Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xiaoping Peng | -- | 1944 | 2022-12-03 04:46:06 | | | |
| 2 | Rita Xu | Meta information modification | 1944 | 2022-12-05 02:43:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Guo, R.; Li, G.; Zhang, Z.; Peng, X. Secondary Metabolites from Trichoderma harzianum. Encyclopedia. Available online: https://encyclopedia.pub/entry/37889 (accessed on 07 February 2026).
Guo R, Li G, Zhang Z, Peng X. Secondary Metabolites from Trichoderma harzianum. Encyclopedia. Available at: https://encyclopedia.pub/entry/37889. Accessed February 07, 2026.
Guo, Rui, Gang Li, Zhao Zhang, Xiaoping Peng. "Secondary Metabolites from Trichoderma harzianum" Encyclopedia, https://encyclopedia.pub/entry/37889 (accessed February 07, 2026).
Guo, R., Li, G., Zhang, Z., & Peng, X. (2022, December 03). Secondary Metabolites from Trichoderma harzianum. In Encyclopedia. https://encyclopedia.pub/entry/37889
Guo, Rui, et al. "Secondary Metabolites from Trichoderma harzianum." Encyclopedia. Web. 03 December, 2022.
Copy Citation
The biocontrol fungus Trichoderma harzianum, from both marine and terrestrial environments, has attracted considerable attention. T. harzianum has a tremendous potential to produce a variety of bioactive secondary metabolites (SMs), which are an important source of new herbicides and antibiotics.
natural products
Trichoderma harzianum
marine sources
bioactivity
1. Introduction
The unique marine environment with high pressure, high salinity, and low temperature, breeds unique marine microorganisms [1][2]. Secondary metabolites obtained from marine-derived fungi have attracted considerable attention in recent years for potential use in the discovery of unique structures and diverse biological properties [3][4].
The biocontrol fungi Trichoderma spp. (sordariomycetes) are widely spread in the environment [5], such as in the ocean. With the deepening of marine science and technology exploration, more and more Trichoderma sp. strains have been discovered from marine sources. From marine and terrestrial environments, there are no fewer than 250 Trichoderma species discovered so far [6]. Trichoderma species are famous for producing plentiful secondary metabolites [7]. Among them, Trichoderma harzianum probably contributed the most secondary metabolites (SMs) originating from Trichoderma species [8][9]. The SMs from T. harzianum showed antifungal activity [10]. Additionally, cytotoxicity [11] and antimicrobial activity [12], and so on, have also been found in its SMs.
The SMs of T. harzianum have not been summarized in detail or systematically. Up to now, nearly 200 compounds of T. harzianum have been reported. The secondary metabolites of T. harzianum include terpenoids, polyketides, peptides, alkaloids, and lactones.
2. Structural and Biological Activity Studies
2.1. Terpenoids
Seven new potent phytotoxic harziane diterpenes harzianelactones A and B (1 and 2), harzianones A–D (3–6) and harziane (9) were isolated from the soft coral-derived fungus T. harzianum XS-20090075 [13]. Compounds 1 and 2 belonged to a unique class of terpenes with a 6-5-7-5-fused carbocyclic core and a lactone ring. Harzianones A–D (3–6) consisted of a fused tetracyclic 6-5-7-4-fused tetra-cyclic skeleton. Chemical epigenetic manipulation was applied to activate silent genes of T. harzianum XS-20090075 by appending a histone deacetylase (HDAC) inhibitor. With this experimental technique, two new diterpenoids harzianone E (7) and harzianolic acid A (41), and one new sesquiterpenoid 3,7,11-trihydroxy-cycloneran (16) were isolated from the same strain T. harzianum XS-20090075. At the same time, 11 known sesquiterpenoids, methyl 3,7-dihydroxy-15-cycloneranate (17), catenioblinc (18), ascotrichic acid (19), cyclonerotriol (20), (10E)-12-acetoxy-10-cycloneren-3,7-diol (21), cyclonerodiol (22), cyclonerodiol oxide (27), epicyclonerodiol oxide (28), ent-trichoacorenol (29), trichoacorenol (30), and ophioceric acid (40) were isolated from T. harzianum XS-20090075 [14]. It was the first time for obtaining cleistanthane diterpenoid from T. harzianum XS-20090075. Trichodermanins C–H (10–15) were new diterpenes with a rare fused 6-5-6-6 ring system, and have been isolated from a fungus T. harzianum OUPS-111D-4 [15][16]. This strain was separated from a piece of sponge Halichondria okadai. Compounds 10–15 were evaluated for their cytotoxicity by using murine P388 leukemia, human HL-60 leukemia, and murine L1210 leukemia cell lines. Compound 10 with a fused 6-5-6-6 ring system exhibited potent cytotoxic activity [15], and compounds 12 and 13 exhibited modest activity [16]. Six new terpenes, including one harziane diterpene, 3R-hydroxy-9R,10R-dihydroharzianone (8), three cyclonerane sesquiterpenes, methyl 3,7-dihydroxy-15-cycloneranate (17), 11-methoxy-9-cycloneren-3,7-diol (23), 10-cycloneren-3,5,7-triol (25), and one acorane sesquiterpene, 8-acoren-3,11-diol (36), and one cyclonerane 11R-methoxy-5,9,13-proharzitrien-3-ol (42), together with four known sesquiterpenes, cyclonerodio (22), 9-cycloneren-3,7,11-triol (24), trichoacorenol (30) and trichoacorenol B (37) were isolated from T. harzianum X-5 [17]. The strain X-5 was an endophytic fungus isolated from the marine brown alga Laminaria japonica. The above six new compounds (8, 17, 23, 25, 36, and 42) were evaluated to inhibit four marine phytoplankton species and four marine-derived pathogenic bacteria [17]. Compounds 23 and 42 exhibited potent inhibition activity [17]. Harzianoic acid A (38) is a sesquiterpene, and harzianoic acid B (39) is a norsesquiterpene with a cyclobutane nucleus. They were isolated from a sponge-isolated fungus, T. harzianum LZDX-32-08 [18], and were found to have new natural scaffolds to exert anti-HCV activity for their capability to inhibit multi-targets, including those for virus replication and entry [18]. (10E)-12-Acetoxy-10-cycloneren-3,7-diol (21) and 12-acetoxycycloneran-3,7-diol (26) were two new cyclonerane sesquiterpenoids, which were isolated from the marine sediment-derived fungus T. harzianum P1-4 [9]. A new acorane-type sesquiterpene, 15-hydroxyacorenone (31), was isolated from T. harzianum [19], together with acorenone (32), acorenone-B (33), 4-epiacorenone (34), and 4-epiacorenone-B (35). Stigmasta-7,22-dien-3β,5α,6α-triol (43) was isolated from T. harzianum XS-20090075, cultivated by the Czapekʹs culture [20]. Compound 43 exhibited antifouling activity with an EC50 value of 39.2 μg/mL and Topo I inhibitory activity with an MIC value of 50.0 μM [20]. Two fungal strains of T. harzianum T-4 and T. harzianum T-5 were obtained from Palampur, Himachal Pradesh (India). Stigmasterol (44) and β-sitosterol (45) were isolated from T. harzianum T-4 [21]. Ergosterol (46) was isolated from T. harzianum T-5 [21]. Trichosordarin A (47), a unique norditerpene aglycone, was isolated from T. harzianum R5 [22]. Compound 47 was toxic to the marine zooplankton Artemia salina with an LC50 value of 233 µM [22] (Figure 1).
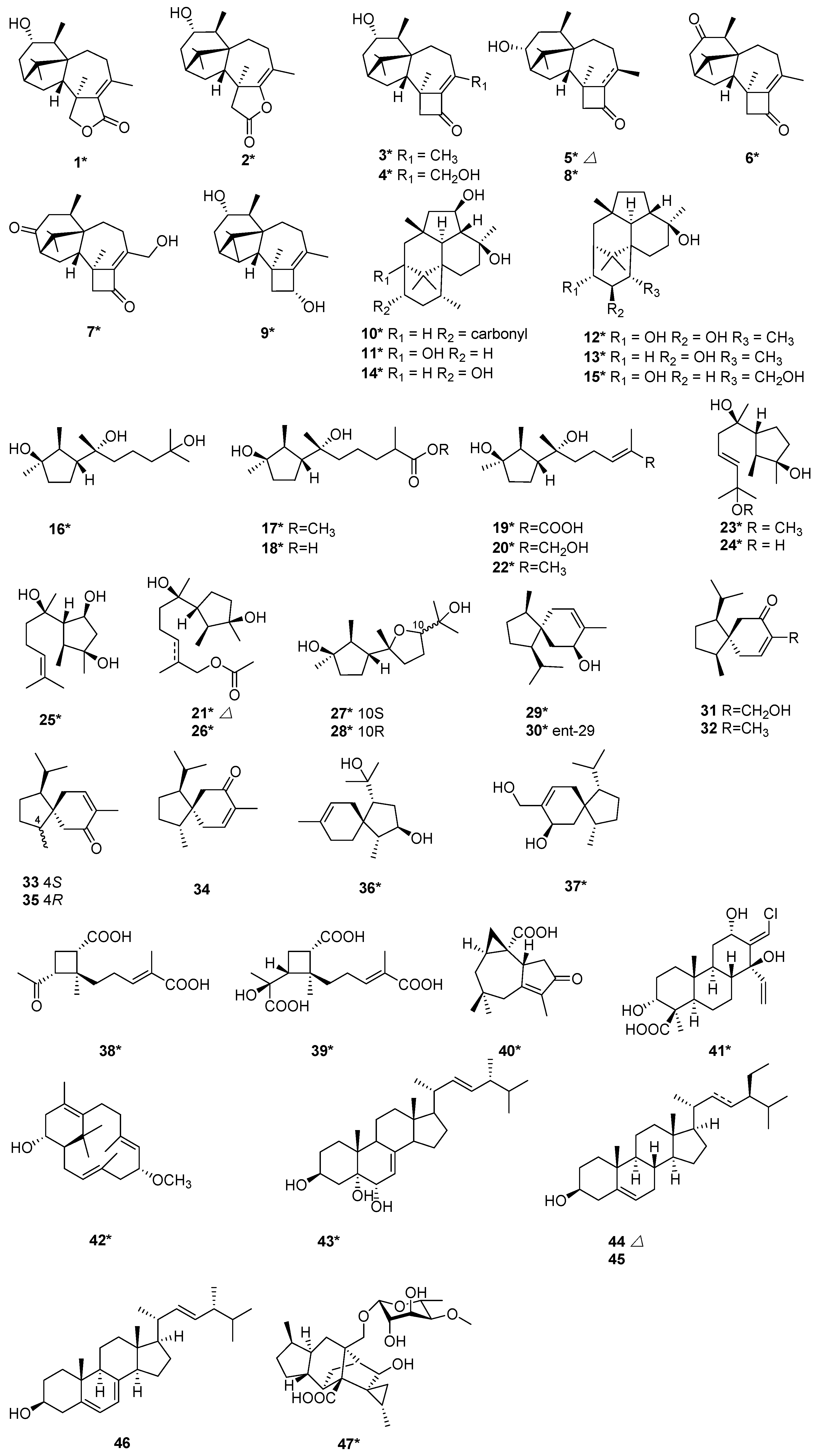
Figure 1. Chemical structures of terpenoids (1–47) from T. harzianum. * Means marine source compounds.
2.2. Polyketides
The fermentation of a sponge-associated fungus T. harzianum HMS-15-3 led to the isolation of four pairs of new C13 lipid enantiomers harzianumols A–H (48–55) [23]. Four polyketides, trichoharzin B (56), methyl-trichoharzin (57), trichoharzin (58), and eujavanicol A (59), were isolated from T. harzianum XS-20090075 [20], which was fermented in rice medium by one strain many compounds (OSMAC) strategy. New naphthalene compound 57, and known naphthalene compound 58 exhibited antifouling activity with the EC50 values of 29.8 and 35.6 μg/mL [20]. Six new tandyukisins, tandyukisins A–F (60–65), were isolated from T. harzianum OUPS-111D-4 [11][24][25], which were initially derived from the sponge Halichondria okadai. Among the tandyukisins A–F (60–65), compounds 60, 64 and 65 exhibited cytotoxicity against murine P388 leukemia, human HL-60 leukemia, and murine L1210 leukemia cell lines inferior to the control 5-fluorouracil [24]. Compounds 61–63 showed slightly selective growth inhibition against the central nervous system cancer SNB-75 cell line in the HCC panel [25]. Compounds 64 and 65 exhibited significant cytotoxicity against the cancer cell lines P388, HL-60, and L1210 [24]. The structure-activity relationship may be relevant to the terminals of the side chains. T. harzianum T-4 was obtained from Palampur, Himachal Pradesh in India, and a polyketide palmitic acid (66) was isolated from the T-4 [21]. Harzianum A (67), was a new trichothecene isolated from the soil-borne fungus T. harzianum in 1994 [26]. Harziphilone (68) was a new polyketide isolated from T. harzianum WC 47695 [27], which was isolated from sandy soil with plant debris collected in Fort Lauderdale. The REV/RRE binding assay and HIV assay revealed that compound 68 showed inhibitory activity against REV-protein binding to RRE RNA with IC50 values of 2.0 μM. In contrast, this compound did not show protection against HIV infection at concentration levels up to 200 μg/mL. The cytotoxicity assay on the murine tumor cell line M-109 showed that 68 exhibited cytotoxicity at 38 μM [27]. Seven polyketides, keto triol 3 (69), keto diol 7 (70), keto diol 6 (71), keto diol 8 (72), triacetate 9 (73), triol 10 (74) and acetal diol 2 (75) were isolated from T. harzianum [28]. One new trichoharzin (58), and two known compounds, tribenzoate (76) and triacetate (77), were isolated from T. harzianum Rifai in 1993 [29]. A new polyketide, T22azaphilone (78), was isolated from T. harzianum T22 [30]. A new compound, trichoharzianol (79), isolated from T. harzianum F031, exhibited antifungal activity against Colletotrichum gloeosporioides with a MIC of 128 μg/mL [31]. Three novel polyketides trichodenones A–C (80–82) were isolated from T. harzianum OUPS-N115 [32]. This strain was separated from the sponge Halichondria okadai. Trichodenones A–C (80–82) showed cytotoxicities against P388 cell line with the ED50 values of 0.21, 1.21, and 1.45 μg/mL, respectively. Homodimericin A (83) was isolated from T. harzianum WC13 [33][34]. In their model, compound 83 was the biologically inert aftermath of a fungal counter to a bacterial attack. The discovery of cryptenol (84) from T. harzianum WC13 [34] indicated that the interactions among microbes in a termite nest were not bipartite but a multipartite system.
The structure and activity relationships of anthraquinones (AQs) in T. harzianum have been studied. AQs represent an important class of SMs occurring in T. harzianum strains, which exhibited a variety of biological functions [12]. The alkylating functionalities in the AQs maximize the anticancer activity by binding tightly with DNA to disrupt the DNA function [35]. Moreover, anthraquinone derivatives were proposed to have an anticancer function by inhibiting protein kinase CK2 [36]. Pachybasin (85) and chrysophanol (86) were isolated from T. harzianum ETS 323 [37]. 1,7-Dihydroxy-3-hydroxymethyl-9,10-anthraquinone (87), 1,5-dihydroxy-3-hydroxymethyl-9,10-anthraquinone (88), emodin (89), and ω-hydroxypachybasin (90) were isolated from T. harzianum strain Th-R16 [38]. These compounds exhibited effective antifungal activity against Botrytis cinerea (Ascomycete) and Rhizoctonia solani (Basidiomycete). At a 500 μg/mL concentration, compound 88 showed comparatively higher activity against R. solani and B. cinerea than 89 [38]. Phomarin (91), (+)-2′S-isorhodoptilometrin (92), 1,6-dihydroxy-3-(hydroxymethyl)anthracene-9,10-dione (93), harzianumnone A (94) and harzianumnone B (95) were isolated from the soft coral-derived fungus T. harzianum XS-20090075 [12]. Compounds 94 and 95 were identified as a pair of epimers, the first example of hydroanthraquinones from T. harzianum XS-20090075. Compound 92 with Topo I inhibition activity, was further assessed for cytotoxic activity against human tumor cell lines. It exhibited cytotoxic activity against HepG2 cell line with an IC50 value of 2.10 µM, and showed cytotoxicity against Hela cell with an IC50 value of 8.59 µM [12] (Figure 2 and Figure 3).
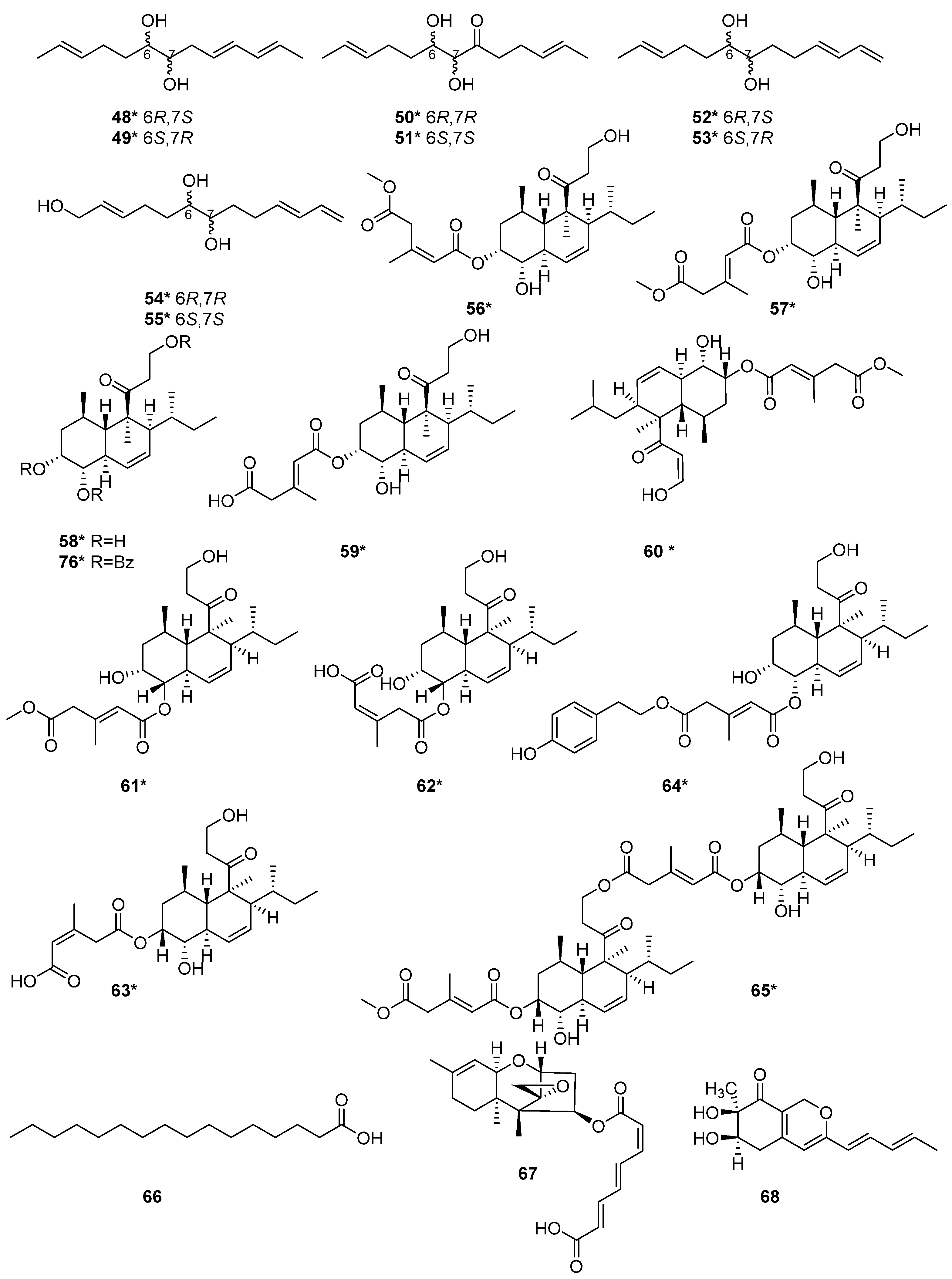
Figure 2. Chemical structures of polyketides (48–68 and 76) from T. harzianum. * Means marine source compounds.
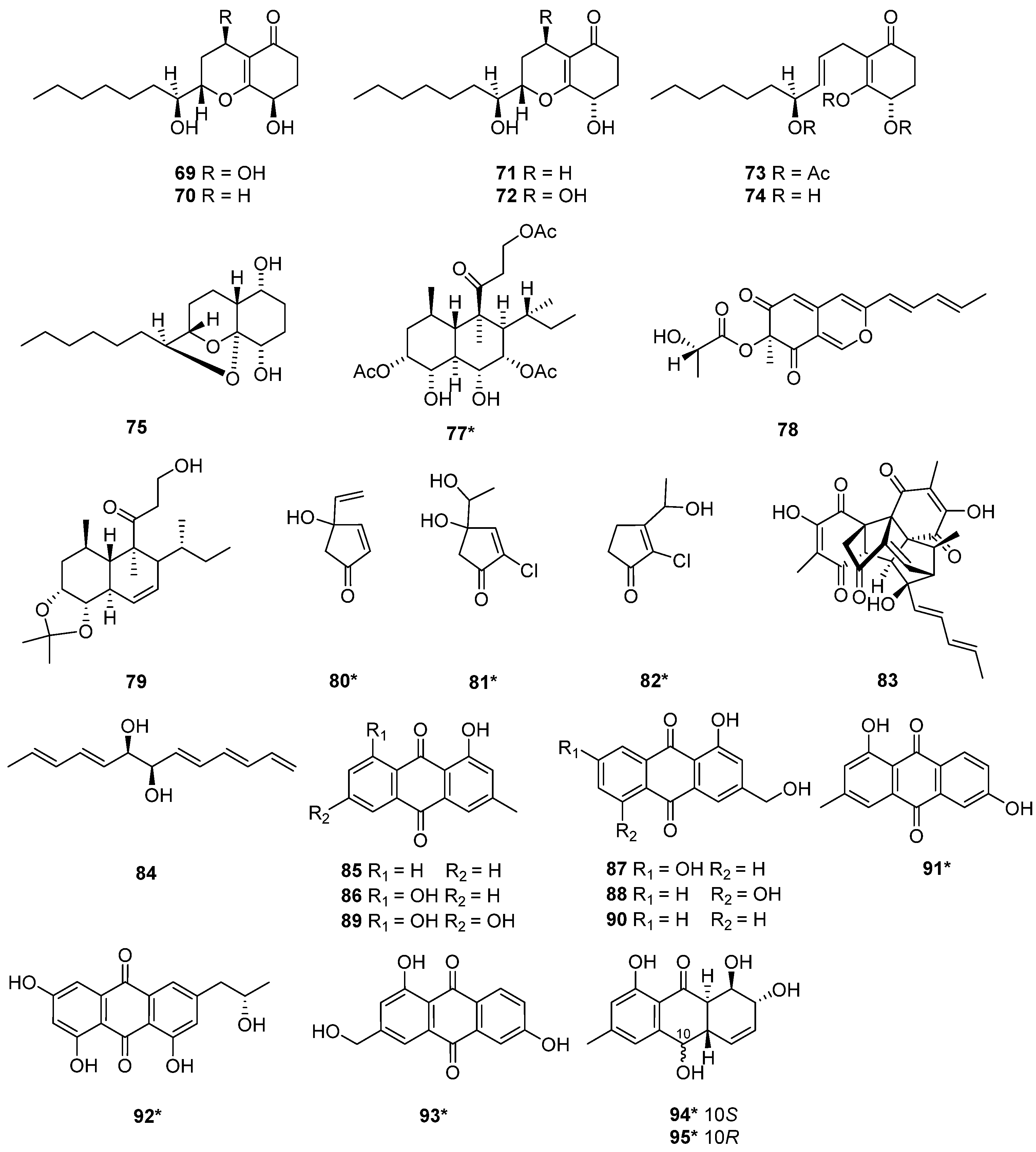
Figure 3. Chemical structures of polyketides (69–75 and 77–95) from T. harzianum. * Means marine source compounds.
2.3. Peptides
Peptaibols are linear antibiotic peptides consisting of 5 to 20 amino acids [39]. It could be biosynthesized by T. harzianum. Peptaibols were characterized by the structures of alpha-aminoisobutyric acid (Aib), and C-terminal hydroxylated amino acid. Two new series peptaibols, trichokindins (TKs) and trichorozins (TZs), were isolated from T. harzianum collected at Nara in Japan. TKs and TZs comprised 18 and 11 amino acid residues, respectively, while TKs were rich in isovaline (Iva). TK-VII (106) is the most hydrophobic of TKs with 18-residue peptides. Compound 106 induced Ca2+-dependent catecholamine secretion from bovine adrenal medullary chromaffin cells [40]. TKs (96–106), with a single peak on HPLC and typical IR absorptions at 3300, 1600, and 1530 cm−1, were confirmed as peptaibols by polarization transfer spectra [40]. With incubating 10 μM of TK-VII (106), 27% of the total catecholamines in bovine adrenal chromaffin cells were secreted in the presence of the Ca2+. In contrast, only 5% of the total catecholamines were secreted without Ca2+ [40]. Hydrophobicity is vital to the interaction between membranes and peptaibols [41]. HB I (107) was isolated from T. harzianum M-903603 [42]. Trichorzins HA (108–113) and MA (114–116) were isolated from T. harzianum M-903602 and T. harzianum M-922835, respectively. Compounds 108–116 are a series of 18-residue peptides [43]. Bioassays on the antifungal activity of trichorzins and harzianins on the phytopathogenic fungus Sclerotium cepivorum revealed that trichorzins were more potent (75% inhibition at 100 μg/mL) than harzianins (40% inhibition at 100 μg/mL) [44]. Research on the structured-activity relationships (SARs) revealed that the peptide chain length and superhydrophobicity played an essential part in the peptide/membrane interaction and the subsequent permeability by perturbing the ironic balance of the cell [44]. As new membrane-modifying peptides isolated from T. harzianum, trichorozins I–IV (117–120), belonged to peptaibols with 11 residues. It was reported that compounds 117–120 exhibited voltage-dependent ion channel-like activity in lipid bilayers [45]. Eleven peptides were isolated from T. harzianum M-903603, and named harzianins HC (121–131) [46]. The detailed study of such proline-rich 14-residue peptaibols revealed that harzianins HC increased the permeability of liposomes and improved voltage-dependent conductance [46]. An exogenous amino acid supply simplified the microheterogeneous peptide mixtures when Aib, Glu, or Arg was added to the fermentation media of T. harzianum M-902608. Harzianin PCU4 (132), trichorzin PAU4 (133), trichorzin PA II (134), trichorzin PA IV–VIII (135–139) and trichorzin PA IX (140) were isolated from this T. harzianum M-902608 [47]. When cultured in the Aib-enriched media, compounds 132 and 133 were isolated, while trichorzins PA was obtained from the standard culture media [47]. Trichorzianines A (TA) and B (TB) are peptaibols isolated from T. harzianum. TA IIIc (141) induced the growth inhibition and lysis of the amoeba Dictyostelium [48]. With the aid of positive ion FAB mass spectrometry, COSY and NOESY experiments, seven peptides of trichorzianines B isolated from T. harzianum were identified, and these peptides included trichorzianine TB IIa (142), trichorzianine TB IIIc (143), trichorzianine TB IVb (144), trichorzianine TB Vb (145), trichorzianine TB VIa (146), trichorzianine TB VIb (147) and trichorzianine TB VII (148) [49]. From a mangrove-derived fungus, T. harzianum D13, a novel heterocyclic dipeptide trichodermamide G (149), two known biogenetically related compounds, trichodermamide A (150) and aspergillazin A (151) were isolated. A unique sulfur bridge was observed in the structures of compounds 149 and 151 [50] (Figure 4).
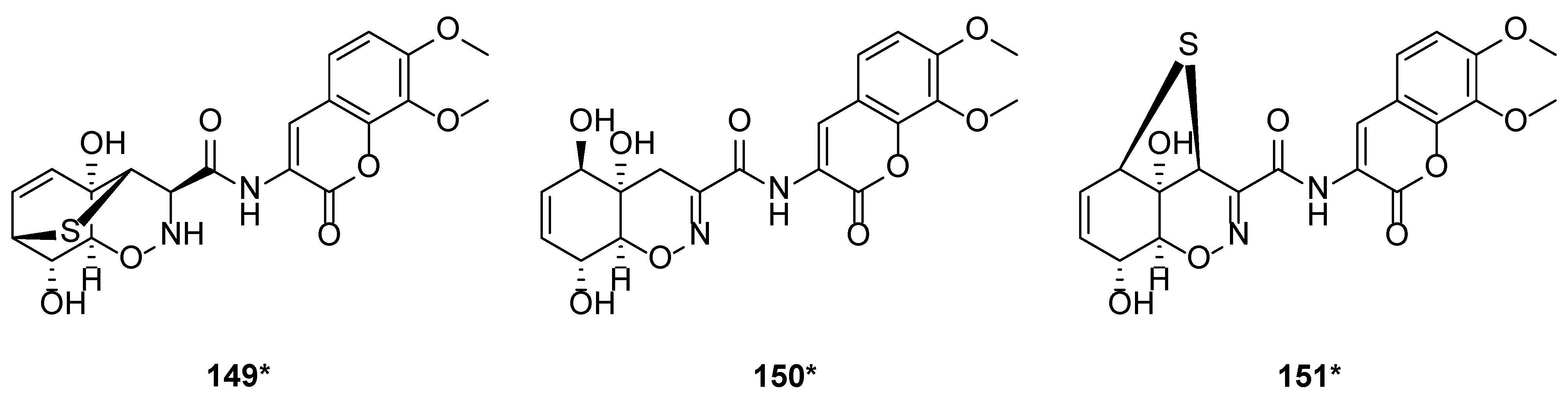
Figure 4. Chemical structures of peptides (149–151) from T. harzianum. * Means marine source compounds.
References
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2021, 38, 362–413.
- Sutak, R.; Camadro, J.M.; Lesuisse, E. Iron uptake mechanisms in marine phytoplankton. Front. Microbiol. 2020, 11, 566691.
- Pang, X.; Lin, X.; Yang, J.; Zhou, X.; Yang, B.; Wang, J.; Liu, Y. Spiro-phthalides and isocoumarins isolated from the marinesponge-derived fungus Setosphaeria sp. SCSIO41009. J. Nat. Prod. 2018, 81, 1860–1868.
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2022, 39, 1122–1171.
- Barra, L.; Dickschat, J.S. Harzianone biosynthesis by the biocontrol fungus Trichoderma. ChemBioChem 2017, 18, 2358–2365.
- Bissett, J.; Gams, W.; Jaklitsch, W.; Samuels, G.J. Accepted Trichoderma names in the year 2015. IMA Fungus 2015, 6, 263–295.
- Khan, R.A.A.; Najeeb, S.; Hussain, S.; Xie, B.; Li, Y. Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic fungi. Microorganisms 2020, 8, 817.
- Han, M.; Qin, D.; Ye, T.; Yan, X.; Wang, J.; Duan, X.; Dong, J. An endophytic fungus from Trichoderma harzianum SWUKD3.1610 that produces nigranoic acid and its analogues. Nat. Prod. Res. 2019, 33, 2079–2087.
- Fang, S.T.; Wang, Y.J.; Ma, X.Y.; Yin, X.L.; Ji, N.Y. Two new sesquiterpenoids from the marine-sediment-derived fungus Trichoderma harzianum P1–4. Nat. Prod. Res. 2019, 33, 3127–3133.
- Vinale, F.; Nigro, M.; Sivasithamparam, K.; Flematti, G.; Ghisalberti, E.L.; Ruocco, M.; Varlese, R.; Marra, R.; Lanzuise, S.; Eid, A.; et al. Harzianic acid: A novel siderophore from Trichoderma harzianum. FEMS Microbiol. Lett. 2013, 347, 123–129.
- Yamada, T.; Mizutani, Y.; Umebayashi, Y.; Inno, N.; Kawashima, M.; Kikuchi, T.; Tanaka, R. Tandyukisin, a novel ketoaldehyde decalin derivative, produced by a marine sponge-derived Trichoderma harzianum. Tetrahedron Lett. 2014, 55, 662–664.
- Shi, T.; Hou, X.-M.; Li, Z.-Y.; Cao, F.; Zhang, Y.-H.; Yu, J.-Y.; Zhao, D.-L.; Shao, C.-L.; Wang, C.-Y. Harzianumnones A and B: Two hydroxyanthraquinones from the coral-derived fungus Trichoderma harzianum. RSC Adv. 2018, 8, 27596–27601.
- Zhao, D.L.; Yang, L.J.; Shi, T.; Wang, C.Y.; Shao, C.L.; Wang, C.Y. Potent phytotoxic harziane diterpenes from a soft coral-derived strain of the fungus Trichoderma harzianum XS-20090075. Sci. Rep. 2019, 9, 13345.
- Shi, T.; Shao, C.L.; Liu, Y.; Zhao, D.L.; Cao, F.; Fu, X.M.; Yu, J.Y.; Wu, J.S.; Zhang, Z.K.; Wang, C.Y. Terpenoids from the coral-derived fungus Trichoderma harzianum (XS-20090075) induced by chemical epigenetic manipulation. Front. Microbiol. 2020, 11, 572.
- Yamada, T.; Suzue, M.; Arai, T.; Kikuchi, T.; Tanaka, R. Trichodermanins C-E, new diterpenes with a fused 6-5-6-6 ring system produced by a marine sponge-derived fungus. Mar. Drugs 2017, 15, 169.
- Yamada, T.; Fujii, A.; Kikuchi, T. New diterpenes with a fused 6-5-6-6 ring system isolated from the marine sponge-derived fungus Trichoderma harzianum. Mar. Drugs 2019, 17, 480.
- Song, Y.P.; Fang, S.T.; Miao, F.P.; Yin, X.L.; Ji, N.Y. Diterpenes and sesquiterpenes from the marine algicolous Fungus Trichoderma harzianum X-5. J. Nat. Prod. 2018, 81, 2553–2559.
- Li, B.; Li, L.; Peng, Z.; Liu, D.; Si, L.; Wang, J.; Yuan, B.; Huang, J.; Proksch, P.; Lin, W. Harzianoic acids A and B, new natural scaffolds with inhibitory effects against hepatitis C virus. Bioorg. Med. Chem. 2019, 27, 560–567.
- Tezuka, Y.; Tasaki, M.; Huang, Q.; Hatanaka, Y.; Kikuchi, T. 15-Hydroxyacorenone: New acorane-type sesquiterpene from the culture broth of the mycoparasitic fungus Trichoderma harzianum. Liebigs Ann. Recl. 1997, 12, 2579–2580.
- Yu, J.Y.; Shi, T.; Zhou, Y.; Xu, Y.; Zhao, D.L.; Wang, C.Y. Naphthalene derivatives and halogenate quinoline from the coral-derived fungus Trichoderma harzianum (XS-20090075) through OSMAC approach. J. Asian. Nat. Prod. Res. 2021, 23, 250–257.
- Ahluwalia, V.; Kumar, J.; Rana, V.S.; Sati, O.P.; Walia, S. Comparative evaluation of two Trichoderma harzianum strains for major secondary metabolite production and antifungal activity. Nat. Prod. Res. 2015, 29, 914–920.
- Liang, X.R.; Ma, X.Y.; Ji, N.Y. Trichosordarin A, a norditerpene glycoside from the marine-derived fungus Trichoderma harzianum R5. Nat. Prod. Res. 2020, 34, 2037–2042.
- Li, B.; Huang, Q.X.; Gao, D.; Liu, D.; Ji, Y.B.; Liu, H.G.; Lin, W.H. New C13 lipids from the marine-derived fungus Trichoderma harzianum. J. Asian. Nat. Prod. Res. 2015, 17, 468–474.
- Suzue, M.; Kikuchi, T.; Tanaka, R.; Yamada, T. Tandyukisins E and F, novel cytotoxic decalin derivatives isolated from a marine sponge-derived fungus. Tetrahedron Lett. 2016, 57, 5070–5073.
- Yamada, T.; Umebayashi, Y.; Kawashima, M.; Sugiura, Y.; Kikuchi, T.; Tanaka, R. Determination of the chemical structures of tandyukisins B-D, isolated from a marine sponge-derived fungus. Mar. Drugs 2015, 13, 3231–3240.
- Corley, D.G.; Miller-Wideman, M.; Durley, R.C. Isolation and structure of harzianum A: A new trichothecene from Trichoderma harzianum. J. Nat. Prod. 1994, 57, 422–425.
- Qian-Cutrone, J.; Huang, S.; Chang, L.P.; Pirnik, D.M.; Klohr, S.E.; Dalterio, R.A.; Hugill, R.; Lowe, S. Harziphilone and fleephilone, two new HIV REV/RRE binding inhibitors produced by Trichoderma harzianum. J. Antibiot. 1996, 49, 990–997.
- Ghisalberti, E.L.; Rowland, C.Y. Antifungal metabolites from Trichoderma harzianum. J. Nat. Prod. 1993, 56, 1799–1804.
- Kobayashi, M.; Uehara, H.; Matsunami, K.; Aoki, S.; Kitagawa, I. Trichoharzin, a new polyketide produced by the imperfect fungus Trichoderma harzianum separated from the marine sponge Micale cecilia. Tetrahedron Lett. 1993, 34, 7925–7928.
- Vinale, F.; Marra, R.; Scala, F.; Ghisalberti, E.L.; Lorito, M.; Sivasithamparam, K. Major secondary metabolites produced by two commercial Trichoderma strains active against different phytopathogens. Lett. Appl. Microbiol. 2006, 43, 143–148.
- Jeerapong, C.; Phupong, W.; Bangrak, P.; Intana, W.; Tuchinda, P. Trichoharzianol, a new antifungal from Trichoderma harzianum F031. J. Agric. Food Chem. 2015, 63, 3704–3708.
- Amagata, T.; Usami, Y.; Minoura, K.; Ito, T.; Numata, A. Cytotoxic substances produced by a fungal strain from a sponge: Physico-chemical properties and structures. J. Antibiot. 1998, 51, 33–40.
- Mevers, E.; Saurí, J.; Liu, Y.; Moser, A.; Ramadhar, T.R.; Varlan, M.; Williamson, R.T.; Martin, G.E.; Clardy, J. Homodimericin A: A complex hexacyclic fungal metabolite. J. Am. Chem. Soc. 2016, 138, 12324–12327.
- Mevers, E.; Chouvenc, T.; Su, N.-Y.; Clardy, J. Chemical interaction among termite-associated microbes. Chem. Eng. J. 2017, 43, 1078–1085.
- Koyama, M.; Kelly, T.R.; Watanabe, K.A. Novel type of potential anticancer agents derived from chrysophanol and emodin. Some structure-activity relationship studies. J. Med. Chem. 1988, 31, 283–284.
- De Moliner, E.; Moro, S.; Sarno, S.; Zagotto, G.; Zanotti, G.; Pinna, L.A.; Battistutta, R. Inhibition of protein kinase CK2 by anthraquinone-related compounds. J. Biol. Chem. 2003, 278, 1831–1836.
- Liu, S.-Y.; Lo, C.-T.; Chen, C.; Liu, M.-Y.; Chen, J.H.; Peng, K.C. Efficient isolation of anthraquinone-derivatives from Trichoderma harzianum ETS 323. J. Biochem. Biophys. Methods 2007, 70, 391–395.
- Liu, S.-Y.; Lo, C.-T.; Shibu, M.A.; Leu, Y.-L.; Jen, B.-Y.; Peng, K.-C. Study on the anthraquinones separated from the cultivation of Trichoderma harzianum strain Th-R16 and their biological activity. J. Agric. Food Chem. 2009, 57, 7288–7292.
- Hou, X.; Sun, R.; Feng, Y.; Zhang, R.; Zhu, T.; Che, Q.; Zhang, G.; Li, D. Peptaibols: Diversity, bioactivity, and biosynthesis. Eng. Microbiol. 2022, 2, 100026.
- Iida, A.; Sanekata, M.; Fujita, T.; Tanaka, H.; Enoki, A.; Fuse, G.; Kanai, M.; Rudewicz, P.J.; Tachikawa, E. Fungal metabolites. XVI. Structures of new peptaibols, trichokindins I-VII, from the fungus Trichoderma harzianum. Chem. Pharma. Bull. 1994, 42, 1070–1075.
- Tsantrizos, Y.S.; Pischos, S.; Sauriol, F.; Widden, P. Peptaibol metabolites of Tolypocladium geodes. Can. J. Chem. 1996, 74, 165–172.
- Augeven-Bour, I.; Rebuffat, S.; Auvin, C.; Goulard, C.; Prigent, Y.; Bodo, B. Harzianin HB I, an 11-residue peptaibol from Trichoderma harzianum: Isolation, sequence, solution synthesis and membrane activity. J. Chem. Soc. Perkin Trans. 1997, 1, 1587–1594.
- Hlimi, S.; Rebuffat, S.; Goulard, C.; Duchamp, S.; Bodo, B. Trichorzins HA and MA, antibiotic peptides from Trichoderma harzianum II. Sequence determination. J. Antibiot. 1995, 48, 1254–1261.
- Goulard, C.; Hlimi, S.; Rebuffat, S.; Bodo, B. Trichorzins HA and MA, antibiotic peptides from Trichoderma harzianum I. Fermentation, isolation and biological properties. J. Antibiot. 1995, 48, 1248–1253.
- Iida, A.; Sanekata, M.; WADA, S.-I.; Fujita, T.; Tanaka, H.; Enoki, A.; Fuse, G.; Kanai, M.; Asami, K. Fungal metabolities. XVIII. New membrane-modifying peptides, trichorozins I-IV, from the fungus trichoderma harzianum. Chem. Pharm. Bull. 1995, 43, 392–397.
- Rebuffat, S.; Goulard, C.; Bodo, B. Antibiotic peptides from Trichoderma harzianum: Harzianins HC, proline-rich 14-residue peptaibols. J. Chem. Soc. Perkin Trans. 1995, 1, 1849–1855.
- Leclerc, G.; Rebuffat, S.; Goulard, C.; Bodo, B. Directed biosynthesis of peptaibol antibiotics in two Trichoderma strains I. Fermentation and isolation. J. Antibiot. 1998, 51, 170–177.
- Hajji, M.E.; Rebuffat, S.; Doan, T.L.; Klein, G.; Satre, M.; Bodo, B. Interaction of trichorzianines A and B with model membranes and with the amoeba Dictyostelium. Biochim. Biophys. Acta 1989, 978, 97–104.
- Rebuffat, S.; Hajji, M.E.; Hennig, P.; Davoust, D.; Bodo, B. Isolation, sequence, and conformation of seven trichorzianines B from Trichoderma harzianum. Int. J. Pept. Protein Res. 1989, 34, 200–210.
- Zhao, D.-L.; Zhang, X.-F.; Huang, R.-H.; Wang, D.; Wang, X.-Q.; Li, Y.-Q.; Zheng, C.-J.; Zhang, P.; Zhang, C.-S. Antifungal nafuredin and epithiodiketopiperazine derivatives from the mangrove-derived fungus Trichoderma harzianum D13. Front. Microbiol. 2020, 11, 1495.
More
Information
Subjects:
Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
05 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




