau Protein. Cell. Mol. Life Sci. CMLS 2020, 77, 1721–1744.
- Annadurai, N.; De Sanctis, J.B.; Hajdúch, M.; Das, V. Tau Secretion and Propagation: Perspectives for Potential Preventive Interventions in Alzheimer’s Disease and Other Tauopathies. Exp. Neurol. 2021, 343, 113756.
- Ruiz-Gabarre, D.; Carnero-Espejo, A.; Ávila, J.; García-Escudero, V. What’s in a Gene? The Outstanding Diversity of MAPT. Cells 2022, 11, 840.
- Miguel, L.; Rovelet-Lecrux, A.; Feyeux, M.; Frebourg, T.; Nassoy, P.; Campion, D.; Lecourtois, M. Detection of All Adult Tau Isoforms in a 3D Culture Model of IPSC-Derived Neurons. Stem Cell Res. 2019, 40, 101541.
- Brandt, R.; Trushina, N.I.; Bakota, L. Much More Than a Cytoskeletal Protein: Physiological and Pathological Functions of the Non-Microtubule Binding Region of Tau. Front. Neurol. 2020, 11, 590059.
- Andreadis, A. Tau Gene Alternative Splicing: Expression Patterns, Regulation and Modulation of Function in Normal Brain and Neurodegenerative Diseases. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2005, 1739, 91–103.
- Kolarova, M.; García-Sierra, F.; Bartos, A.; Ricny, J.; Ripova, D. Structure and Pathology of Tau Protein in Alzheimer Disease. Int. J. Alzheimers Dis. 2012, 2012, 731526.
- Gauthier-Kemper, A.; Suárez Alonso, M.; Sündermann, F.; Niewidok, B.; Fernandez, M.-P.; Bakota, L.; Heinisch, J.J.; Brandt, R. Annexins A2 and A6 Interact with the Extreme N Terminus of Tau and Thereby Contribute to Tau’s Axonal Localization. J. Biol. Chem. 2018, 293, 8065–8076.
- da Costa, P.J.; Hamdane, M.; Buée, L.; Martin, F. Tau MRNA Metabolism in Neurodegenerative Diseases: A Tangle Journey. Biomedicines 2022, 10, 241.
- Sibille, N.; Huvent, I.; Fauquant, C.; Verdegem, D.; Amniai, L.; Leroy, A.; Wieruszeski, J.-M.; Lippens, G.; Landrieu, I. Structural Characterization by Nuclear Magnetic Resonance of the Impact of Phosphorylation in the Proline-Rich Region of the Disordered Tau Protein. Proteins 2012, 80, 454–462.
- He, H.J.; Wang, X.S.; Pan, R.; Wang, D.L.; Liu, M.N.; He, R.Q. The Proline-Rich Domain of Tau Plays a Role in Interactions with Actin. BMC Cell Biol. 2009, 10, 81.
- Xia, Y.; Prokop, S.; Gorion, K.-M.M.; Kim, J.D.; Sorrentino, Z.A.; Bell, B.M.; Manaois, A.N.; Chakrabarty, P.; Davies, P.; Giasson, B.I. Tau Ser208 Phosphorylation Promotes Aggregation and Reveals Neuropathologic Diversity in Alzheimer’s Disease and Other Tauopathies. Acta Neuropathol. Commun. 2020, 8, 88.
- Goedert, M. Tau Filaments in Neurodegenerative Diseases. FEBS Lett. 2018, 592, 2383–2391.
- Janning, D.; Igaev, M.; Sündermann, F.; Brühmann, J.; Beutel, O.; Heinisch, J.J.; Bakota, L.; Piehler, J.; Junge, W.; Brandt, R. Single-Molecule Tracking of Tau Reveals Fast Kiss-and-Hop Interaction with Microtubules in Living Neurons. Mol. Biol. Cell 2014, 25, 3541–3551.
- Trushina, N.I.; Bakota, L.; Mulkidjanian, A.Y.; Brandt, R. The Evolution of Tau Phosphorylation and Interactions. Front. Aging Neurosci. 2019, 11, 256.
- Gu, J.-L.; Liu, F. Tau in Alzheimer’s Disease: Pathological Alterations and an Attractive Therapeutic Target. Curr. Med. Sci. 2020, 40, 1009–1021.
- Wegmann, S.; Biernat, J.; Mandelkow, E. A Current View on Tau Protein Phosphorylation in Alzheimer’s Disease. Curr. Opin. Neurobiol. 2021, 69, 131–138.
- Drummond, E.; Wisniewski, T. Alzheimer’s Disease: Experimental Models and Reality. Acta Neuropathol. 2017, 133, 155–175.
- Goedert, M.; Spillantini, M.G. Propagation of Tau Aggregates. Mol. Brain 2017, 10, 18.
- Jeganathan, S.; von Bergen, M.; Brutlach, H.; Steinhoff, H.-J.; Mandelkow, E. Global Hairpin Folding of Tau in Solution. Biochemistry 2006, 45, 2283–2293.
- Hefti, M.M.; Farrell, K.; Kim, S.; Bowles, K.R.; Fowkes, M.E.; Raj, T.; Crary, J.F. High-Resolution Temporal and Regional Mapping of MAPT Expression and Splicing in Human Brain Development. PLoS ONE 2018, 13, e0195771.
- Qian, W.; Liu, F. Regulation of Alternative Splicing of Tau Exon 10. Neurosci. Bull. 2014, 30, 367–377.
- Couchie, D.; Shelanski, M.L.; Nunez, J. Primary Structure of High Molecular Weight Tau Present in the Peripheral Nervous System. Proc. Natl. Acad. Sci. USA 1992, 89, 4378–4381.
- Goedert, M.; Spillantini, M.G.; Crowther, R.A. Cloning of a Big Tau Microtubule-Associated Protein Characteristic of the Peripheral Nervous System. Proc. Natl. Acad. Sci. USA 1992, 89, 1983–1987.
- Oblinger, M.M.; Argasinski, A.; Wong, J.; Kosik, K.S. Tau Gene Expression in Rat Sensory Neurons during Development and Regeneration. J. Neurosci. Off. J. Soc. Neurosci. 1991, 11, 2453–2459.
- Wei, M.-L.; Andreadis, A. Splicing of a Regulated Exon Reveals Additional Complexity in the Axonal Microtubule-Associated Protein Tau. J. Neurochem. 1998, 70, 11.
- LaPointe, N.E.; Horowitz, P.M.; Guillozet-Bongaarts, A.L.; Silva, A.; Andreadis, A.; Binder, L.I. Tau 6D and 6P Isoforms Inhibit Polymerization of Full-Length Tau In Vitro. Biochemistry 2009, 48, 12290–12297.
- Luo, M.; Leski, M.L.; Andreadis, A. Tau Isoforms Which Contain the Domain Encoded by Exon 6 and Their Role in Neurite Elongation. J. Cell. Biochem. 2004, 91, 880–895.
- Wei, M.-L.; Memmott, J.; Screaton, G.; Andreadis, A. The Splicing Determinants of a Regulated Exon in the Axonal MAP Tau Reside within the Exon and in Its Upstream Intron. Mol. Brain Res. 2000, 80, 207–218.
- Leroy, O.; Wang, J.; Maurage, C.-A.; Parent, M.; Cooper, T.; Buée, L.; Sergeant, N.; Andreadis, A.; Caillet-Boudin, M.-L. Brain-Specific Change in Alternative Splicing of Tau Exon 6 in Myotonic Dystrophy Type 1. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2006, 1762, 460–467.
- Wilson, D.M.; Binder, L.I. Free Fatty Acids Stimulate the Polymerization of Tau and Amyloid Beta Peptides. In Vitro Evidence for a Common Effector of Pathogenesis in Alzheimer’s Disease. Am. J. Pathol. 1997, 150, 2181–2195.
- García-Escudero, V.; Ruiz-Gabarre, D.; Gargini, R.; Pérez, M.; García, E.; Cuadros, R.; Hernández, I.H.; Cabrera, J.R.; García-Escudero, R.; Lucas, J.J.; et al. A New Non-Aggregative Splicing Isoform of Human Tau Is Decreased in Alzheimer’s Disease. Acta Neuropathol. 2021, 142, 159–177.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Revisions:
3 times
(View History)
Update Date:
27 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




