Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | MARCO ARESE | -- | 1729 | 2022-12-05 09:32:27 | | | |
| 2 | Conner Chen | Meta information modification | 1729 | 2022-12-06 07:04:51 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Arese, M.; Bussolino, F.; Pergolizzi, M.; Bizzozero, L. Perineural Invasion and the Perineural Niche. Encyclopedia. Available online: https://encyclopedia.pub/entry/38000 (accessed on 07 February 2026).
Arese M, Bussolino F, Pergolizzi M, Bizzozero L. Perineural Invasion and the Perineural Niche. Encyclopedia. Available at: https://encyclopedia.pub/entry/38000. Accessed February 07, 2026.
Arese, Marco, Federico Bussolino, Margherita Pergolizzi, Laura Bizzozero. "Perineural Invasion and the Perineural Niche" Encyclopedia, https://encyclopedia.pub/entry/38000 (accessed February 07, 2026).
Arese, M., Bussolino, F., Pergolizzi, M., & Bizzozero, L. (2022, December 05). Perineural Invasion and the Perineural Niche. In Encyclopedia. https://encyclopedia.pub/entry/38000
Arese, Marco, et al. "Perineural Invasion and the Perineural Niche." Encyclopedia. Web. 05 December, 2022.
Copy Citation
Perineural invasion (PNI) is a very well known process by pathologists and one the most actively studied events in cancer–nerve relations at the molecular level. The discrete microenvironment surrounding an invaded nerve, also called the perineural niche, is formed by neural cells, supporting cells, inflammatory cells, extracellular matrix, blood vessels, and immune components.
tumor–nervous system molecular crosstalk
autocrine/paracrine interactions
signal transduction
1. Introduction
Tumors are not only composed of transformed cells; they also contain stable cell types that collectively form a complex biosystem. Endothelial cells, immunocompetent cells, cancer-associated fibroblasts (CAFs), neurons, nerves, and other elements with their products (e.g., the extracellular matrix) influence the transformed cells within the tumor microenvironment (TME) and in such a way affect tumor biology [1].
Targeting the TME rather the tumor cells alone has significant clinical repercussions. Indeed, drugs targeting angiogenesis, CAFs, immunocompetent cells, and some related signaling pathways are in clinical trials (reviewed in [2]). Altogether, it appears that people will better understand and tackle many open questions related to cancer only with a detailed knowledge of microenvironmental factors, their sources, and their impacts.
The concept that the nervous system affects the progression of cancer was initially put forward by the Greek physician Galen, who recognized that “melancholic” women were more prone to developing breast cancer than other women (reviewed in [3][4]). The most recent indications from the burgeoning field of cancer neuroscience [5] suggest that nerves are significant players in the tumor microenvironment, engendering a selective pressure on cancer cells by modulating a complex network of mediators related to tumor progression.
There has been a considerable increase in the published research on cancer–nervous system relations in the last three years, while many excellent reviews in the last year alone [6][7][8][9][10][11] have approached the field from different perspectives.
Nerve and cancer cells relate in many ways, some of which are known only at the descriptive level. Taking into account the “manifest” of cancer neuroscience [5], it can be cited the four main interfaces through which cancer and the nervous system influence each other: (1) Paracrine signaling between nerve cells and cancer cells, such as neuronal-activity-dependent neurotransmitter or growth factor release, regulates cancer growth in a variety of tissues. The influence of neurons on malignant cells may be mediated by other cell types in the tumor microenvironment. Paracrine factors derived from cancer remodel the nervous system to promote increased neural activity in the tumor microenvironment. (2) Synaptic communication between neurons and brain cancer cells (e.g., malignant glioma), through voltage-regulated mechanisms, can regulate cancer growth. (3) Cancer-related circulating factors can influence nervous system functions, such as sleep, whereas the nervous system can influence cancer progression via circulating factors, such as hormones and progenitor cells, or altered immune system function. (4) Cancer treatments frequently result in nervous system toxicity, ranging from peripheral neuropathy to cognitive impairment (Figure 1).
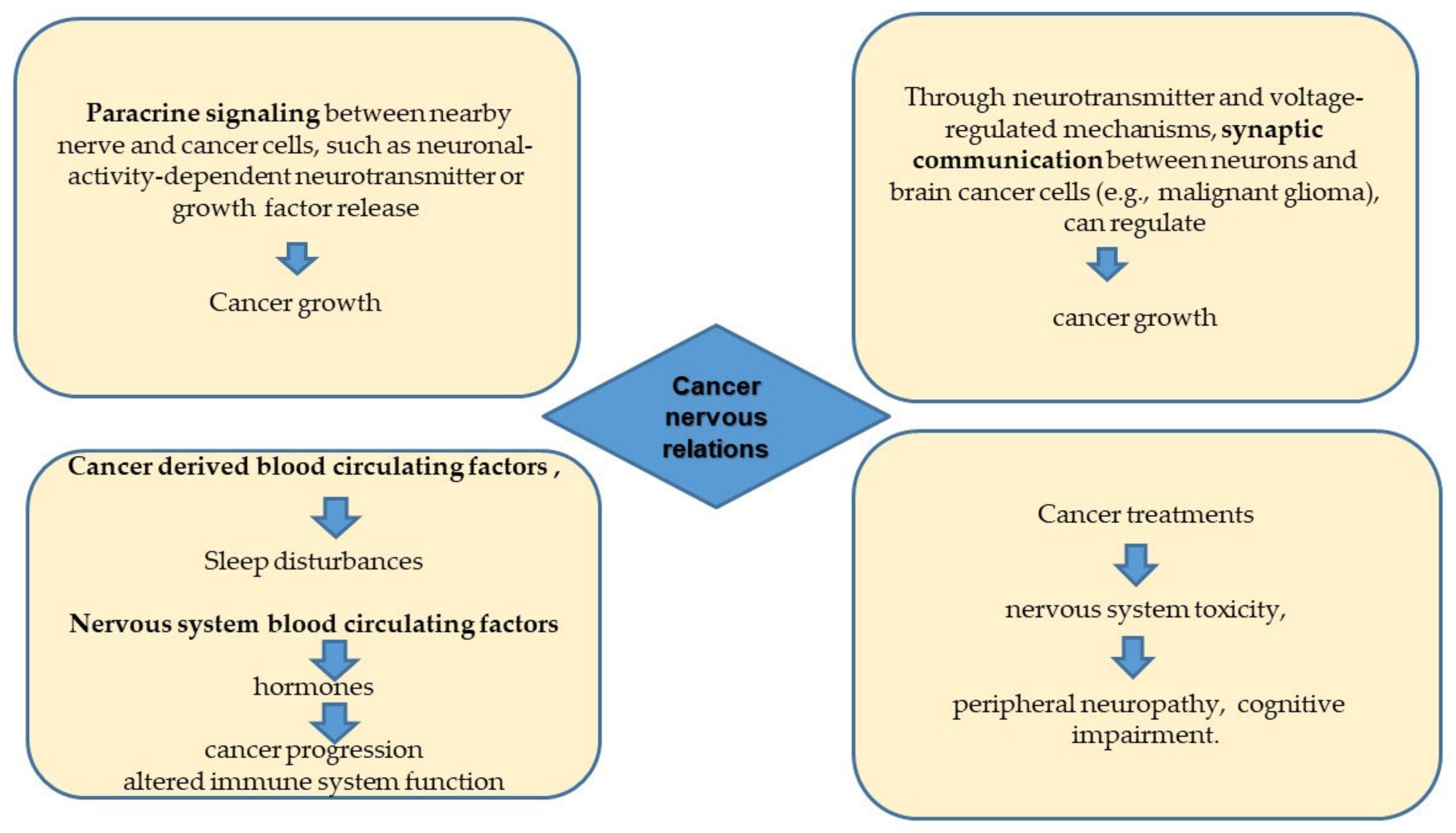

Figure 1. The four main areas of cancer/nervous system interactions.
2. Perineural Invasion and the Perineural Niche
Perineural invasion (PNI) is a very well known process by pathologists and one the most actively studied events in cancer–nerve relations at the molecular level. Nonetheless, the current understanding of PNI is very limited and is still highly debated. Macroscopically, it happens as cancer cells infiltrate the surrounding nerve endings, invading them through the destruction of the perineurium and entry via the perforating vessels [12].
While virtually all peripheral cancer types display PNI, the process has mostly been studied for head and neck, prostatic, and pancreatic cancers. For many of these, PNI is linked with considerable morbidity, poor outcomes, and decreased survival (reviewed in [13]).
The discrete microenvironment surrounding an invaded nerve, also called the perineural niche, is formed by neural cells, supporting cells, inflammatory cells, extracellular matrix, blood vessels, and immune components. The discovery of the innervated niche as a new specialized microenvironment has opened exciting new avenues of research into bidirectional neural–cancer interactions [14]. The migration of cancer cells along nerves is supported by multiple growth factors and chemokines secreted by these cell types. Nerve injury and repair, in a continuous process, seem to guide this migration (also called tracking) through the secretion of growth factors and chemokines simulating damaged and dying nerves [15]. Amit et al. [15] proposed that PNI occurs in seven stages: tumor cell survival, the formation of a neural steady state, an inflammatory reaction, the recruitment of tumor cells to nerves, the neogenesis of nerves, the adhesion of tumor cells and neurolemma, and nerve invasion. This theory is supported by data from in vitro studies, as the coculture of human prostate cancer (PCa) cells, stromal cells, and mouse dorsal root ganglia increases tumor colony and neurite outgrowth [16].
The molecular characterization of the perineural niche is a burgeoning field of research [17] that would require a whole review by itself.
Most studies emphasize the importance of neural components in tumors, making it critical to investigate the mechanism of nerve emergence in cancer. Strikingly, neurogenesis is not only a local process. Neural progenitors from the brain that express doublecortin (DCX+) can migrate to prostate tumors and initiate neurogenesis, leading to tumor growth and metastasis [18][19]. High expression of the transcription factor Snail in PCa cells improves adherence to nerve cells and promotes neurite outgrowth [19]. As a result, the neurogenesis program is crucial in prostate tumor–nerve crosstalk. A recent single-cell sequencing study discovered that Neurexin 1 and Neuroligin 1 (see below), two genes encoding neuronal synaptic molecules, are strongly linked to PCa progression [20]. Other neurotrophic factors, such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF), have been linked to PNI [21]. PNI in PCa is thought to be characterized by N-CAM upregulation in nerves. Li et al. proposed that cancer cells induce N-CAM upregulation in nerves via a paracrine loop that accelerates cancer cell migration towards nerves via the nuclear factor kappa B (NFkB) pathway [22]. NFkB is involved in many different types of cancer. In PCa, NFkB nuclear translocation has been found to be critical for inhibiting apoptosis and increased tumor cell proliferation in PNI lesions. Semaphorin 4F (SEMA4F), a member of the semaphorin family of axon guidance molecules (see below), is another molecule that correlates with the NFkB pathway in PNI. The SEMA4F expression level has been linked to nerve density and PNI diameter, in addition to the antiapoptotic effect induced by interacting with the NFkB pathway, as the overexpression of SEMA4F can induce neurogenesis [23]. Another semaphorin family member, semaphorin 3C (SEMA3C), has been shown to be involved in PNI in PCa. SEMA3C is activated by monoamine oxidase A (MAOA) in a Twist-1-dependent manner and interacts with its receptor and coreceptor PlexinA2 as well as neuropilin-1 (NRP1), stimulating c-MET. This SEMA3C/PlexinA2/NRP1-cMET axis promotes PNI in PCa and demonstrates the close relationship between PNI and nerve-related signals [24][25]. Many other molecular cues promoting aggressive behavior have been identified in the perineural niche. In pancreatic cancer, the cell adhesion molecule L1CAM and a metalloproteinase mediate paracrine communication between Schwann cells and cancer cells, thus promoting cancer invasion into nerves [26]. In head and neck cancer, the neuropeptide galanin (GAL) activates its receptor, GALR2, on cancer cells and stimulates the transcription of cyclooxygenase-2 and GAL itself. In turn, the cyclooxygenase-2 product prostaglandin E2 promotes cancer invasion, while the GAL released by cancer stimulates neurite outgrowth, promoting PNI [27]. In the same tumor model, the NGF-TrkA axis triggers the epithelial–mesenchymal transition (EMT) and confers resistance to the epidermal growth factor receptor tyrosine kinase (EGFR) inhibitor erlotinib [28]. Moreover, in PCa, nerve-produced glial cell line derived neurotrophic factor receptor alpha (GFRα1) activates the receptor tyrosine kinase RET, thus promoting transformation-associated phenotypes [29].
From a therapeutic perspective targeting the events at the perineural niche, two approaches are worth mentioning here. One is targeting NGF and its receptors. In prostate cancer, this may reduce nerve infiltration, inhibit tumor cell growth, and alleviate cancer-related pain (reviewed in [30]). Another possible strategy is to target the interaction of the nerve and immune cells in the tumor microenvironment. It has been reported that tumor-associated nerves in prostatic cancer have high levels of programmed cell death ligand-1 (PD-L1) expression and inhibit the function of immune cells, providing a new perspective for the use of immune checkpoint inhibitors [31].
A novel and highly investigated player in nerve cancer crosstalk is autophagy. Autophagy is a conserved cellular self-degradation process that is critical for balancing energy sources during neuronal development and nutrient stress [32][33]. Autophagy has recently been identified as a double-edged sword that plays an important role in the innervated niche [34]. The question of whether upregulated autophagy is beneficial or harmful to tumor progression in the innervated niche is still being debated. Autophagy stimulates tumor progression in part because of its ability to maintain innervated niche homeostasis and even promote innervated niche development [35][36]. In contrast, dysfunctional autophagy may result in axon breakdown, dendritic degradation, somal stress, and glial cell death, ultimately leading to innervated niche disorder [37]. As more is learned about autophagy in the innervated niche, new treatment strategies can be developed. Changing the innervated niche by regulating the autophagy pathway has emerged as a novel therapeutic opportunity for cancer treatment and a novel window for drug repurposing in this context. Following an escalating era for drug discovery, repurposing old drugs to treat new indications is becoming an increasingly appealing proposition because it is a time-saving and cost-effective method with high success rates [38][39]. A large number of preclinical trials have shown that multiple noncancer drugs (antipsychotics, cardiovascular drugs, etc.) have off-label antitumor effects [40] Based on the autophagy-mediated innervated niche, repurposing drugs such as beta-adrenergic antagonists is a promising pharmacological approach in cancer neuroscience [41][42]. Hence, it is now recognized that the innervated niche is a complex and heterogeneous site that can influence cancer development [43]. Finally, the role of a specific type of autophagy in glioblastoma progression should be cited here. Pericytes (PCs), contractile perivascular cells, are seized by glioblastoma (GB) to aid tumor progression. The work of Valdor et al. [44] showed that chaperone-mediated autophagy (CMA), a process that transports specific cytosolic proteins to lysosomes for degradation and has pro-oncogenic effects, is significantly upregulated in PCs in response to the oxidative burst that occurs after PC-GB cell interactions. The upregulation of CMA activity in PCs by GB is required for their effective interaction with GB cells, which aids tumor growth. These findings point to abnormal CMA upregulation as a mechanism by which GB cells elicit the immunosuppressive function of PCs and stabilize the GB-PC interactions required for tumor cell survival.
References
- Lacina, L.; Coma, M.; Dvovránková, B.; Kodet, O.; Melegova, N.; Gal, P.; Smetana, K. Evolution of Cancer progression in the context of Darwinism. Anticancer Res. 2019, 39, 1–16.
- Gal, P.; Varinska, L.; Faber, L.; Novákv, S.; Szabo, P.; Mitrengova, P.; Mirossay, A.; Muvcaji, P.; Smetana, K. How signaling molecules regulate tumor microenvironment: Parallels to wound repair. Molecules 2017, 22, 1818.
- Fife, A.; Beasley, P.J.; Fertig, D.L. Psychoneuroimmunology and cancer: Historical perspectives and current research. Adv. Neuroimmunol. 1996, 6, 179–190.
- Kiecolt-Glaser, J.K.; Robles, T.; Heffner, K.; Loving, T.; Glaser, R. Psycho-oncology and cancer: Psychoneuroimmunology and cancer. Ann. Oncol. 2002, 13, 165–169.
- Monje, M.; Borniger, J.C.; D’Silva, N.J.; Deneen, B.; Dirks, P.B.; Fattahi, F. Roadmap for the Emerging Field of Cancer Neuroscience. Cell 2020, 181, 219–222.
- Li, J.; Xu, Y.; Zhu, H.; Wang, Y.; Li, P.; Wang, D. The dark side of synaptic proteins in tumours. Br. J. Cancer 2022, 3, 1184–1192.
- Shi, D.D.; Guo, J.A.; Hoffman, H.I.; Su, J.; Mino-Kenudson, M.; Barth, J.L.; Schenkel, J.M.; Loeffler, J.S.; Shih, H.A.; Hong, T.S.; et al. Therapeutic avenues for cancer neuroscience: Translational frontiers and clinical opportunities. Lancet Oncol. 2022, 23, e62–e74.
- Hernandez, S.; Serrano, A.G.; Solis Soto, L.M. The Role of Nerve Fibers in the Tumor Immune Microenvironment of Solid Tumors. Adv. Biol. 2022, 6, 2200046.
- Pan, C.; Winkler, F. Insights and opportunities at the crossroads of cancer and neuroscience. Nat. Cell Biol 2022, 24, 1454–1460.
- Amit, M. Cancer Neuroscience: Evidence for a New Hallmark of Cancer. Adv. Biol. 2022, 6, 2200192.
- Silverman, D.A.; Martinez, V.K.; Dougherty, P.M.; Myers, J.N.; Calin, G.A.; Amit, M. Cancer-Associated Neurogenesis and Nerve-Cancer Cross-talk. Cancer Res. 2021, 81, 1431–1440.
- Kayahara, M.; Nakagawara, H.; Kitagawa, H.; Ohta, T. The nature of neural invasion by pancreatic cancer. Pancreas 2007, 35, 218.
- Liebig, C.; Ayala, G.; Wilks, J.A.; Berger, D.H.; Albo, D. Perineural invasion in cancer. Cancer 2009, 115, 3379–3391.
- Zahalka, A.H.; Frenette, P.S. Nerves in cancer. Nat. Rev. Cancer 2020, 20, 143–157.
- Amit, M.; Na’ara, S.; Gil, Z. Mechanisms of cancer dissemination along nerves. Nat. Rev. 2016, 16, 399.
- Ayala, G.E.; Wheeler, T.M.; Shine, H.D.; Schmelz, M.; Frolov, A.; Chakraborty, S.; Rowley, D. In vitro dorsal root ganglia and human prostate cell line interaction: Redefining perineural invasion in prostate cancer. Prostate 2001, 49, 213–223.
- Niu, Y.; Förster, S.; Muders, M. The Role of Perineural Invasion in Prostate Cancer and Its Prognostic Significance. Cancers 2022, 14, 4065.
- Mauffrey, P.; Tchitchek, N.; Barroca, V.; Bemelmans, A.-P.; Firlej, V.; Allory, Y.; Romeo, P.-H.; Magnon, C. Progenitors from the central nervous system drive neurogenesis in cancer. Nature 2019, 569, 672–678.
- Edwards, G.; Campbell, T.; Henderson, V.; Danaher, A.; Wu, D.; Srinivasan, R.; Rezvani, K.; Odero-Marah, V.A. SNAIL Transctiption factor in prostate cancer cells promotes neurite outgrowth. Biochimie 2021, 180, 1–9.
- Eksi, S.E.; Chitsazan, A.; Sayar, Z.; Thomas, G.V.; Fields, A.J.; Kopp, R.P.; Spellman, P.T.; Adey, A.C. Epigenetic loss of heterogeneity from low to high grade localized prostate tumours. Nat. Commun. 2021, 12, 7292.
- Frunza, A.; Slavescu, D.; Lascar, I. Perineural invasion in head and neck cancers-a review. J. Med. Life 2014, 7, 121.
- Li, R.; Wheeler, T.; Dai, H.; Ayala, G. Neural cell adhesion molecule is upregulated in nerves with prostate cancer invasion. Hum. Pathol. 2003, 34, 457–461.
- Ding, Y.; He, D.; Florentin, D.; Frolov, A.; Hilsenbeck, S.; Ittmann, M.; Kadmon, D.; Miles, B.; Rowley, D.; Ayala, G. Semaphorin 4F as a Critical Regulator of Neuroepithelial Interactions and a Biomarker of Aggressive Prostate CancerSemaphorin 4F and Nerves. Clin. Cancer Res. 2013, 19, 6101–6111.
- Yin, L.; Li, J.; Wang, J.; Pu, T.; Wei, J.; Li, Q.; Wu, B.J. MAOA promotes prostate cancer cell perineural invasion through SEMA3C/PlexinA2/NRP1-cMET signaling. Oncogene 2021, 40, 1362–1374.
- Adekoya, T.O.; Richardson, R.M. Cytokines and chemokines as mediators of prostate cancer metastasis. Int. J. Mol. Sci. 2020, 21, 4449.
- Na’ara, S.; Amit, M.; Gil, Z. L1CAM induces perineural invasion of pancreas cancer cells by upregulation of metalloproteinase expression. Oncogene 2019, 38, 596–608.
- Scanlon, C.S.; Banerjee, R.; Inglehart, R.C.; Liu, M.; Russo, N.; Hariharan, A.; Van Tubergen, E.A.; Corson, S.L.; Asangani, I.A.; Mistretta, C.M.; et al. Galanin modulates the neural niche to favour perineural invasion in head and neck cancer. Nat. Commun 2015, 6, 6885.
- Lin, C.; Ren, Z.; Yang, X.; Yang, R.; Chen, Y.; Liu, Z.; Dai, Z.; Zhang, Y.; He, Y.; Zhang, C.; et al. Nerve growth factor (NGF)-TrkA axis in head and neck squamous cell carcinoma triggers EMT and confers resistance to the EGFR inhibitor erlotinib. Cancer Lett. 2020, 472, 81–96.
- Ban, K.; Feng, S.; Shao, L.; Ittmann, M. RET signaling in prostate cancer. Clin. Cancer Res. 2017, 23, 4885–4896.
- Jobling, P.; Pundavela, J.; Oliveira, S.M.; Roselli, S.; Walker, M.M.; Hondermarck, H. Nerve-Cancer Cell Cross-talk: A Novel Promoter of Tumor Progression. Cancer Res. 2015, 75, 1777–1781.
- Mo, R.-J.; Han, Z.-D.; Liang, Y.-K.; Ye, J.-H.; Wu, S.-L.; Lin, S.X.; Zhang, Y.-Q.; Song, S.-D.; Jiang, F.-N.; Zhong, W.-D.; et al. Expression of PD-L1 in tumor-associated nerves correlates with reduced CD8+ tumor-associated lymphocytes and poor prognosis in prostate cancer. Int. J. Cancer 2019, 144, 3099–3110.
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075.
- Morishita, H.; Mizushima, N. Diverse cellular roles of autophagy. Annu. Rev. Cell Dev. Biol. 2019, 35, 453–475.
- Cai, Q.; Ganesan, D. Regulation of neuronal autophagy and the implications in neurodegenerative diseases. Neurobiol. Dis. 2021, 4, 105582.
- Yazdankhah, M.; Ghosh, S.; Shang, P.; Stepicheva, N.; Hose, S.; Liu, H.; Chamling, X.; Tian, S.; Sullivan, M.L.; Calderon, M.J.; et al. BNIP3L-mediated mitophagy is required for mitochondrial remodeling during the differentiation of optic nerve oligodendrocytes. Autophagy 2021, 17, 3140–3159.
- Griffey, C.J.; Yamamoto, A. Macroautophagy in CNS health and disease. Nat. Rev. Neurosci. 2022, 23, 411–427.
- Wang, P.; Chen, X.; Wang, Y.; Jia, C.; Liu, X.; Wang, Y.; Wu, H.; Cai, H.; Shen, H.-M.; Le, W. Essential role for autophagy protein VMP1 in maintaining neuronal homeostasis and preventing axonal degeneration. Cell Death Dis. 2021, 12, 116.
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58.
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674.
- Regulska, K.; Regulski, M.; Karolak, B.; Murias, M.; Stanisz, B. Can cardiovascular drugs support cancer treatment? The rationale for drug repurposing. Drug Discov. Today 2019, 24, 1059–1065.
- Demir, I.E.; Reyes, C.M.; Alrawashdeh, W.; Ceyhan, G.O.; Deborde, S.; Friess, H.; Görgülü, K.; Istvanffy, R.; Jungwirth, D.; Kuner, R.; et al. Future directions in preclinical and translational cancer neuroscience research. Nature 2020, 1, 1027–1031.
- Shaw, V.; Srivastava, S.; Srivastava, S.K. Repurposing antipsychotics of the diphenylbutylpiperidine class for cancer therapy. Semin. Cancer Biol. 2021, 1, 75–83.
- Shi, J.; Xu, J.; Li, Y.; Li, B.; Ming, H.; Nice, E.C.; Huang, C.; Li, Q.; Wang, C. Drug repurposing in cancer neuroscience: From the viewpoint of the autophagy-mediated innervated niche. Front. Pharmacol. 2022, 13, 990665.
- Valdor, R.; Garc’ia-Bernal, D.; Riquelme, D.; Martinez, C.M.; Moraleda, J.M.; Cuervo, A.M.; Macian, F.; Martinez, S. Glioblastoma ablates pericytes antitumor immune function through aberrant up-regulation of chaperone-mediated autophagy. Proc. Natl. Acad. Sci. USA 2019, 116, 20655–20665.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
898
Revisions:
2 times
(View History)
Update Date:
06 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




