Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ivan Aprahamian | -- | 1392 | 2022-12-16 02:17:33 | | | |
| 2 | Sirius Huang | Meta information modification | 1392 | 2022-12-16 08:10:50 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Costa, A.C.; Joaquim, H.P.G.; Pedrazzi, J.F.C.; Pain, A.D.O.; Duque, G.; Aprahamian, I. Cannabinoid Systems and the Brain. Encyclopedia. Available online: https://encyclopedia.pub/entry/38853 (accessed on 07 February 2026).
Costa AC, Joaquim HPG, Pedrazzi JFC, Pain ADO, Duque G, Aprahamian I. Cannabinoid Systems and the Brain. Encyclopedia. Available at: https://encyclopedia.pub/entry/38853. Accessed February 07, 2026.
Costa, Alana C., Helena P. G. Joaquim, João F. C. Pedrazzi, Andreia De O. Pain, Gustavo Duque, Ivan Aprahamian. "Cannabinoid Systems and the Brain" Encyclopedia, https://encyclopedia.pub/entry/38853 (accessed February 07, 2026).
Costa, A.C., Joaquim, H.P.G., Pedrazzi, J.F.C., Pain, A.D.O., Duque, G., & Aprahamian, I. (2022, December 16). Cannabinoid Systems and the Brain. In Encyclopedia. https://encyclopedia.pub/entry/38853
Costa, Alana C., et al. "Cannabinoid Systems and the Brain." Encyclopedia. Web. 16 December, 2022.
Copy Citation
The use of cannabinoids as therapeutic drugs has increased among aging populations. Age-related changes in the endogenous cannabinoid system could influence the effects of therapies that target the cannabinoid system. At the preclinical level, cannabidiol (CBD) induces anti-amyloidogenic, antioxidative, anti-apoptotic, anti-inflammatory, and neuroprotective effects. These findings suggest a potential therapeutic role of cannabinoids to neurodegenerative disorders such as Parkinson’s disease (PD) and Alzheimer.
cannabis
cannabinoids
THC
CBD
neurological disorders
elderly
1. Introduction
A better understanding of age-related changes in CB1 receptor expression and function and the subsequent changes in behavioral effects of cannabinoid agonists may impact the use of cannabinoids in aging populations. There is increasing interest in the therapeutic use of cannabinoids such as cannabidiol (CBD), synthetic tetrahydrocannabinol (THC) and Cannabis extract, among the aged for various indications including pain, inflammation and multiple sclerosis [1][2][3].
Research about cannabis compounds use among older adults is increasing. Health conditions commonly researched concerning cannabis use among older adults include pain management [4], sleep assistance [5], appetite stimulation [6], and managing behaviors of dementia such as agitation [7]. Data from a survey of 568 volunteers (>years) showed that, for the ones who started using cannabis later in life, it was closely connected to medicinal purpose for issues such as pain management, sleep improvement, and to address anxiety and depression symptoms [8]. Interestingly, cannabis has been employed to replace both prescribed or over-the-counter medications [9][10].
Those data corroborate with research that explored beliefs toward cannabis use. Sixty percent of the older adults surveyed, strongly agreed that the use of medical cannabis was acceptable [11], but he favorability of cannabis decreased as age increased. Notwithstanding, most of older adults consider recreational cannabis as risky and a potential gateway drug [11]. In addition, another study showed that older adults who use cannabis medically or recreationally recognize that there is still a stigma attached to cannabis use regardless of its legality [12]. On the other hand, older adults are less worried about the potential perceived risk of using cannabinoids. Between 2015 and 2019, older Americans showed an 18.8% relative decrease in the perceived risk [13].
2. Cannabinoid Systems and the Brain
The endocannabinoid system (ECS) is the most widespread endogenous signaling neurotransmitter system in the brain [14][15][16][17][18][19][20]. This system can regulate feeding behavior, memory, anxiety, and stress response [21][22][23].
The discovery of the ECS is relatively recent. From experiments carried out with molecules isolated from the plant, it was observed that delta-9-tetrahydrocannabinol (Δ9-THC), through its connection with CB1 receptors, is responsible for the neuropsychological and psychopathological effects [15]. These findings triggered countless other studies that allowed the cloning of the cannabinoid receptor 2 (CB2) receptor [16] and the identification of endogenous molecules that compose the system [17]. The ECS consists mainly of cannabinoid receptors CB1 and CB2; endogenous ligands anandamide (AEA) and 2-arachidonoylglycerol (2-AG); synthesis enzymes such as N-acyl phosphatidylethanolamine (NAPE) and diacylglycerol lipases (DAGL) and degradation or reuptake enzymes as fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL) [24][25][26][27].
AEA and 2-AG are both endocannabinoids synthesized on demand from arachidonic acid [19]. Once released in the extracellular space, endocannabinoids act near the synthesis as retrograde synaptic messengers at presynaptic receptors [14]. AEA acts as a partial agonist at CB1 and CB2 [28], but also works on selective cation channels. Transient receptor potential cation channel subfamily V member (TRPV1), a key element in inflammatory conditions and pain [29]. AEA has a notable role in several physiological and neurobehavioral processes, such as pain perception [30], emotional behavior [31] and energy metabolism [32]. 2-AG is the most abundant endocannabinoid in the brain and is considered a full agonist of CB1 and CB2 [33]. It has been implicated in numerous physiological processes [34], including several forms of neuroplasticity [35] and its generation and degradation is part of the lipid metabolism [14].
AEA and 2-AG are the most studied and investigated endogenous ligands. These compounds, unlike classical neurotransmitters, are not synthesized at presynaptic terminals or stored in vesicles but are formed based on demand at postsynaptic terminals. AEA and 2-AG act on presynaptic CB1 or CB2 receptors, inhibiting neurotransmitter release. Because the ECS is widely present in the central nervous system, it plays an essential role in the neurobiology of neurodegenerative diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD). Several approaches, whether in vitro assays, animal models, and clinical studies, suggest that ECS modulation can reduce proteins involved in AD pathophysiology, such as tau and beta-amyloid [36] and alpha-synuclein form Lewy bodies in PD. The increased reactivity of microglia and astrocytes, as well as the pro-inflammatory [37] markers TNF-α, IL-17, IFN-γ, iNOS, IL-1β, and NF-κB, are factors implicated in these diseases, where ECS modulation can be a critical pharmacological and molecular target. Furthermore, endocannabinoid modulation can prevent mitochondrial damage, facilitate homeostasis, and decrease excitotoxicity, as well as reactive oxygen species (ROS), culminating in restoring memory and cognitive function, prevalent in the diseases mentioned earlier [38][39][40]. As seen in the image (Figure 1), adequate functioning of the ECS can be an essential tool in the homeostasis of inflammatory responses, in glial reactivity, in the proper functioning of mitochondrial complexes, and the control of the expression of proteins implicated in the pathophysiology of AD (Table 1) and PD. Furthermore, this system and its complex machinery have also participated in synaptic plasticity and neurogenesis events [39]. Both CBD and THC have potential targets for therapeutic effects on neurodegenerative diseases, since they can modulate ECS. CBD acts as an agonist of the receptors TRPV1, PPARγ, and mAChR and as an antagonist of the receptor GPR55 [41][42]. This compound is suggested to act as a negative allosteric modulator of CB1 and CB2 receptors [43]. Finally, CBD inhibits the enzyme FAAH, with a consequent increase in AEA levels. Moreover, AEA can activate CB1, CB2, and TRPV1 receptors (Table 2). CBD is relevant for treating neurodegenerative diseases since it can increase the activity of mitochondrial complexes and shows antioxidant and anti-inflammatory effects that are partially mediated by its actions on TRPV1, mitochondria, and PPARγ. On the other hand, THC is a partial agonist of CB1 and CB2 receptors, an agonist of GPR55 and PPARγ, which, just like CBD, can exert anti-inflammatory effects. Figure 1 summarizes the effects of cannabinoids in dementias.
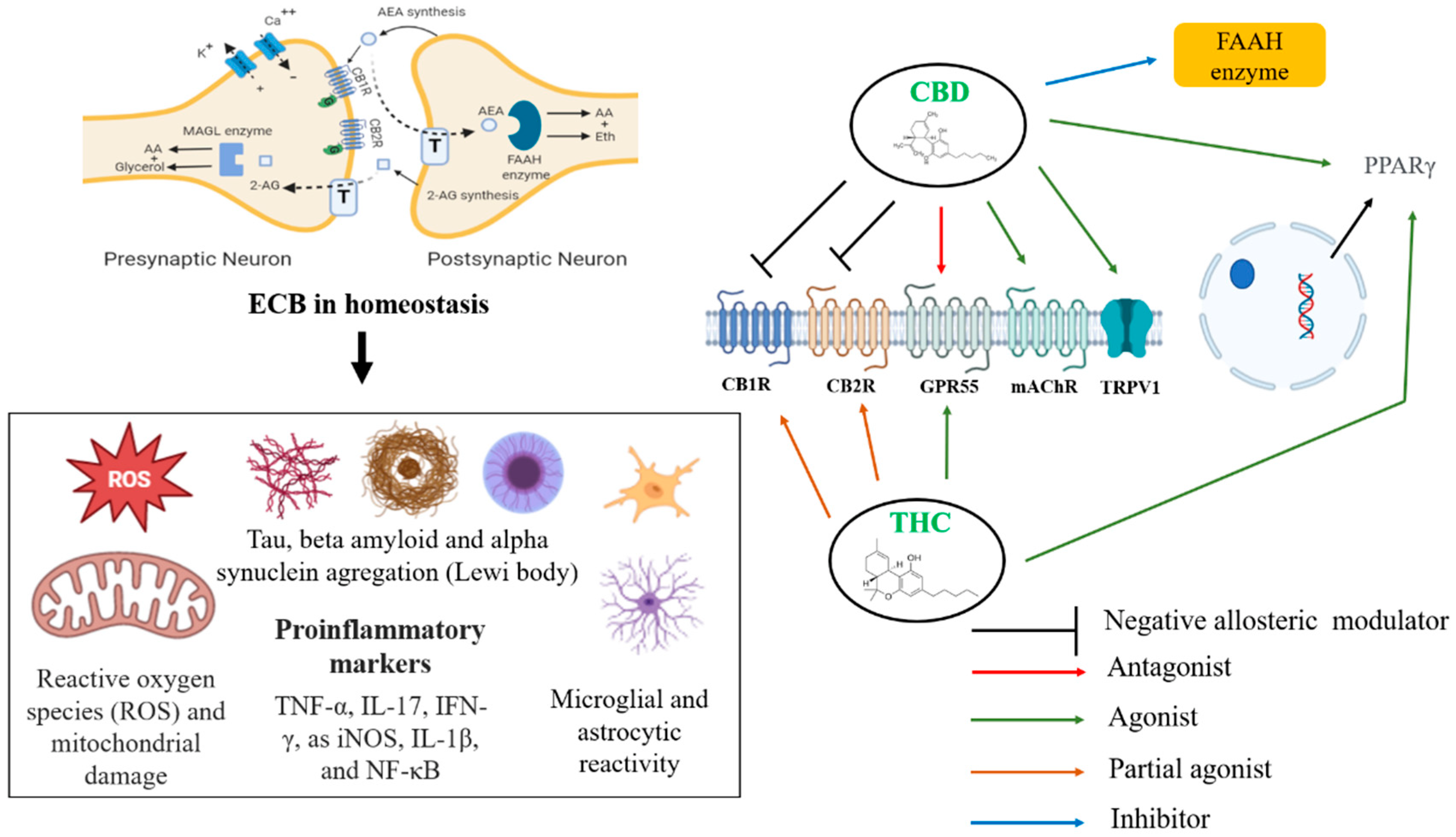
Figure 1. Potential targets and therapeutic effect of CBD in dementias. Legend: TRPV1, transient receptor potential vanilloid type 1; PPARγ, peroxisome proliferator-activated receptor gamma; GPR55, G-protein-coupled receptor 55; CB1, cannabinoid receptor type 1; CB2, cannabinoid receptor type 2; FAAH, fatty acid amide hydrolase; mAChR, muscarinic acetylcholine receptor; 2-AG, 2-arachidonoylglycerol; AEA, anandamide; T, transporter.
Table 1. Potential targets and therapeutic effect of CBD in dementias.
| Receptors | Action | Pharmacology Propriety |
|---|---|---|
| CB1 | Direct antagonist and negative allosteric modulation antagonist | Attenuation of learning deficit, memory, and psychotic effects of THC |
| CB2 | Antagonist & reverse agonist | Anti-inflammatory |
| GPR55 | Antagonist | Antitumor |
| 5HT1A | Agonist | Analgesia and anxiolytic |
| mAChR | Agonist | Cognition improvement |
| TRPV1 | Agonist | Anti-inflammatory and analgesia |
| PPARγ | Agonist | Antioxidant and anti-inflammatory |
Legend: CB1 Cannabinoid receptor type 1; CB2, cannabinoid receptor type 2; GPR55, G-protein-coupled receptor 55; 5HT1A Serotonin 1A receptor; mAChR, muscarinic acetylcholine receptor; TRPV1, transient receptor potential vanilloid type 1; PPARγ, peroxisome proliferator-activated receptor gamma.
Table 2. Practical management with cannabinoids in Parkinson’s and Alzheimer’s disease.
| Neuropsychiatric Disorder | Potential (Off Label) Indication | Suggested Dose Regimen |
|---|---|---|
| Parkinson’s disease | Resistant tremor or dyskinesia | Starting dose: CBD (<0.3% THC) 5 mg once daily. Increase 5 mg every 3 days. Maximum dose: 20 mg twice a week. |
| Resistant anxiety | Starting dose: CBD (<0.3% THC) 5 mg once daily. Increase 5 mg every 3 days. May split the dose in two or three intakes. Maximum dose: 90 mg twice a week (CBD monotherapy). 1 mg of THC can be initiated with CBD or after 20 mg of CBD without a positive effect. Increase THC to a maximum of 20 mg combined to a maximum of 40 mg of CBD. |
|
| Agitation due psychosis partially treated with quetiapine or clozapine | ||
| Persisted sleeping disturbance albeit treated with two first-line treatment | Starting dose: CBD (<0.3% THC) 5 mg at night. Increase 5 mg every 3 days. Maximum dose: 20 mg |
|
| Alzheimer’s disease | Persisting agitation or aggression besides non-pharmacologic and first-line drug treatment implemented | Starting dose: CBD (<0.3% THC) 5 mg once daily. Increase 5 mg every 3 days. May split the dose in two or three intakes. Maximum dose: 20 mg twice a week. 1 mg of THC can be initiated with CBD or after 20 mg of CBD without a positive effect. Increase THC to a maximum of 20 mg |
| Major adverse event with first-line drug treatment for agitation, anxiety, or aggression | ||
| Persisting anorexia albeit traditional treatment for dementia and exclusion of secondary causes | Starting dose: CBD (<0.3% THC) 5 mg once daily. Increase 5 mg every 3 days. Maximum dose: 10 mg twice daily |
Note: CBD = cannabidiol; THC = delta-9-tetrahydrocannabinol.
References
- Minerbi, A.; Häuser, W.; Fitzcharles, M.-A. Medical Cannabis for Older Patients. Drugs Aging 2019, 36, 39–51.
- Alessandria, G.; Meli, R.; Infante, M.T.; Vestito, L.; Capello, E.; Bandini, F. Long-Term Assessment of the Cognitive Effects of Nabiximols in Patients with Multiple Sclerosis: A Pilot Study. Clin. Neurol. Neurosurg. 2020, 196, 105990.
- Ueberall, M.A.; Essner, U.; Vila Silván, C.; Mueller-Schwefe, G.H.H. Comparison of the Effectiveness and Tolerability of Nabiximols (THC:CBD) Oromucosal Spray versus Oral Dronabinol (THC) as Add-on Treatment for Severe Neuropathic Pain in Real-World Clinical Practice: Retrospective Analysis of the German Pain e-Registry. J. Pain Res. 2022, 15, 267–286.
- Abuhasira, R.; Schleider, L.B.-L.; Mechoulam, R.; Novack, V. Epidemiological Characteristics, Safety and Efficacy of Medical Cannabis in the Elderly. Eur. J. Intern. Med. 2018, 49, 44–50.
- Bachhuber, M.; Arnsten, J.H.; Wurm, G. Use of Cannabis to Relieve Pain and Promote Sleep by Customers at an Adult Use Dispensary. J. Psychoact. Drugs 2019, 51, 400–404.
- Han, B.H.; Sherman, S.; Mauro, P.M.; Martins, S.S.; Rotenberg, J.; Palamar, J.J. Demographic Trends among Older Cannabis Users in the United States, 2006–2013. Addiction 2017, 112, 516–525.
- Stella, F.; Valiengo, L.C.L.; de Paula, V.J.R.; Lima, C.A.d.M.; Forlenza, O.V. Medical Cannabinoids for Treatment of Neuropsychiatric Symptoms in Dementia: A Systematic Review. Trends Psychiatry Psychother. 2021, 43, 243–255.
- Yang, K.H.; Kaufmann, C.N.; Nafsu, R.; Lifset, E.T.; Nguyen, K.; Sexton, M.; Han, B.H.; Kim, A.; Moore, A.A. Cannabis: An Emerging Treatment for Common Symptoms in Older Adults. J. Am. Geriatr. Soc. 2021, 69, 91–97.
- Baumbusch, J.; Sloan Yip, I. Exploring New Use of Cannabis among Older Adults. Clin. Gerontol. 2021, 44, 25–31.
- Manning, L.; Bouchard, L. Medical Cannabis Use: Exploring the Perceptions and Experiences of Older Adults with Chronic Conditions. Clin. Gerontol. 2021, 44, 32–41.
- Arora, K.; Qualls, S.H.; Bobitt, J.; Lum, H.D.; Milavetz, G.; Croker, J.; Kaskie, B. Measuring Attitudes toward Medical and Recreational Cannabis among Older Adults in Colorado. Gerontologist 2020, 60, e232–e241.
- Bobitt, J.; Qualls, S.H.; Schuchman, M.; Wickersham, R.; Lum, H.D.; Arora, K.; Milavetz, G.; Kaskie, B. Qualitative Analysis of Cannabis Use Among Older Adults in Colorado. Drugs Aging 2019, 36, 655–666.
- Han, B.H.; Funk-White, M.; Ko, R.; Al-Rousan, T.; Palamar, J.J. Decreasing Perceived Risk Associated with Regular Cannabis Use among Older Adults in the United States from 2015 to 2019. J. Am. Geriatr. Soc. 2021, 69, 2591–2597.
- Ahn, K.; McKinney, M.K.; Cravatt, B.F. Enzymatic Pathways That Regulate Endocannabinoid Signaling in the Nervous System. Chem. Rev. 2008, 108, 1687–1707.
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a Cannabinoid Receptor and Functional Expression of the Cloned CDNA. Nature 1990, 346, 561–564.
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular Characterization of a Peripheral Receptor for Cannabinoids. Nature 1993, 365, 61–65.
- Silver, R.J. The Endocannabinoid System of Animals. Animals 2019, 9, 686.
- Wolf, S.A.; Ullrich, O. Endocannabinoids and the Brain Immune System: New Neurones at the Horizon? J. Neuroendocrinol. 2008, 20 (Suppl. 1), 15–19.
- Katona, I.; Freund, T.F. Multiple Functions of Endocannabinoid Signaling in the Brain. Annu. Rev. Neurosci. 2012, 35, 529–558.
- Joshi, N.; Onaivi, E.S. Endocannabinoid System Components: Overview and Tissue Distribution. In Recent Advances in Cannabinoid Physiology and Pathology; Springer: Cham, Switzerland, 2019; Volume 1162, pp. 1–12.
- Di Marzo, V.; Melck, D.; Bisogno, T.; De Petrocellis, L. Endocannabinoids: Endogenous Cannabinoid Receptor Ligands with Neuromodulatory Action. Trends Neurosci. 1998, 21, 521–528.
- Matias, I.; Bisogno, T.; Di Marzo, V. Endogenous Cannabinoids in the Brain and Peripheral Tissues: Regulation of Their Levels and Control of Food Intake. Int. J. Obes. 2006, 30 (Suppl. 1), S7–S12.
- Ruehle, S.; Rey, A.A.; Remmers, F.; Lutz, B. The Endocannabinoid System in Anxiety, Fear Memory and Habituation. J. Psychopharmacol. 2012, 26, 23–39.
- Van Egmond, N.; Straub, V.M.; van der Stelt, M. Targeting Endocannabinoid Signaling: FAAH and MAG Lipase Inhibitors. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 441–463.
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis Sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19.
- O’brien, C.P. Endocannabinoids: The Brain and Body’s Marijuana and Beyond-Preface; Onaivi, E.S., Sugiura, T., Di Marzo, V., Eds.; Taylor and Francis: Boca Raton, FL, USA, 2006.
- Uhl, G.R.; Ishiguro, H.; Onaivi, E.S.; Zhang, P.-W.; Akinshola, B.E.; Lin, Z.; Hope, B.; Leonard, C.M.; Liu, Q.-R. Molecular Neurobiological Methods in Marijuana-Cannabinoid Research. In Marijuana and Cannabinoid Research; Humana Press: Totowa, NJ, USA, 2006; Volume 123, pp. 1–17.
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and Structure of a Brain Constituent That Binds to the Cannabinoid Receptor. Science 1992, 258, 1946–1949.
- Oliveira, A.B.; Ribeiro, R.T.; Mello, M.T.; Tufik, S.; Peres, M.F.P. Anandamide Is Related to Clinical and Cardiorespiratory Benefits of Aerobic Exercise Training in Migraine Patients: A Randomized Controlled Clinical Trial. Cannabis Cannabinoid Res. 2019, 4, 275–284.
- Piscitelli, F.; Di Marzo, V. “Redundancy” of Endocannabinoid Inactivation: New Challenges and Opportunities for Pain Control. ACS Chem. Neurosci. 2012, 3, 356–363.
- Micale, V.; Di Marzo, V.; Sulcova, A.; Wotjak, C.T.; Drago, F. Endocannabinoid System and Mood Disorders: Priming a Target for New Therapies. Pharmacol. Ther. 2013, 138, 18–37.
- Silvestri, C.; Di Marzo, V. The Endocannabinoid System in Energy Homeostasis and the Etiopathology of Metabolic Disorders. Cell Metab. 2013, 17, 475–490.
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Nakane, S.; Shinoda, A.; Itoh, K.; Yamashita, A.; Waku, K. 2-Arachidonoylglycerol: A Possible Endogenous Cannabinoid Receptor Ligand in Brain. Biochem. Biophys. Res. Commun. 1995, 215, 89–97.
- Murataeva, N.; Straiker, A.; Mackie, K. Parsing the Players: 2-Arachidonoylglycerol Synthesis and Degradation in the CNS. Br. J. Pharmacol. 2014, 171, 1379–1391.
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-Mediated Control of Synaptic Transmission. Physiol. Rev. 2009, 89, 309–380.
- Esposito, G.; De Filippis, D.; Carnuccio, R.; Izzo, A.A.; Iuvone, T. The Marijuana Component Cannabidiol Inhibits Beta-Amyloid-Induced Tau Protein Hyperphosphorylation through Wnt/Beta-Catenin Pathway Rescue in PC12 Cells. J. Mol. Med. 2006, 84, 253–258.
- Giuliano, C.; Francavilla, M.; Ongari, G.; Petese, A.; Ghezzi, C.; Rossini, N.; Blandini, F.; Cerri, S. Neuroprotective and Symptomatic Effects of Cannabidiol in an Animal Model of Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 8920.
- Vasincu, A.; Rusu, R.-N.; Ababei, D.-C.; Larion, M.; Bild, W.; Stanciu, G.D.; Solcan, C.; Bild, V. Endocannabinoid Modulation in Neurodegenerative Diseases: In Pursuit of Certainty. Biology 2022, 11, 440.
- Tadijan, A.; Vlašić, I.; Vlainić, J.; Đikić, D.; Oršolić, N.; Jazvinšćak Jembrek, M. Intracellular Molecular Targets and Signaling Pathways Involved in Antioxidative and Neuroprotective Effects of Cannabinoids in Neurodegenerative Conditions. Antioxidants 2022, 11, 2049.
- Lipina, C.; Hundal, H.S. Modulation of Cellular Redox Homeostasis by the Endocannabinoid System. Open Biol. 2016, 6, 150276.
- Karl, T.; Garner, B.; Cheng, D. The Therapeutic Potential of the Phytocannabinoid Cannabidiol for Alzheimer’s Disease. Behav. Pharmacol. 2017, 28, 142–160.
- Coles, M.; Steiner-Lim, G.Z.; Karl, T. Therapeutic Properties of Multi-Cannabinoid Treatment Strategies for Alzheimer’s Disease. Front. Neurosci. 2022, 16, 962922.
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.M.; Denovan-Wright, E.M. Cannabidiol Is a Negative Allosteric Modulator of the Cannabinoid CB1 Receptor. Br. J. Pharmacol. 2015, 172, 4790–4805.
More
Information
Subjects:
Geriatrics & Gerontology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
955
Revisions:
2 times
(View History)
Update Date:
16 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




