Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Liu, Y.; Guo, Z.; Zhou, X. Chinese Cordyceps in the Antitumor Mechanisms. Encyclopedia. Available online: https://encyclopedia.pub/entry/37165 (accessed on 07 February 2026).
Liu Y, Guo Z, Zhou X. Chinese Cordyceps in the Antitumor Mechanisms. Encyclopedia. Available at: https://encyclopedia.pub/entry/37165. Accessed February 07, 2026.
Liu, Yan, Zhi-Jian Guo, Xuan-Wei Zhou. "Chinese Cordyceps in the Antitumor Mechanisms" Encyclopedia, https://encyclopedia.pub/entry/37165 (accessed February 07, 2026).
Liu, Y., Guo, Z., & Zhou, X. (2022, November 29). Chinese Cordyceps in the Antitumor Mechanisms. In Encyclopedia. https://encyclopedia.pub/entry/37165
Liu, Yan, et al. "Chinese Cordyceps in the Antitumor Mechanisms." Encyclopedia. Web. 29 November, 2022.
Copy Citation
Chinese Cordyceps is a valuable source of natural products with various therapeutic effects. It is rich in various active components, of which adenosine, cordycepin and polysaccharides have been confirmed with significant immunomodulatory and antitumor functions.
Chinese Cordyceps
antitumor
immunomodulatory
bioactive components
mechanism
1. Enhancing Antitumor Immune Responses
Studies have shown that the occurrence and development of tumors are closely related to immune surveillance. Immunotherapy has been proved to be an effective method to treat a variety of cancers [1] and increasing data has shown that antitumor effects of enhancing immunity may be associated with its action for the regulation of the tumor immune environment [2][3]. Immune cells show different phenotypes in response to various environmental cues (microbial products, damaged cells, cytokines, etc.). Chinese Cordyceps has a biphasic regulatory effect on the immune-cell phenotype and can increase antitumor immune activity in the tumor immune microenvironment (TIM): increasing the proinflammatory phenotypic while reversing the suppressive phenotype (Table 1).
Table 1. Antitumor immunity effects and mechanisms of Chinese Cordyceps in various models.
| Bioactive Component | Pharmacological Effects | Models | Major Mediating Signaling Pathways | Mechanism of Action | Ref. |
|---|---|---|---|---|---|
| Cordycepin | ↑Antitumor immunity responses ↓CT 26 cell migration ↑CT 26 cell apoptosis |
CT 26 cells in mice | ↑CD4+ T, CD8+ T cells ↑NK cells ↑M1 macrophages ↑CD11b+, F4/80+ ↓CD47 |
[4] | |
| JLM 0636 (cordycepin-enriched extract of C. militaris) |
↑Th 1 cells ↑Immune responses ↓Treg cells ↓Immunosuppression |
FM3A murine breast cancer cells, derived from C3H/He mouse | ↑CD8+ T cells ↑IFN-γ ↓CD4+CD25+ T cells ↓IL-2 ↓TGF-β |
[5] | |
| WECS (Nucleoside extract of C. sinensis) |
↓MDA-MB-231 cells ↓4T1 cells ↑M1 macrophages ↑Immune responses |
MDA-MB-231, 4T1 breast cancer cells co-cultured with macrophages | NF-κB | ↑CD38 ↑iNOS ↑IL-1β ↑IL-12p70 ↑TNF-α ↑IL-6 ↑IFN-γ ↑NO |
[6] |
| EPSP | ↑M1 macrophages ↑Spleen lymphocyte ↑Immune response ↓Tumor migration |
B16 melanoma-bearing mice | ↓Bcl-2 | [7] | |
| APSF | ↑M1 macrophages ↑Immune response ↓M2 macrophages ↓Immunosuppression |
Ana-1 mouse macrophages co-cultured with H22 cells | NF-κB | ↑TNF-α ↑IL-12 ↑iNOS ↓IL-10 ↓SR ↓MR |
[8] |
| CMPB90-1 | ↑M1 macrophages ↑Immune response ↓M2 macrophages ↓Immunosuppression |
IL-4, tumor cell supernatant-induced RAW264.7 cells | NF-κB Akt MAPK (p38 and ERK) |
↓IL-10 ↓TGF-β ↓Arg-1 ↑IL-12 ↑iNOS |
[9] |
Akt, serine/threonine kinase; Arg-1, arginase-1; ERK, extracellular-signal-regulated kinases; IFN-γ, interferon-gamma; IL, interleukin; iNOS, inducible nitric oxide synthase; IPS, intracellular polysaccharide; MAPKs, mitogen-activated protein kinases; MR, mannose receptor; NK, natural killer cell; NF-κB, nuclear factor kappaB; NO, nitric oxide; TGF-β, transforming growth factor-beta; TNF-α, tumor necrosis factor-alpha; SR, scavenger receptor.
Chinese Cordyceps as an immunomodulator has suppressive effects on the immune system. Cordycepin has demonstrated to inhibit the differentiation of T cells into regulatory T cells (Treg, a suppressive phenotype of T cells) and delay tumor growth in tumor-bearing mice [5]. The further investigation reveals that cordycepin decreased the secretions of interleukin-2 (IL-2) and transforming growth factor-β (TGF-β) which were essential for Treg cells’ proliferation and differentiation. In addition, macrophages are the key player in the immune system which can engulf and destroy foreign pathogens and cancer cells. Tumor-associated macrophages (TAM), taking up 50% of the infiltrated cells at the tumor site, can be differentiated into two phenotypes: M1 phenotype (classic activation polarization) or M2 phenotype (alternative activation polarization), based on the stimulatory signals from the tumor microenvironment (TME) [10]. Macrophages in the TME are predominantly in an M2 state [11], and currently M2 macrophages are potential targets for the treatment of cancer. Activated M2 macrophages would suppress the immune system and promote tumor progression by releasing immunosuppressive cytokines (i.e., IL-4 and IL-10) and recruiting Th2 and Treg cells [12][13]. Clinical studies have proposed a new strategy where reversing M2 into the M1 phenotype is an effective approach to enhance antitumor immunity [14][15]. Chinese Cordyceps are capable to of resetting the macrophage phenotype repolarizing M2 to M1 macrophages. A study by Chen et al. [8] showed that APSF, a polysaccharide isolated from the fruiting bodies of O. sinensis, reversed M2 to the M1 phenotype through reducing the expression of IL-10 and increasing the expression of tumor necrosis factor-alpha (TNF-α), IL-12 and inducible nitric oxide synthase (iNOS), and downregulating the expressions of SR and MR (Scavenger Receptor and Mannose Receptor, M2 markers), in Ana-1 mouse macrophages co-cultured with a supernatant of H22 cells. Additionally, a novel polysaccharide CMPB90-1 from C. militaris was found to remodel TAMs from M2 to the M1 phenotype through decreasing the mRNA expression level of immunosuppressive cytokines (IL-10, TGF-β and Arg-1 (arginase 1), M2 markers) while increasing the mRNA expression levels of IL-12 and iNOS (M1 markers). Additionally, a further investigation revealed that the signaling pathways of p38, extracellular-signal-regulated kinases (ERK), Akt and nuclear factor kappaB (NF-κB) were activated [9]. These findings demonstrated that Chinese Cordyceps and its bioactive constituents could promote immune cells’ antitumor function by enhancing immune responses and downregulating immune suppression (Figure 1).
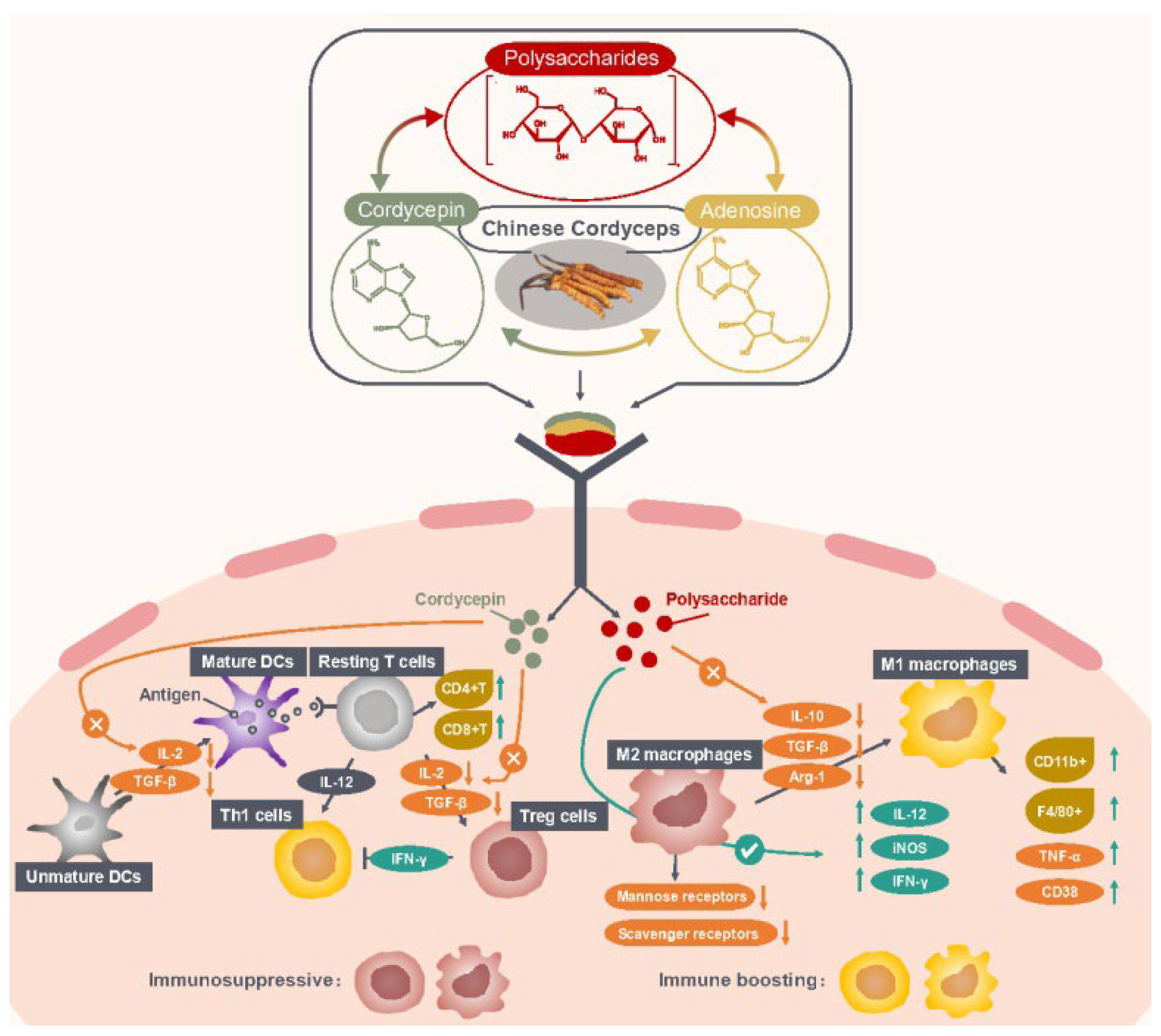
Figure 1. Mechanism of Chinese Cordyceps regulating immune cells in TIM. Abbreviations: Arg-1, arginase-1; CD38, CD11b+ and F4/80+, M1 macrophage markers; IFN-γ, interferon-gamma; IL, interleukin; iNOS, inducible nitric oxide synthase; TGF-β, transforming growth factor-beta; TIM, tumor immune microenvironment; TNF-α, tumor necrosis factor-alpha.
2. Direct Antitumor Effects
To date, increasing studies have shown that Chinese Cordyceps has significant antitumor effects. Although the mechanisms of action are complicated, the possible mechanisms of antitumor action of Chinese Cordyceps are summarized and presented in Table 2.
Table 2. Antitumor effects and mechanisms of Chinese Cordyceps in various cancer models.
| Cancer | Bioactive Component | Pharmacological Effects | Cell line | Major Mediating Signaling Pathways | Mechanism of Action | Ref. |
|---|---|---|---|---|---|---|
| Bladder cancer | ||||||
| Cordycepin | ↓Migration and invasion | TNF-α-induced 5637 and T-24 cells | NF-κB AP-1 |
↓MMP-9 | [16] | |
| Breast cancer | ||||||
| Cordycepin | ↑Apoptosis | MDA-MB-231 cells | Caspase | ↑Bax (mitochondria) ↑Cytochrome c (cytosol) ↑PARP ↑c-caspases-9, -3 ↑DNA fragmentation |
[17] | |
| Cordycepin | ↑Autophagy | MCF-7 cells | Autophagy | ↑LC3-II ↑Autophagosome-like structure |
[17] | |
| Cordycepin | ↑Apoptosis | MDA-MB-435 and T47D cells | ↑DNA fragmentation ↑Histone γH2AX ↓RNA synthesis |
[18] | ||
| C. militaris extract | ↑Apoptosis | MCF-7 cells | Caspase | ↑Bax/Bcl-2 ↑c-caspase-7, -8 |
[19] | |
| Cordycepin | ↑Apoptosis | C6 glioma cells | A2AR Caspase |
↑Caspase-7 ↑p-p53 ↑PARP |
[20] | |
| Cervical cancer | ||||||
| Cordycepin | ↑Apoptosis ↓Cell cycle |
SiHa cells HeLa cells |
↓CDK-2 ↓Cyclin-E1 ↓Cyclin-A2 ↑ROS |
[21] | ||
| CCP (C. cicadae polysaccharides) |
↑Apoptosis ↓Cell cycle |
hela cells | Akt | ↑Bak ↑Bax ↑Caspase-3, -7, -9 ↓P21 ↓P27 ↓CDK2 ↓Cyclin E1 ↓Cyclin A2 ↓Bcl-2 ↓Bcl-xl ↓PARP |
[22] | |
| Colon cancer | ||||||
| CSP | ↑Autophagy, ↑Apoptosis |
HCT116 cells | Autophagy mTOR Caspase |
↑LC3B-II ↑Caspase-8, -3 |
[23][24] | |
| Cordycepin | ↓Cell cycle | HCT116 cells | JNK MAPK | ↑p21WAF1 ↓Cyclin B1 ↓Cdc25c ↓Cdc2 |
[25] | |
| Colorectal cancer | ||||||
| C. militaris extract | ↓Cell cycle | RKO cells | ↑Bax ↑Bim ↑Bak ↑Bad ↑PARP ↑p-p53 ↑c-caspase -9, -3 |
[26] | ||
| Gastric cancer | ||||||
| Cordycepin | ↑Apoptosis | AGS cells | PI3K/Akt | ↑Caspase-9, -3, -7 ↑Bax ↓Bcl-2 |
[27] | |
| CECJ (C. jiangxiensis extract) |
↑Apoptosis ↓Cell cycle |
SGC-7901 cells | Caspase | ↑Caspase-3 | [28] | |
| Liver cancer | ||||||
| Adenosine | ↑Apoptosis | HepG2 cells | Caspase | ↑TNF ↑TRADD ↑TRAIL-R2 ↑FADD ↑Caspase-9, -8, -3 |
[29] | |
| Adenosine | ↑Apoptosis | BEL-7404 cells | Caspase | ↑Caspase-8, -9, -3 ↑c-PARP ↑Bak ↑Mcl1 ↑Bcl-xl |
[30] | |
| Adenosine | ↑Apoptosis | HuH-7 Fas-deficient cells | Caspase | ↑AMP ↓Caspase-3, -8 ↓c-FLIP |
[31] | |
| CMF (C. militaris extract) |
↓Migration and invasion ↓Tumor growth |
SMMC-7721 cells | Akt ERK |
↓p-VEGFR2 ↓p-Akt ↓p-ERK |
[32] | |
| Lung cancer | ||||||
| AECS1, AECS2 (C. sinensis nucleosides extract) |
↓Tumor growth | Lewis xenograft mouse | Akt NF-κB |
↓p-Akt ↓MMP2 ↓MMP9 ↓p-IκBα ↓TNF-α ↓COX-2 ↓Bcl2 ↓Bcl-xl ↑Bax |
[33] | |
| CS (C. sinensis extract) |
↑Apoptosis | H157 NSCLC cells | ↓VEGF ↓bFGF |
[34] | ||
| C. militaris extract | ↑Apoptosis ↓Cell cycle |
NCI-H460 cells | ↑P53 ↑P21 ↑53BP1 |
[35] | ||
| Mouse melanoma | ||||||
| Cordycepin | ↓Proliferation | B16-BL6 cells | A3R | ↑GSK-3β ↓Cyclin D1 protein |
[36] | |
| CME-1 | ↓Tumor migration | B16-F10 cells | NF-κB MAPK (ERK and p38) |
↓MMP-1 | [37] | |
| EPSP | ↓Tumor migration | B16 cells | ↓c-Myc ↓c-Fos ↓VEGF |
[38] | ||
| Myeloma cancer | ||||||
| Cordycepin | ↑Apoptosis | MM.1S cells | Caspase | ↑Caspase-9, -3, -8 ↓RNA synthesis |
[29] | |
| Oral cancer | ||||||
| CMP (C. militaris polysaccharides) |
↑Apoptosis ↓Cell cycle |
4NAOC-1 cells | STAT3 ERK |
↓ki-67 ↓EGFR ↓IL-17A ↓Cyclin B1 ↓DNA synthesis |
[39] | |
| WECM (C. militaris extract) |
↑Apoptosis ↓Cell cycle |
SCC-4 cells | ↓PCNA ↓VEGF ↓Caspase-3 ↓c-fos |
[40] | ||
| Ovarian cancer | ||||||
| CME (C. militaris extract) |
↑Apoptosis ↓Migration |
SKOV-3 cells | NF-κB | ↓TNF-1R ↓Bcl-2 ↑Bcl-xl |
[41] | |
| ↑Autophagy ↓Tumor growth |
A2780 and OVCAR3 cells | ENT1-AMPK-mTOR | ↑LC3II/LC3I ↑p-AMPK |
[42] | ||
| Prostate cancer | ||||||
| Cordycepin | ↓Migration and invasion | LNCaP cells | PI3K/Akt | ↑TIMP-1 ↑TIMP-2 ↓MMP-2 ↓MMP-9 |
[43] | |
| Testicular cancer | ||||||
| Cordycepin | ↑Apoptosis ↓Cell cycle |
MA-10 cells | Caspase | ↑Caspase-9, -3, -7 ↑DNA fragmentation |
[44] |
AMP, activated protein kinase; A2AR, adenine 2A receptor; A3R, adenine 3 receptor; c-FLIP, cellular FADD-like interleukin-1β-converting enzyme inhibitory protein; c-PARP, cleaved-poly ADP-ribose polymerase; COX-2, cyclooxygenase-2; c-Fos, c-Myc, cellular proto-oncogenes; cyclin B1, Cdc25c and Cdc2, cell cycle regulatory proteins; ERK, extracellular signal-regulated kinases; FADD, fas-associated death domain; JNK, Jun N terminal kinase; LC3-II, the lipidated form of LC3B; MMP, mitochondrial membrane potential; mTOR, mechanistic target of rapamycin; MAPK, mitogen-activated protein kinases; p-Akt, phosphorylated serine/threonine kinase; p21WAF1, cyclin-dependent kinase inhibitor; TNF, tumor necrosis factor; TRADD, TNF receptor-associated death domain; TRAIL-R2, TNF-related apoptosis-inducing ligand receptor 2; VEGF, vascular endothelial growth factor.
2.1. Inducing Apoptosis and Autophagy
Apoptosis is a form of programmed cell death and essential for the development and homeostasis of organisms, and its abnormal regulation is perhaps related to tumor formulation. Inducing apoptosis involves two major pathways: the intrinsic pathway (particularly mitochondrial stress) and extrinsic signal pathway. The Fas/FasL system plays an important role in apoptosis regulation. Fas and its ligand FasL are mainly expressed on the cell membrane surface. When external FasL is expressed by cytotoxic T lymphocytes and combines with Fas which is expressed by target cells, Fas-associated death domain (FADD) will be formed. FADD triggers apoptosis through recruiting extrinsic stimuli and death receptors (DRs) [45][46]. A study by Lee et al. [47] showed that cordycepin inhibited proliferation and induced HT-29 colon cancer cells’ apoptosis by increasing expression of DR3, caspase-8, -1 and -3. Caspases is a family of cysteine proteases and acts on proteins or enzymes related to the cytoskeleton or DNA and is responsible for apoptosis. DR3 activated apoptosis through triggering TRADD, FADD and caspase-8 [48], and caspase-8 further activated downstream effectors caspase-1 and caspase-3, resulting in cell death [49][50]. Similarly, cordycepin induced apoptosis in human prostate carcinoma LNCaP cells via the caspase pathway by increasing the expression of Fas, DR5, caspase-8, -9 and -3, and causing a dose-dependent increase in pro-apoptotic Bax and decrease in anti-apoptotic Bcl-2 [51]. Changes in Bax and Bcl-2 levels trigger a collapse of mitochondrial membrane potential and activation of caspase-9 and -3. A study by Balk et al. [52] revealed that cordycepin increased the levels of Fas, FasL and TRAIL (related apoptosis-inducing ligand) of U87MG cells, and decreased Bcl-2 level, indicating that cordycepin induced apoptosis via the Fas/FasL pathway. Lee et al. [19] found that C. militaris extract induced apoptosis by increasing the protein expression ratio of Bax/Bcl-2 and the cleavage of caspase-7, -8 and -9 in MCF-7 cells. In addition, cordycepin has been demonstrated to inhibit the proliferation of B16-BL6 mouse melanoma cells through combination with adenosine A3 receptor (A3R) on the B16 cell membrane, reducing expression of cyclin D1 protein and activating glycogen synthase kinase-3β (GSK-3β) [36]. In addition, cordycepin is also thought to induce apoptosis through A3R and A2AR. The apoptosis induction of cordycepin is possibly mediated by A3R in human bladder cancer T24 cells since both overexpression of A3R and cordycepin treatment decreased cell survival since the apoptosis-inducing effect of cordycepin is abolished with the depletion of adenosine receptors [53]. Moreover, adenosine was found to induce apoptosis and upregulate mRNAs of TNF, FADD, TRADD, and TRAIL-2 by activating caspase-3, -8 and -9 in human hepatoma HepG2 cells [29]. Ma et al. [30] found a novel apoptosis mechanism that extracellular adenosine could trigger apoptosis by increasing reactive oxygen species (ROS) production and mitochondrial membrane dysfunction in BEL-7404 liver cancer cells. Choi et al. [17] found that cordycepin induced MDA-MB-231 cells’ apoptosis through increasing translocation of Bax in the mitochondrion and triggering cytosolic release of cytochrome c and activation of caspases-9 and -3. These studies indicate that Chinese Cordyceps induces apoptosis via both the mitochondrion-mediated intrinsic pathway and extrinsic Fas/FasL and ARs pathways.
Autophagy, mediated by an intracellular suicide program, plays important roles in antitumor responses. A study by Qi et al. [23] showed that a polysaccharide named CSP from O. sinensis mycelia inhibited the proliferation of HCT116 human colon cancer cells through inducing apoptosis and autophagy. On one hand, CSP induced apoptosis by activating caspase-8 and -3, on the other hand, it inhibited lysosome formation, blocked autophagy flux and accumulated autophagosomes, resulting in autophagy. The further investigation revealed that signaling pathways of PI3K-AKT-mTOR and AMPK-mTOR-ULK1 were all involved. Cordycepin was also found to induce autophagic cell death and formation of a large membranous vacuole in MCF-7 human breast cancer cells, accompanied with the increase in autophagosome marker LC3-II levels [17]. Generally, autophagy occurs before apoptosis under certain stress stimuli, and autophagy will be inactivated when stress exceeds the intensity threshold or critical duration, followed by the activation of apoptosis [54].
2.2. Blocking Cell Cycle
The cell cycle can be mainly divided into four phases, of which G1, S and G2/M phases are crucial checkpoints in the cell cycle processes. Cell cycle arrest in the G1, S and G2/M phases can lead to the inhibition of tumor cells’ proliferation and induction of apoptosis. Adenosine, cordycepin and polysaccharides were found to cause cell cycle arrest at certain checkpoints. Cordycepin inhibited the growth of 5637 and T-24 bladder cancer cells and HCT116 colon cancer cells, through G2/M cell-cycle arrest. The expression of p21WAF1 (a universal key inhibitor in regulating cell-cycle progression) was upregulated and cyclin B1, Cdc25c and Cdc2 (G2/M cell-cycle regulatory proteins) were downregulated, through the JNK1 signal pathway [55][56]. Cells blocked in the G2/M phase failed to enter mitosis, resulting in cell growth inhibition. Moreover, cordycepin induced an increase in subG1 cell number and the decrease in G1 and G2/M cell numbers and cell viability through inducing caspase-9, -3 and -7 expression as subG1 phase accumulation could be partly suppressed using a caspase inhibitor, which indicated that the caspase-9 pathway is involved in cordycepin-induced subG1 phase arrest [44].
In addition, cordycepin is a transcription and polyadenylation inhibitor and affects RNA synthesis. A study showed that cordycepin caused accumulation of the corresponding triphosphate derivative, 3′dATP, which might lead to the incorporation of analogue into nascent nucleic acid oligonucleotides and RNA synthesis inhibition [57][58]. This might illustrate that cordycepin affects the cell cycle from another perspective. An extract of Cordyceps cicadae was identified as a nucleoside mixture containing adenine, adenosine, uridine and N6-(2-Hydroxyethyl)-adenosine, induced S phase arrest in human gastric cancer SGC-7901 cells, which was related to downregulation of CDK2 expression and upregulation of expression of transcription factor E2F1 (cyclin/CDK complexes, regulating G1/S phase transition), cyclin A2 and cyclin E [59].
2.3. Inhibiting Migration, Invasion and Metastasis
Metastasis refers to the movement of cancer cells from primary tumor sites to other organs and tissues and is the end result of multiple interactions including invasion between the tumor and host, indicating the uncontrolled spread of the tumor cells. Epithelial–mesenchymal transition (EMT)-related proteins such as matrix metalloproteinases (MMPs) play an important role in metastasis. For example, MMP-2 and MMP-9 can lead to the degradation of extracellular matrix (ECM) components and tissue invasion [60][61][62]. A study showed that cordycepin inhibited 5637 and T-24 cells’ invasion through decreasing MMP-9 expression and the transcriptional activity of activator protein-1 (AP-1), which were identified by gel-shift assay as cis-elements for TNF-α activation of the MMP-9 promoter via the NF-κB/MMP-9 pathway [63]. In addition, a novel polysaccharide CME-1 isolated from O. sinensis was found to inhibit migration of B16-F10 melanoma cells, and the mechanism was that CME-1 reduced MMP-1 expression and downregulated the phosphorylation level of ERK1/2 and p38 MAPK [37].
Angiogenesis is vital for organ growth and repair, and essential for tumor growth. The vascular endothelial growth factor (VEGF) family plays an important role in angiogenesis. VEGF, a key angiogenic growth factor, has a higher expression level in tumor tissues and can accelerate the differentiation, proliferation, and migration of endothelial cells. Chinese Cordyceps has been demonstrated to inhibit the VEGF/VEGFR2 signaling pathway and exert antiangiogenesis function [32]. Moreover, the overexpression of proto-oncogenes c-Myc and c-Fos may promote tumor cell proliferation under growth-promoting stimulation. c-Myc, encoding a ubiquitous transcription factor and promoting cell division, is related to apoptosis and the occurrence and development of various tumors. c-Fos, essential for cell proliferation, can upregulate the cell cycle by induction of cyclin D1 [64]. c-Fos is expressed at a low level in normal cells while it is overexpressed in tumor cells. Yang et al. [38] found that EPSF isolated from C.sinensis could downregulate the expression of VEGF, c-Myc and c-Fos, which was the important factor to inhibit tumor growth, invasion and metastasis.
References
- Zigler, M.; Shir, A.; Levitzki, A. Targeted cancer immunotherapy. Curr. Opin. Pharmacol. 2013, 13, 504–510.
- Xu, L.; Feng, J.M.; Li, J.X.; Zhu, J.M.; Song, S.S.; Tong, L.J.; Chen, Y.; Yang, X.Y.; Shen, Y.Y.; Lian, F.L.; et al. Tanshinone-1 induces tumor cell killing, enhanced by inhibition of secondary activation of signaling networks. Cell Death Dis. 2013, 4, e905.
- Jia, Y.; Guan, Q.; Guo, Y.; Du, C. Reduction of inflammatory hyperplasia in the intestine in colon cancer-prone mice by water-extract of Cistanche deserticola. Phytother. Res. 2012, 26, 812–819.
- Deng, Q.; Li, X.; Fang, C.; Li, X.; Zhang, J.; Xi, Q.; Li, Y.; Zhang, R. Cordycepin enhances anti-tumor immunity in colon cancer by inhibiting phagocytosis immune checkpoint CD47 expression. Int. Immunopharmacol. 2022, 107, 108695.
- Jeong, M.H.; Lee, C.M.; Lee, S.W.; Seo, S.Y.; Seo, M.J.; Kang, B.W.; Jeong, Y.K.; Choi, Y.J.; Yang, K.M.; Jo, W.S. Cordycepin-enriched Cordyceps militaris induces immunomodulation and tumor growth delay in mouse-derived breast cancer. Oncol. Rep. 2013, 30, 1996–2002.
- Li, J.; Cai, H.; Sun, H.; Qu, J.; Zhao, B.; Hu, X.; Li, W.; Qian, Z.; Yu, X.; Kang, F.; et al. Extracts of Cordyceps sinensis inhibit breast cancer growth through promoting M1 macrophage polarization via NF-κB pathway activation. J. Ethnopharmacol. 2020, 260, 112969.
- Zhang, W.Y.; Yang, J.Y.; Chen, J.P.; Hou, Y.Y.; Han, X.D. Immunomodulatory and antitumour effects of an exopolysaccharide fraction from cultivated Cordyceps sinensis (Chinese caterpillar fungus) on tumor-bearing mice. Biotechnol. Appl. Biochem. 2005, 42, 9–15.
- Chen, W.; Yuan, F.; Wang, K.; Song, D.; Zhang, W. Modulatory effects of the acid polysaccharide fraction from one of anamorph of Cordyceps sinensis on Ana-1 cells. J. Ethnopharmacol. 2012, 142, 739–745.
- Bi, S.; Huang, W.; Chen, S.; Huang, C.; Li, C.; Guo, Z.; Yang, J.; Zhu, J.; Song, L.; Yu, R. Cordyceps militaris polysaccharide converts immunosuppressive macrophages into M1-like phenotype and activates T lymphocytes by inhibiting the PD-L1/PD-1 axis between TAMs and T lymphocytes. Int. J. Biol. Macromol. 2020, 150, 261–280.
- Guo, Q.; Li, J.; Lin, H. Effect and molecular mechanisms of traditional Chinese medicine on regulating tumor immunosuppressive microenvironment. Biomed. Res. Int. 2015, 2015, 261620.
- Liu, M.; Luo, F.; Ding, C.; Albeituni, S.; Hu, X.; Ma, Y.; Cai, Y.; Mcnally, L.; Sanders, M.A.; Jain, D.; et al. Dectin-1 activation by a natural product β-glucan converts immunosuppressive macrophages into an M1-like phenotype. J. Immunol. 2015, 195, 5055–5065.
- Ji, Y.; Sun, S.; Xu, A.; Bhargava, P.; Yang, L.; Lam, K.S.L.; Gao, B.; Lee, C.H.; Kersten, S.; Qi, L. Activation of natural killer T cells promotes M2 macrophage polarization in adipose tissue and improves systemic glucose tolerance via interleukin-4 (IL-4)/STAT6 protein signaling axis in obesity. J. Biol. Chem. 2012, 287, 13561–13571.
- Yuan, F.; Fu, X.; Shi, H.; Chen, G.; Dong, P.; Zhang, W. Induction of murine macrophage M2 polarization by cigarette smoke extract via the JAK2/STAT3 pathway. PLoS ONE 2014, 9, e107063.
- Isidro, R.A.; Appleyard, C.B. Colonic macrophage polarization in homeostasis, inflammation, and cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G59–G73.
- Tariq, M.; Zhang, J.; Liang, G.; Ding, L.; He, Q.; Yang, B. Macrophage polarization: Anti-cancer strategies to target tumor-associated macrophage in breast cancer. J. Cell. Biochem. 2017, 118, 2484–2501.
- Lee, E.J.; Kim, W.J.; Moon, S.K. Cordycepin suppresses TNF-alpha-induced invasion, migration and matrix metalloproteinase-9 expression in human bladder cancer cells. Phytother. Res. 2010, 24, 1755–1761.
- Choi, S.; Lim, M.H.; Kim, K.M.; Jeon, B.H.; Song, W.O.; Kim, T.W. Cordycepin-induced apoptosis and autophagy in breast cancer cells are independent of the estrogen receptor. Toxicol. Appl. Pharmacol. 2011, 257, 165–173.
- Lee, H.J.; Burger, P.; Vogel, M.; Friese, K.; Bruening, A. The nucleoside antagonist cordycepin causes DNA double strand breaks in breast cancer cells. Investig. New Drugs 2012, 30, 1917–1925.
- Lee, D.; Lee, W.Y.; Jung, K.; Kwon, Y.S.; Kim, D.; Hwang, G.S.; Kim, C.E.; Lee, S.; Kang, K.S. The inhibitory effect of cordycepin on the proliferation of MCF-7 breast cancer cells, and its mechanism/ an investigation using network pharmacology-based analysis. Biomology 2019, 9, 414.
- Chen, Y.; Yang, S.H.; Hueng, D.Y.; Syu, J.P.; Liao, C.C.; Wu, Y.C. Cordycepin induces apoptosis of C6 glioma cells through the adenosine 2A receptor-p53-caspase-7-PARP pathway. Chem. Biol. Interact. 2014, 216, 17–25.
- Tania, M.; Shawon, J.; Saif, K.; Kiefer, R.; Khorram, M.S.; Halim, M.A.; Khan, M.A. Cordycepin Downregulates Cdk-2 to Interfere with Cell Cycle and Increases Apoptosis by Generating ROS in Cervical Cancer Cells: In vitro and in silico Study. Curr. Cancer Drug Targets 2019, 19, 152–159.
- Xu, J.; Tan, Z.C.; Shen, Z.Y.; Shen, X.J.; Tang, S.M. Cordyceps cicadae polysaccharides inhibit human cervical cancer hela cells proliferation via apoptosis and cell cycle arrest. Food Chem. Toxicol. 2021, 148, 111971.
- Qi, W.; Zhou, X.; Wang, J.; Zhang, K.; Zhou, Y.; Chen, S.; Nie, S.; Xie, M. Cordyceps sinensis polysaccharide inhibits colon cancer cells growth by inducing apoptosis and autophagy flux blockage via mTOR signaling. Carbohydr. Polym. 2020, 237, 116113.
- Wang, J.; Nie, S.; Kan, L.; Chen, H.; Cui, S.W.; Phillips, A.O.; Phillips, G.O.; Xie, M. Comparison of structural features and antioxidant activity of polysaccharides from natural and cultured Cordyceps sinensis. Food Sci. Biotechnol. 2017, 26, 55–62.
- Lee, S.J.; Moon, G.S.; Jung, K.H.; Kim, W.J.; Moon, S.K. c-Jun N-terminal kinase 1 is required for cordycepin-mediated induction of G2/M cell-cycle arrest via p21WAF1 expression in human colon cancer cells. Food Chem. Toxicol. 2010, 48, 277–283.
- Lee, W.H.; Lee, S.; Lee, K.; Shin, Y.S.; Kang, H.; Cho, H. Anti-cancer effect of Cordyceps militaris in human colorectal carcinoma RKO cells via cell cycle arrest and mitochondrial apoptosis. DARU J. Pharm. Sci. 2015, 23, 35.
- Lee, H.H.; Jeong, J.W.; Choi, Y.H. Inhibition of PI3K/AKT signaling pathway enhances cordycepin-induced apoptosis in human gastric cancer cells. J. Korean Soc. Food Sci. Nutr. 2016, 45, 835–842.
- Xiao, J.H.; Chen, D.X.; Fang, N.; Liu, Z.L.; Zhang, T. Growth arrest of human gastric adenocarcinoma cells by bioactive compounds of Cordyceps jiangxiensis (CaoMuWang) through induction of apoptosis. J. Food Agric. Environ. 2006, 4, 66–73.
- Yang, D.; Yaguchi, T.; Lim, C.R.; Ishizawa, Y.; Nakano, T.; Nishizaki, T. Tuning of apoptosis-mediator gene transcription in HepG2 human hepatoma cells through an adenosine signal. Cancer Lett. 2010, 291, 225–229.
- Ma, Y.F.; Zhang, J.; Zhang, Q.; Chen, P.; Song, J.; Yu, S.; Liu, H.; Liu, F.; Song, C.; Yang, D.; et al. Adenosine induces apoptosis in human liver cancer cells through ROS production and mitochondrial dysfunction. Biochem. Biophys. Res. Commun. 2014, 448, 8–14.
- Yang, D.; Yaguchi, T.; Yamamoto, H.; Nishizaki, T. Intracellularly transported adenosine induces apoptosis in HuH-7 human hepatoma cells by downregulating c-FLIP expression causing caspase-3/-8 activation. Biochem. Pharmacol. 2007, 73, 1665–1675.
- Li, Z.; Guo, Z.; Zhu, J.; Bi, S.; Luo, Y.; Yu, R.; Huang, W.; Song, L. Cordyceps militaris fraction inhibits angiogenesis of hepatocellular carcinoma in vitro and in vivo. Pharmacogn. Mag. 2020, 16, 169–176.
- Huo, X.; Liu, C.; Bai, X.; Li, W.; Li, J.; Hu, X.; Cao, L. Aqueous extract of Cordyceps sinensis potentiates the antitumor effect of DDP and attenuates therapy-associated toxicity in non-small cell lung cancer via IkBa/NFkB and AKT/MMP2/MMP9 pathways. RSC Adv. 2017, 66, 37743–37754.
- Yao, L.S.; Li, Y.; He, W.; Yi, K.S.; Huang, M. Polysaccharide of Cordyceps sinensis Enhances Cisplatin Cytotoxicity in Non–Small Cell Lung Cancer H157 Cell Line. Integr. Cancer Ther. 2011, 10, 359–367.
- Bizarro, A.; Ferreira, I.C.F.R.; Soković, M.; Van, G.; Leo, J.L.D.; Sousa, D.; Vasconcelos, M.H.; Lima, R.T. Cordyceps militaris (L.) Link Fruiting Body Reduces the Growth of a Non-Small Cell Lung Cancer Cell Line by Increasing Cellular Levels of p53 and p21. Molecules 2015, 20, 13927–13940.
- Yoshikawa, N.; Yamada, S.; Takeuchi, C.; Kagota, S.; Shinozuka, K.; Kunitomo, M.; Nakamura, K. Cordycepin (3′-deoxyadenosine) inhibits the growth of B16-BL6 mouse melanoma cells through the stimulation of adenosine A3 receptor followed by glycogen synthase kinase-3β activation and cyclin D1 suppression. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2008, 377, 591–595.
- Jayakumar, T.; Chiu, C.C.; Wang, S.H.; Chou, D.S.; Huang, Y.K.; Sheu, J.R. Anti-cancer effects of CME-1, a novel polysaccharide, purified from the mycelia of Cordyceps sinensis against B16-F10 melanoma cells. J. Cancer Res. Ther. 2014, 10, 43–49.
- Yang, J.; Zhang, W.; Shi, P.; Chen, J.; Han, X.; Wang, Y. Effects of exopolysaccharide fraction (EPSF) from a cultivated Cordyceps sinensis fungus on c-Myc, c-Fos, and VEGF expression in B16 melanoma-bearing mice. Pathol. Res. Pract. 2005, 201, 745–750.
- Hsu, P.Y.; Lin, Y.H.; Yeh, E.L.; Lo, H.C.; Hsu, T.H.; Su, C.C. Cordycepin and a preparation from Cordyceps militaris inhibit malignant transformation and proliferation by decreasing EGFR and IL-17RA signaling in a murine oral cancer model. Oncotarget 2017, 8, 93712–93728.
- Lin, L.T.; Lai, Y.J.; Wu, S.C.; Hsu, W.H.; Tai, C.J. Optimal conditions for cordycepin production in surface liquid-cultured Cordyceps militaris treated with porcine liver extracts for suppression of oral cancer. J. Food Drug Anal. 2018, 26, 135–144.
- Jo, E.; Jang, H.J.; Yang, K.E.; Jang, M.S.; Huh, Y.H.; Yoo, H.S.; Park, J.S.; Jang, I.S.; Park, S.J. Cordyceps militaris induces apoptosis in ovarian cancer cells through TNF-α/TNFR1-mediated inhibition of NF-κB phosphorylation. BMC Complement. Med. Ther. 2020, 20, 1.
- Yoon, S.Y.; Lindroth, A.M.; Kwon, S.; Park, S.J.; Par, Y.J. Adenosine derivatives from Cordyceps exert antitumor effects against ovarian cancer cells through ENT1-mediated transport, induction of AMPK signaling, and consequent autophagic cell death. Biomed. Pharmcother. 2022, 153, 113491.
- Jeong, J.W.; Jin, C.Y.; Park, C.; Han, M.H.; Kim, G.Y.; Moon, S.K.; Kim, C.G.; Jeong, Y.K.; Kim, W.J.; Lee, J.D.; et al. Inhibition of migration and invasion of LNCaP human prostate carcinoma cells by cordycepin through inactivation of Akt. Int. J. Oncol. 2012, 40, 1697–1704.
- Jen, C.Y.; Lin, C.Y.; Huang, B.M.; Leu, S.F. Cordycepin induced MA-10 mouse Leydig tumor cell apoptosis through caspase-9 pathway. Evid. Based Complement. Altern. Med. 2011, 2011, 984537.
- Algeciras-Schimnich, A.; Shen, L.; Barnhart, B.C. Molecular ordering of the initial signaling events of CD95. Mol. Cell. Biol. 2002, 22, 207–220.
- Villa-Morales, M.; Fernandez-Piqueras, J. Targeting the Fas FasL signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 85–101.
- Lee, S.Y.; Debnath, T.; Kim, S.K.; Lim, B.O. Anti-cancer effect and apoptosis induction of cordycepin through DR3 pathway in the human colonic cancer cell HT-29. Food Chem. Toxicol. 2013, 60, 439–447.
- Ashkenazi, A.; Dixit, V.M. Death receptors: Signaling and modulation. Science 1998, 281, 1305–1308.
- Gao, L.; Abu, K.Y. Hijacking of apoptotic pathways by bacterial pathogens. Microbes Infect. 2002, 2, 1705–1719.
- Detjen, K.M.; Farwig, K.; Welzel, M.; Wiedenmann, B.; Rosewicz, S. Interferon gamma inhibits growth of human pancreatic carcinoma cells via caspase-1 dependent induction of apoptosis. Gut 2001, 49, 251–262.
- Lee, H.H.; Kim, S.O.; Kim, G.Y.; Moon, S.K.; Kim, W.J.; Jeong, Y.K.; Yoo, Y.H.; Choi, Y.H. Involvement of autophagy in cordycepin-induced apoptosis in human prostate carcinoma LNCaP cells. Environ. Toxicol. Pharmacol. 2014, 38, 239–250.
- Balk, J.S.; Mun, S.W.; Kim, K.S.; Park, S.J.; Yoon, H.K.; Kim, D.H.; Park, M.K.; Kim, C.H.; Lee, Y.C. Apoptotic effects of cordycepin through the extrinsic pathway and p38 MAPK activation in human glioblastoma U87MG cells. J. Microbiol. Biotechnol. 2016, 26, 309–314.
- Cao, H.L.; Liu, Z.J.; Chang, Z. Cordycepin induces apoptosis in human bladder cancer cells via activation of A3 adenosine receptors. Tumor Biol. 2017, 39, 7.
- Marino, G.; Niso-Santano, M.; Baehrecke, E.H.; Guido, K. Self-consumption: The interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014, 15, 81–94.
- Lee, J.S.; Kwon, J.S.; Yun, J.S.; Pahk, J.W.; Shin, W.C.; Lee, S.Y.; Hong, E.K. Structural characterization of immunostimulating polysaccharide from cultured mycelia of Cordyceps militaris. Carbohydr. Polym. 2010, 80, 1011–1017.
- Lee, S.J.; Kim, S.K.; Choi, W.S.; Kim, W.J.; Moon, S.K. Cordycepin causes p21WAF1-mediated G2/M cell-cycle arrest by regulating c-Jun N-terminal kinase activation in human bladder cancer cells. Arch. Biochem. Biophys. 2009, 490, 103–109.
- Chen, L.S.; Stellrecht, C.M.; Gandhi, V. RNA-directed agent, cordycepin, induces cell death in multiple myeloma cells. Br. J. Haematol. 2008, 140, 682–687.
- Holbein, S.; Wengi, A.; Decourty, L.; Freimoser, F.M.; Jacquier, A.; Dichtl, B. Cordycepin interferes with 3′ end formation in yeast independently of its potential to terminate RNA chain elongation. RNA 2009, 15, 837–849.
- Xie, H.; Li, X.; Chen, Y.; Lang, M.; Shen, Z.; Shi, L. Ethanolic extract of Cordyceps cicadae exerts antitumor effect on human gastric cancer SGC-7901 cells by inducing apoptosis, cell cycle arrest and endoplasmic reticulum stress. J. Ethnopharmacol. 2019, 231, 230–240.
- Hunter, K.W.; Crawford, N.P.S.; Alsarraj, J. Mechanisms of metastasis. Breast Cancer Res. 2008, 10, S2.
- Tao, X.; Ning, Y.; Zhao, X.; Pan, T. The effects of cordycepin on the cell proliferation, migration and apoptosis in human lung cancer cell lines A549 and NCI-H460. J. Pharm. Pharmacol. 2016, 68, 901–911.
- Nakamura, K.; Shinozuka, K.; Yoshikawa, N. Anticancer and antimetastatic effects of cordycepin, an active component of Cordyceps sinensis. J. Pharm. Sci. 2015, 127, 53–56.
- Lee, S.J.; Cho, J.Y.; Hong, E.K. Study on macrophage activation and structural characteristics of purified polysaccharide from the liquid culture broth of Cordyceps militaris. Carbohydr. Polym. 2010, 82, 982–988.
- Phuchareon, J.; Tokuhisa, T. Deregulated c-Fos/AP-1 modulates expression of the cyclin and the cdk gene in splenic B cells stimulated with lipopolysaccharide. Cancer Lett. 1995, 92, 203–208.
More
Information
Subjects:
Others
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
3 times
(View History)
Update Date:
30 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




