
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mrutyunjaya Panda | -- | 4110 | 2022-11-30 12:26:45 | | | |
| 2 | Lindsay Dong | + 7 word(s) | 4117 | 2022-12-01 08:18:05 | | |
Video Upload Options
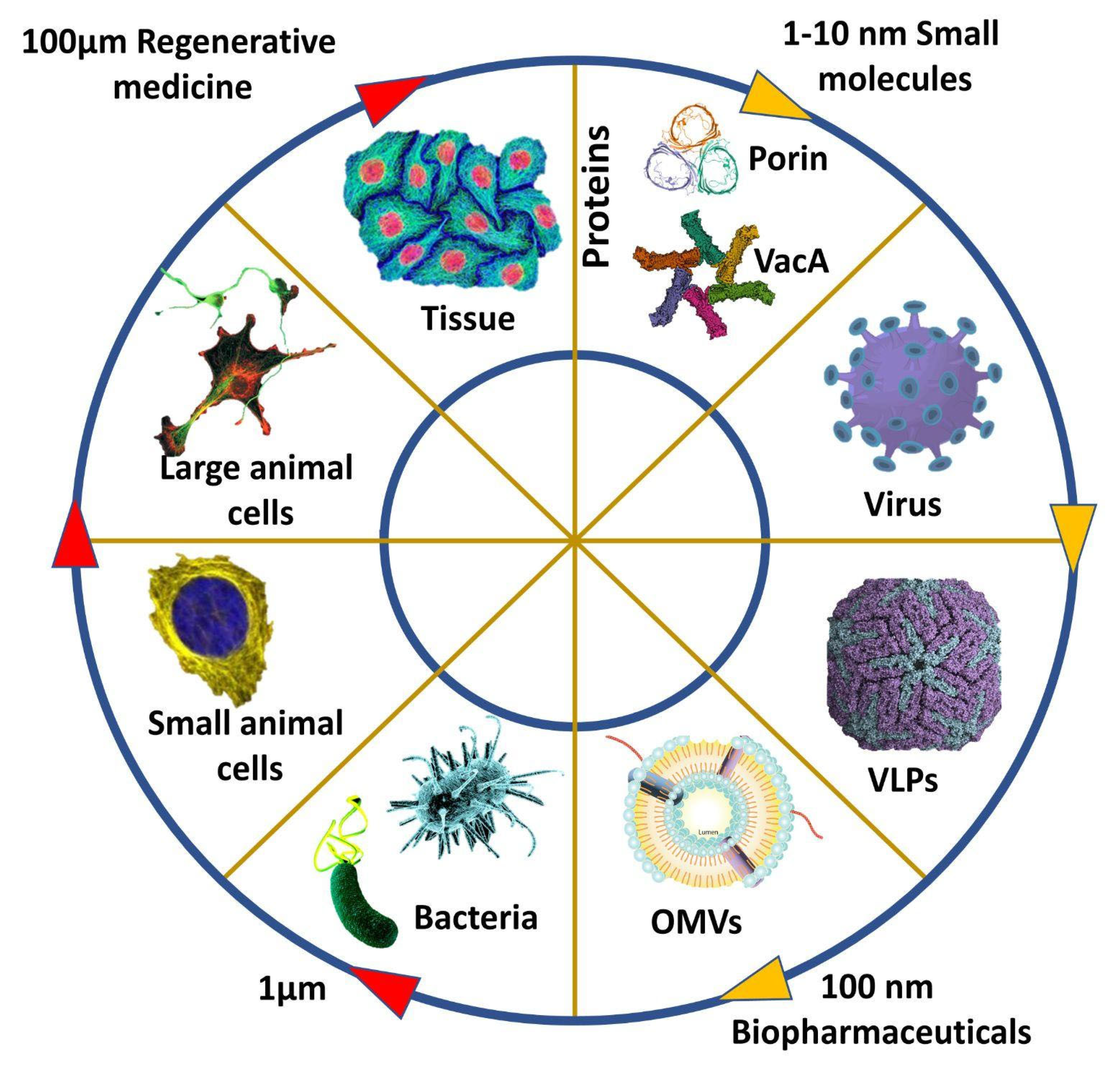
Vaccine adjuvants are substances that improve the immune capacity of a recombinant vaccine to a great extent and have been in use since the early 1900s; they are primarily short-lived and initiate antigen activity, mainly an inflammatory response. With the developing technologies and innovation, early options such as alum were modified, yet the inorganic nature of major vaccine adjuvants caused several side effects. Outer membrane vesicles, which respond to the stressed environment, are small nano-sized particles secreted by gram-negative bacteria. The secretory nature of outer membrane vesicles (OMV) gives us many benefits in terms of infection bioengineering.
1. Introduction
2. Formation of OMVs
3. Species Producing OMVs
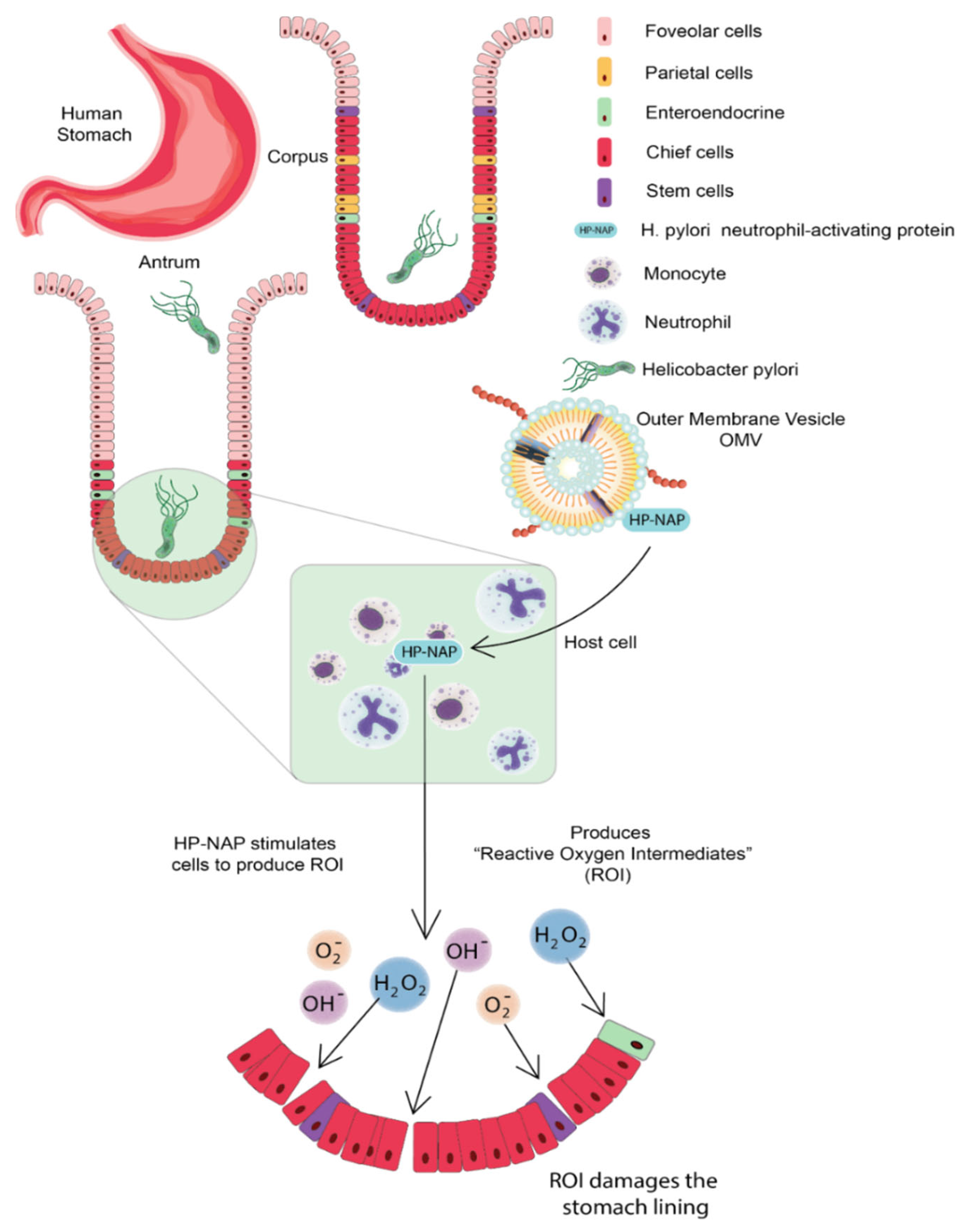
3.1. Helicobacter pylori
3.2. Neisseria meningitidis
3.3. Campylobacter jejuni
4. OMV-Based Vaccine Delivery
Host immune responses are the first line of barrier to stopping any infection, but occasionally immoderate responses could lead to lethargic tissue damage. Thus, there is a need for a system that avoids excessive immune responses. Along with the adjuvant properties, OMVs also provide complete immunity as they carry the antigen of pathogens. Moreover, the non-replicative nature of OMVs makes them advantageous for antigen delivery to the host thus denying any fright of infection associated with whole cell vaccine against disease-causing pathogens. OMVs stimulate the innate immune system of the host via the activation of TLRs and NLRs as they contain various PAMPs such as lipoproteins, LPS, and pathogenic DNA fragments [22].
Host immune responses are the first line of barrier to stopping any infection, but occasionally immoderate responses could lead to lethargic tissue damage. Thus, there is a need for a system that avoids excessive immune responses. Along with the adjuvant properties, OMVs also provide complete immunity as they carry the antigen of pathogens. Moreover, the non-replicative nature of OMVs makes them advantageous for antigen delivery to the host thus denying any fright of infection associated with whole cell vaccine against disease-causing pathogens. OMVs stimulate the innate immune system of the host via the activation of TLRs and NLRs as they contain various PAMPs such as lipoproteins, LPS, and pathogenic DNA fragments [22].
Host immune responses are the first line of barrier to stopping any infection, but occasionally immoderate responses could lead to lethargic tissue damage. Thus, there is a need for a system that avoids excessive immune responses. Along with the adjuvant properties, OMVs also provide complete immunity as they carry the antigen of pathogens. Moreover, the non-replicative nature of OMVs makes them advantageous for antigen delivery to the host thus denying any fright of infection associated with whole cell vaccine against disease-causing pathogens. OMVs stimulate the innate immune system of the host via the activation of TLRs and NLRs as they contain various PAMPs such as lipoproteins, LPS, and pathogenic DNA fragments [22].
5. Uptake of OMVs by Host Cells
OMVs can enter through many ways: macropinocytosis, lipid raft-dependent or independent endocytosis, clathrin, caveolin, and dynamin-dependent entry [23]. LPS is usually delivered through endocytosis; O antigen structural region is crucial to OMV entry; if OMVs lack antigen, they can use clathrin-dependent endocytosis to enter the host cell. PAMPs facilitate TLR signaling to facilitate OMV entry into host cells [24]. OMVs mimic the pathogenicity of bacteria; these invade the epithelial lining of the host cells and present themselves to the body’s immune cells, such as neutrophils, macrophages, and dendritic cells in the submucosa, thus activating the immune response. B and T lymphocytes will also be stimulated, thus, enabling a comprehensive immune response. When treated with E. coli, OMVs can also cause apoptosis of host cells by developing the G2 phase arrest, or they can carry virulence factors that cause cell death of epithelial cells of the gut [25]. Neisseria meningitidis OMVs can stimulate the human neutrophils to produce cytokines and chemokines such as interleukin 1-beta, IL-8, tumor necrosis factor-alpha, macrophage inflammatory proteins 1 alpha, and 1 beta [26]. Gamma interferon-stimulated can maintain or increase the inflammation reaction. E. coli OMVs have cytotoxic necrotizing factors 1; these can reduce the membrane fluidity of polymorphonuclear leukocytes, thereby decreasing the levels of cytokines and chemokines [27]. OMVs make macrophages secrete proinflammatory substances such as chemokines and cytokines; they are phagocytosed by macrophages, activating them, then induce other immune molecules such as interleukin 1-beta, IL-8, tumor necrosis factor-alpha, and macrophage inflammatory proteins 1 alpha and 1 beta [28]. Legionella pneumophila OMVs facilitate the replication of the pathogen in the host macrophages [29].
OMVs can also induce macrophage remodeling leading to dysfunction of immune cells. OMVs can play an important role in secreting anti-inflammatory molecules such as IL 10. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia OMVs phagocytosed by macrophages; they also stimulate the macrophages to release immune molecules such as TNFα, IL-8, and IL-1β and activate the NF-κB complex; they also primed and activated the inflammasome complex [30].
OMVs can also induce macrophage remodeling leading to dysfunction of immune cells. OMVs can play an important role in secreting anti-inflammatory molecules such as IL 10. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia OMVs phagocytosed by macrophages; they also stimulate the macrophages to release immune molecules such as TNFα, IL-8, and IL-1β and activate the NF-κB complex; they also primed and activated the inflammasome complex [30].
6. OMVs-Based Therapeutics
7. Native OMV Vaccines
8. Heterologous Vaccines
9. OMV-Based Novel Adjuvants
OMV as an adjuvant is based on Pathogen Associated Molecular Pattern (PAMP), induction of dangerous molecules, and geographic concept. The PAMPs activate both the Pattern Recognizing Receptors (PRRs) and Toll Signalling Receptors (TLRs); these then recruit the cells into active immunity and stimulate the Antigen Presenting Cells (APCs). It is thus thought that OMVs could increase antigen uptake, cell surface expression, and immunostimulatory molecules, aiding in T cell production [42]. Danger molecules affect host cells, leading to mature T cells engaged in an enhanced immune response [43]. The geographic concept involves diverting antigens from the injection site to the tissue-draining lymph node by dendritic cells. Thus, OMVs have immunogenic properties, act as carriers, and show an inherent adjuvant effect [44].
Meningitidis MenB OMVs was used as an adjuvant with group A meningococcal capsular polysaccharide. Unlike other adjuvants causing hypersensitivity, these vaccines had low toxicity and elicited a strong T cell response. [45]. OMV derived from E. coli can stimulate the humoral and cell-mediated immunity mediated via the IFN-g and IL-17 T cell-dependent response production [46]. In HIV, in addition to inducing IFN-g and IL-17, T cell-dependent response and performing Th1 oriented response. Other particles such as Virus-Like Particles (VLP) induced a high anti-HIV IgG production by using OMV vaccines [47][48]. OMVs have been explored as an adjuvant by mixing them with other known antigens such as keyhole limpet hemocyanin and ovalbumin in the hepatitis B vaccine, triggering an enhanced immune response in the host cells. Lipoprotein OspA in Neisseria meningitidis is a surface-exposed antigen. The surface-exposed antigen elicited a more robust OspA-specific antibody immune response against outer membrane proteins and LPS than the luminal antigen [49].
10. Conclusions
OMVs are an emerging and promising platform for vaccine development, especially in non-regenerative and acellular vaccines. OMVs-based vaccines are more immunogenic in comparison to non-regenerative and VLP vaccines. OMV-based vaccines are safer than the whole pathogen attenuated vaccine as OMVs do not have the self-replicative capacity, and there is no evolutionary escaping. OMVs vaccines are potential candidates where whole-cell treatment approaches are not applicable. Moreover, the cons of OMV-based vaccines are their low yields and endotoxic effects. Notably, there is continuous improvement in extraction methods of OMVs to increase vesicle formation and genetic modifications to avoid possible endotoxicity. Various factors are responsible for elevated spontaneous vesicle formation in bacteria, such as temperature, stress, and antibiotic treatments. Host-associated antimicrobial peptides have immunomodulatory potential; they act by directing and evoking the proinflammatory (Th1/Th17) response. Importantly, OMV-based vaccines are a potential platform for vaccine development against various bacterial infections. Conclusively, OMV-based vaccines are the future of antibacterial vaccine development.
References
- Fulsundar, S.; Harms, K.; Flaten, G.E.; Johnsen, P.J.; Chopade, B.A.; Nielsen, K.M. Gene Transfer Potential of Outer Membrane Vesicles of Acinetobacter Baylyi and Effects of Stress on Vesiculation. Appl. Environ. Microbiol. 2014, 80, 3469–3483.
- Jan, A.T. Outer Membrane Vesicles (OMVs) of Gram-Negative Bacteria: A Perspective Update. Front. Microbiol. 2017, 8, 1053.
- Kulkarni, H.M.; Jagannadham, M.V.Y. Biogenesis and Multifaceted Roles of Outer Membrane Vesicles from Gram-Negative Bacteria. Microbiology 2014, 160, 2109–2121.
- Chatterjee, S.N.; Das, J.Y. Electron Microscopic Observations on the Excretion of Cell-Wall Material by Vibrio Cholerae. Microbiology 1967, 49, 1–11.
- Mayrand, D.; Grenier, D. Biological Activities of Outer Membrane Vesicles. Can. J. Microbiol. 1989, 35, 607–613.
- Hussain, S.; Bernstein, H.D. The Bam Complex Catalyzes Efficient Insertion of Bacterial Outer Membrane Proteins into Membrane Vesicles of Variable Lipid Composition. J. Biol. Chem. 2018, 293, 2959–2973.
- Turner, L.; Bitto, N.J.; Steer, D.L.; Lo, C.; D’Costa, K.; Ramm, G.; Shambrook, M.; Hill, A.F.; Ferrero, R.L.; Kaparakis-Liaskos, M. Helicobacter Pylori Outer Membrane Vesicle Size Determines Their Mechanisms of Host Cell Entry and Protein Content. Front. Immunol. 2018, 9, 1466.
- Bonnington, K.E.; Kuehn, M.J. Protein Selection and Export via Outer Membrane Vesicles. Biochim. Et Biophys. Acta (BBA) Mol. Cell Res. 2014, 1843, 1612–1619.
- Roier, S.; Zingl, F.G.; Cakar, F.; Durakovic, S.; Kohl, P.; Eichmann, T.O.; Klug, L.; Gadermaier, B.; Weinzerl, K.; Prassl, R.; et al. A Novel Mechanism for the Biogenesis of Outer Membrane Vesicles in Gram-Negative Bacteria. Nat. Commun. 2016, 7, 10515.
- Kulp, A.; Kuehn, M.J. Biological Functions and Biogenesis of Secreted Bacterial Outer Membrane Vesicles. Annu. Rev. Microbiol. 2010, 64, 163–184.
- Alfarouk, K.O.; Bashir, A.H.H.; Aljarbou, A.N.; Ramadan, A.M.; Muddathir, A.K.; AlHoufie, S.T.S.; Hifny, A.; Elhassan, G.O.; Ibrahim, M.E.; Alqahtani, S.S.; et al. The Possible Role of Helicobacter Pylori in Gastric Cancer and Its Management. Front. Oncol. 2019, 9, 75.
- Parker, H.; Keenan, J.I. Composition and Function of Helicobacter Pylori Outer Membrane Vesicles. Microbes Infect. 2012, 14, 9–16.
- Chmiela, M.; Walczak, N.; Rudnicka, K. Helicobacter Pylori Outer Membrane Vesicles Involvement in the Infection Development and Helicobacter Pylori-Related Diseases. J. Biomed. Sci. 2018, 25, 78.
- Olofsson, A.; Vallström, A.; Petzold, K.; Tegtmeyer, N.; Schleucher, J.; Carlsson, S.; Haas, R.; Backert, S.; Wai, S.N.; Gröbner, G.; et al. Biochemical and Functional Characterization of Helicobacter Pylori Vesicles. Mol. Microbiol. 2010, 77, 1539–1555.
- Mullaney, E.; Brown, P.A.; Smith, S.M.; Botting, C.H.; Yamaoka, Y.Y.; Terres, A.M.; Kelleher, D.P.; Windle, H.J. Proteomic and Functional Characterization of the Outer Membrane Vesicles from the Gastric Pathogen Helicobacter Pylori. Proteom. Clin. Appl. 2009, 3, 785–796.
- Ismail, S.; Hampton, M.B.; Keenan, J.I. Helicobacter Pylori Outer Membrane Vesicles Modulate Proliferation and Interleukin-8 Production by Gastric Epithelial Cells. Infect. Immun. 2003, 71, 5670–5675.
- Hancock, R.E.W.; Haney, E.F.; Gill, E.E. The Immunology of Host Defence Peptides: Beyond Antimicrobial Activity. Nat. Rev. Immunol. 2016, 16, 321–334.
- Acevedo, R.; Fernandez, S.; Zayas, C.; Acosta, A.; Sarmiento, M.; Ferro, V.; Rosenqvist, E.; Campa, C.; Cardoso, D.; Garcia, L.; et al. Bacterial Outer Membrane Vesicles and Vaccine Applications. Front. Immunol. 2014, 5, 121.
- Holst, J.; Martin, D.; Arnold, R.; Huergo, C.C.; Oster, P.; O’Hallahan, J.; Rosenqvist, E. Properties and Clinical Performance of Vaccines Containing Outer Membrane Vesicles from Neisseria Meningitidis. Vaccine 2009, 27, B3–B12.
- Ge, Z.; Schauer, D.B.; Fox, J.G. In Vivo Virulence Properties of Bacterial Cytolethal-Distending Toxin. Cell. Microbiol. 2008, 10, 1599–1607.
- Lara-Tejero, M.; Galán, J.E. CdtA, CdtB, and CdtC Form a Tripartite Complex That Is Required for Cytolethal Distending Toxin Activity. Infect. Immun. 2001, 69, 4358–4365.
- Kaparakis-Liaskos, M.; Ferrero, R.L. Immune Modulation by Bacterial Outer Membrane Vesicles. Nat. Rev. Immunol. 2015, 15, 375–387.
- Alaniz, R.C.; Deatherage, B.L.; Lara, J.C.; Cookson, B.T. Membrane Vesicles Are Immunogenic Facsimiles of Salmonella Typhimurium That Potently Activate Dendritic Cells, Prime B and T Cell Responses, and Stimulate Protective Immunity in Vivo. J. Immunol. 2007, 179, 7692–7701.
- van der Pol, L.; Stork, M.; van der Ley, P. Outer Membrane Vesicles as Platform Vaccine Technology. Biotechnol. J. 2015, 10, 1689–1706.
- Cheng, K.; Zhao, R.; Li, Y.; Qi, Y.; Wang, Y.; Zhang, Y.; Qin, H.; Qin, Y.; Chen, L.; Li, C.; et al. Bioengineered Bacteria-Derived Outer Membrane Vesicles as a Versatile Antigen Display Platform for Tumor Vaccination via Plug-and-Display Technology. Nat. Commun. 2021, 12, 2041.
- Rabets, A.; Bila, G.; Grytsko, R.; Samborskyy, M.; Rebets, Y.; Vari, S.G.; Pagneux, Q.; Barras, A.; Boukherroub, R.; Szunerits, S.; et al. The Potential of Developing Pan-Coronaviral Antibodies to Spike Peptides in Convalescent COVID-19 Patients. Arch. Immunol. Ther. Exp. 2021, 69, 5.
- Qing, G.; Gong, N.; Chen, X.; Chen, J.; Zhang, H.; Wang, Y.; Wang, R.; Zhang, S.; Zhang, Z.; Zhao, X.; et al. Natural and Engineered Bacterial Outer Membrane Vesicles. Biophys. Rep. 2019, 5, 184–198.
- Klimentová, J.; Stulík, J. Methods of Isolation and Purification of Outer Membrane Vesicles from Gram-Negative Bacteria. Microbiol. Res. 2015, 170, 1–9.
- Dragovic, R.A.; Gardiner, C.; Brooks, A.S.; Tannetta, D.S.; Ferguson, D.J.P.; Hole, P.; Carr, B.; Redman, C.W.G.; Harris, A.L.; Dobson, P.J.; et al. Sizing and Phenotyping of Cellular Vesicles Using Nanoparticle Tracking Analysis. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 780–788.
- Gerritzen, M.J.H.; Salverda, M.L.M.; Martens, D.E.; Wijffels, R.H.; Stork, M. Spontaneously Released Neisseria Meningitidis Outer Membrane Vesicles as Vaccine Platform: Production and Purification. Vaccine 2019, 37, 6978–6986.
- Chandler, C.E.; Ernst, R.K. Bacterial Lipids: Powerful Modifiers of the Innate Immune Response. F1000Research 2017, 6, 1334.
- Ko, S.H.; Rho, D.J.; Jeon, J.I.; Kim, Y.-J.; Woo, H.A.; Kim, N.; Kim, J.M. Crude Preparations of Helicobacter Pylori Outer Membrane Vesicles Induce Upregulation of Heme Oxygenase-1 via Activating Akt-Nrf2 and MTOR–IκB Kinase–NF-ΚB Pathways in Dendritic Cells. Infect. Immun. 2016, 84, 2162–2174.
- Schetters, S.T.T.; Jong, W.S.P.; Horrevorts, S.K.; Kruijssen, L.J.W.; Engels, S.; Stolk, D.; Daleke-Schermerhorn, M.H.; Garcia-Vallejo, J.; Houben, D.; Unger, W.W.J.; et al. Outer Membrane Vesicles Engineered to Express Membrane-Bound Antigen Program Dendritic Cells for Cross-Presentation to CD8+ T Cells. Acta Biomater. 2019, 91, 248–257.
- Durant, L.; Stentz, R.; Noble, A.; Brooks, J.; Gicheva, N.; Reddi, D.; O’Connor, M.J.; Hoyles, L.; McCartney, A.L.; Man, R.; et al. Bacteroides Thetaiotaomicron-Derived Outer Membrane Vesicles Promote Regulatory Dendritic Cell Responses in Health but Not in Inflammatory Bowel Disease. Microbiome 2020, 8, 88.
- Laughlin, R.C.; Alaniz, R.C. Outer Membrane Vesicles in Service as Protein Shuttles, Biotic Defenders, and Immunological Doppelgängers. Gut Microbes 2016, 7, 450–454.
- Vidakovics, M.L.A.P.; Jendholm, J.; Mörgelin, M.; Månsson, A.; Larsson, C.; Cardell, L.-O.; Riesbeck, K. B Cell Activation by Outer Membrane Vesicles—A Novel Virulence Mechanism. PLoS Pathog. 2010, 6, e1000724.
- Bottero, D.; Gaillard, M.E.; Zurita, E.; Moreno, G.; Martinez, D.S.; Bartel, E.; Bravo, S.; Carriquiriborde, F.; Errea, A.; Castuma, C.; et al. Characterization of the Immune Response Induced by Pertussis OMVs-Based Vaccine. Vaccine 2016, 34, 3303–3309.
- Finne, J.; Leinonen, M.; Mäkelä, P.H. ANTIGENIC SIMILARITIES BETWEEN BRAIN COMPONENTS AND BACTERIA CAUSING MENINGITIS: Implications for Vaccine Development and Pathogenesis. Lancet 1983, 322, 355–357.
- Granoff, D.M. Review of Meningococcal Group B Vaccines. Clin. Infect. Dis. 2010, 50 (Suppl. S2), S54–S65.
- Tunheim, G.; Arnemo, M.; Næss, L.M.; Fjeldheim, Å.K.; Nome, L.; Bolstad, K.; Aase, A.; Mandiarote, A.; González, H.; González, D.; et al. Preclinical Immunogenicity and Functional Activity Studies of an A+W Meningococcal Outer Membrane Vesicle (OMV) Vaccine and Comparisons with Existing Meningococcal Conjugate- and Polysaccharide Vaccines. Vaccine 2013, 31, 6097–6106.
- Jäger, J.; Marwitz, S.; Tiefenau, J.; Rasch, J.; Shevchuk, O.; Kugler, C.; Goldmann, T.; Steinert, M. Human Lung Tissue Explants Reveal Novel Interactions during Legionella Pneumophila Infections. Infect. Immun. 2014, 82, 275–285.
- Martins, P.; Machado, D.; Theizen, T.H.; Guarnieri, J.P.O.; Bernardes, B.G.; Gomide, G.P.; Corat, M.A.F.; Abbehausen, C.; Módena, J.L.P.; Melo, C.F.O.R.; et al. Outer Membrane Vesicles from Neisseria Meningitidis (Proteossome) Used for Nanostructured Zika Virus Vaccine Production. Sci. Rep. 2018, 8, 8290.
- Muralinath, M.; Kuehn, M.J.; Roland, K.L.; Curtiss, R. Immunization with Salmonella Enterica Serovar Typhimurium-Derived Outer Membrane Vesicles Delivering the Pneumococcal Protein PspA Confers Protection against Challenge with Streptococcus Pneumoniae. Infect. Immun. 2011, 79, 887–894.
- Schild, S.; Nelson, E.J.; Bishop, A.L.; Camilli, A. Characterization of Vibrio Cholerae Outer Membrane Vesicles as a Candidate Vaccine for Cholera. Infect. Immun. 2009, 77, 472–484.
- Chen, L.; Valentine, J.L.; Huang, C.-J.; Endicott, C.E.; Moeller, T.D.; Rasmussen, J.A.; Fletcher, J.R.; Boll, J.M.; Rosenthal, J.A.; Dobruchowska, J.; et al. Outer Membrane Vesicles Displaying Engineered Glycotopes Elicit Protective Antibodies. Proc. Natl. Acad. Sci. USA 2016, 113, E3609–E3618.
- Chen, D.J.; Osterrieder, N.; Metzger, S.M.; Buckles, E.; Doody, A.M.; DeLisa, M.P.; Putnam, D. Delivery of Foreign Antigens by Engineered Outer Membrane Vesicle Vaccines. Proc. Natl. Acad. Sci. USA 2010, 107, 3099–3104.
- Shen, Y.; Torchia, M.L.G.; Lawson, G.W.; Karp, C.L.; Ashwell, J.D.; Mazmanian, S.K. Outer Membrane Vesicles of a Human Commensal Mediate Immune Regulation and Disease Protection. Cell Host Microbe 2012, 12, 509–520.
- Siegemund, S.; Schütze, N.; Freudenberg, M.A.; Lutz, M.B.; Straubinger, R.K.; Alber, G. Production of IL-12, IL-23 and IL-27p28 by Bone Marrow-Derived Conventional Dendritic Cells Rather than Macrophages after LPS/TLR4-Dependent Induction by Salmonella Enteritidis. Immunobiol. 2008, 212, 739–750.
- Tan, K.; Li, R.; Huang, X.; Liu, Q. Outer Membrane Vesicles: Current Status and Future Direction of These Novel Vaccine Adjuvants. Front. Microbiol. 2018, 9, 783.




