Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jamir Pitton Rissardo | -- | 1307 | 2022-11-28 18:33:51 | | | |
| 2 | Camila Xu | Meta information modification | 1307 | 2022-11-29 07:31:44 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rissardo, J.P.; Caprara, A.L.F. Calcitonin Gene-Related Peptide and Gepants. Encyclopedia. Available online: https://encyclopedia.pub/entry/36915 (accessed on 07 February 2026).
Rissardo JP, Caprara ALF. Calcitonin Gene-Related Peptide and Gepants. Encyclopedia. Available at: https://encyclopedia.pub/entry/36915. Accessed February 07, 2026.
Rissardo, Jamir Pitton, Ana Letícia Fornari Caprara. "Calcitonin Gene-Related Peptide and Gepants" Encyclopedia, https://encyclopedia.pub/entry/36915 (accessed February 07, 2026).
Rissardo, J.P., & Caprara, A.L.F. (2022, November 28). Calcitonin Gene-Related Peptide and Gepants. In Encyclopedia. https://encyclopedia.pub/entry/36915
Rissardo, Jamir Pitton and Ana Letícia Fornari Caprara. "Calcitonin Gene-Related Peptide and Gepants." Encyclopedia. Web. 28 November, 2022.
Copy Citation
Calcitonin gene-related peptide (CGRP) antagonists are a class of medications that act as antagonists of the CGRP receptor or ligand. They can be divided into monoclonal antibodies and non-peptide small molecules, also known as gepants. CGRP antagonists were the first oral agents specifically designed to prevent migraines. The second generation of gepants includes rimegepant (BHV-3000, BMS-927711), ubrogepant (MK-1602), and atogepant (AGN-241689, MK-8031). Zavegepant (BHV-3500, BMS-742413) belongs to the third generation of gepants characterized by different administration routes.
rimegepant
ubrogepant
atogepant
zevagepant
1. Introduction
Migraine is a disabling disorder that affects approximately 15% of the global population [1]. In the United States (US), migraine is encountered among 70 million people and is the second most prevalent neurological disorder. The incidence of migraine headaches was 1722 cases per 100,000 people in 2017. The “Burden of Neurological Disorders Across the US From 1990–2017” estimated that migraine is the third most burdensome neurological disorder in terms of disability-adjusted life-years [2]. Migraine also most commonly occurs at productive age, in which the frequency of migraine headache days correlate with increased disability, leading to a decreased quality of life involving negative psychological impacts on performances within social and familial contexts [3]. Moreover, the estimated mean cost for migraine-related care per outpatient visit is $139.88, per emergency room visit is $775.09, and per inpatient hospitalization is $7317.07 [4]. Therefore, this disease affects an important percentage of the US population and represents a significant burden to the health care system.
The pathophysiology of migraine remains poorly understood. However, some specific vasoactive substances and neurotransmitters probably play a role in the mechanism of neurovascular and cortical spreading depression [5]. Calcitonin gene-related peptide (CGRP), neurokinin A, nitric oxide, and substance P are released with perivascular nerve activity [6]. They likely interact with the blood vessel wall, causing dilation, protein extravasation, and sterile inflammation [7]. The stimulation of the trigeminocervical complex by these chemical substances is relayed to the thalamus and cortex, both of which register the pain [8]. Interestingly, it was observed that the patients with migraine have reduced functioning of endogenous pain-control pathways that gate the pain [9]. Thus, the headache pain process requires not only the activation of nociceptors of pain-producing intracranial structures but also an impairment in its neuromodulation.
2. CGRP and GEPANTS
In 1982, the CGRP structure was discovered by Amara et al [10]. They noted that this compound was mainly synthesized in the hypothalamus, suggesting a hormonal effect for CGRP. A few years later, it was observed that CGRP-specific mRNA could be encountered throughout the central nervous system and in the peripheral nervous system, including the visceral motor functions mediated by the vagus nerve [11]. Rabbit models showed that intradermal injection of CGRP induced microvascular dilatation, resulting in increased blood flow [12]. Interestingly, CGRP is the most potent peripheral and cerebral vasodilator molecule ever discovered [13].
The potential role of the neurovascular system in migraine was hypothesized. In 1988, the first preclinical studies in cat models showed a potential association between CGRP and migraine [14]. It was observed that the irritation of the trigeminovascular system released CGRP in the extra-cerebral circulation. Two years later, Goadsby et al demonstrated dynamic changes in the concentrations of CGRP during migraine attacks in the external jugular but not in the cubital fossa blood [15]. Lassen et al infused CGRP in individuals with migraine to elucidate the mechanism of CGRP and migraine. They observed that intravenous administration of CGRP caused migraine in migraineurs [16].
CGRP receptors are transmembrane G protein-coupled receptors produced by the association between calcitonin receptor-like receptors (CALCRL) and a receptor activity-modifying protein (RAMP1) [17]. CGRP-expressing neurons are either C-fiber or Aδ-fiber [18]. Interestingly, more than thirty percent of trigeminal ganglion neurons exhibit CGRP receptors [19]. CGRP antagonists are a class of medications that act as antagonists of the CGRP receptor or ligand. They can be divided into monoclonal antibodies and non-peptide small molecules, also known as gepants (Table 1).
Table 1. Comparative analyses of pharmacokinetics, dosage, and formulations of rimegepant, ubrogepant, and atogepant.
| Drugs | Rimegepant | Ubrogepant | Atogepant |
|---|---|---|---|
| Other names | BHV-3000, BMS-927711 | MK-1602 | AGN-241689, MK-8031 |
| Trade name | Nurtec®, Nurtec ODT®, Vydura® | Ubrelvy® | Qulipta® |
| Marketed | Biohaven Pharmaceuticals, Pfizer | Allergan Pharmaceuticals | Allergan Pharmaceuticals |
| FDA approval | February 2020 (acute); May 2021 (prevention) | December 2019 | September 2021 |
| Molecule | 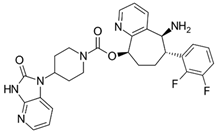 |
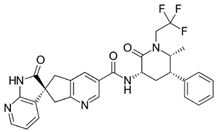 |
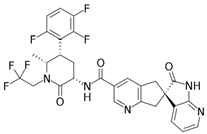 |
| Indication | Acute treatment of migraine with or without aura in adults. Preventive treatment of episodic migraine in adults | Acute treatment of migraine with or without aura in adults | Preventive treatment of episodic migraine in adults |
| Dose | 75 mg PO as needed. Not to exceed 75 mg/ 24 h. Safety of >18 doses/30 days has not been established | 50 or 100 mg PO as needed. If needed, may take second dose at least 2 hrs after initial dose. Not to exceed 200 mg/24 h. Safety of treating >8 migraines/30 days has not been established | 10, 30, or 60 mg PO |
| Dose adjustments | Renal impairment (CrCl < 15 mL/min); hepatic impairment (Child–Pugh > C); drug interactions | Renal impairment (CrCl < 30 mL/min); hepatic impairment (Child–Pugh ≥ C); drug interactions | Renal impairment (CrCl < 30 mL/min); hepatic impairment (Child–Pugh ≥ C); drug interactions |
| Route of administration | Oral | Oral | Oral |
| Contraindications | History of hypersensitivity | Concomitant use with strong CYP3A4 inhibitors | None |
| Adverse effects | Nausea (2%), hypersensitivity (including delayed) | Nausea (2–4%), somnolence (2–3%), dry mouth (2%) | Nausea (5–9%), constipation (6%), somnolence (4–6%), elevated AST/ALT (1%) |
| Pregnancy and lactation | Data not available | Data not available | Data not available |
| Pediatric | Data not available | Data not available | Data not available |
| Considerations | Orally disintegrating tablet. It can be swallowed without additional liquid | High-fat meal delayed plasma concentration | High-fat meal effect was not significant. |
The first generation of gepants raised concerns about liver toxicity and poor oral availability [20]. These drugs were BI 44370 TA (BI 44370), MK-3207, olcegepant (BIBN-4096BS), and telcagepant (MK-0974). Thus, the studies in the early 2010s were terminated, and the results were probably overlooked [21]. After almost a decade, the pharmaceutical industry retrieved and reanalyzed the results with rimegepant. They observed that rimegepant was effective, compared to the placebo and sumatriptan 100 mg for managing acute migraine. Furthermore, rimegepant did not show significant abnormalities in liver enzymes, even with participants receiving a single dose (four capsules of 150 mg each) of rimegepant 600 mg orally within 45 days of randomization once they experienced a migraine headache of moderate to severe intensity [22].
The favorable results of rimegepant’s first clinical trials were followed by ongoing studies with other gepants. In this way, the second generation of gepants emerged, and it includes rimegepant (BHV-3000, BMS-927711), ubrogepant (MK-1602), and atogepant (AGN-241689, MK-8031). These drugs revealed a low number of side effects, with excellent bioavailability due to molecular modifications. In December 2019, the FDA approved ubrogepant (Ubrelvy®) for the acute treatment of migraine with or without aura in adults. This drug was the first oral gepant approved by the FDA [23]. It is worth mentioning that the second generation has an affinity for different receptors besides the CGRP receptors [24]. The first-generation affinity for the human CGRP receptors is also higher than the second generation of gepants [25].
Rimegepant has a unique drug formulation, characterized as an oral dissolving tablet that disperses in the mouth, facilitating medication delivery and adhesion [26]. In addition, this formulation improves patients’ compliance to the medication due to decreased triggers such as nausea and vomiting commonly associated with migraine [27].
The third generation of gepants is characterized by different routes of administration. In this context, zavegepant (BHV-3500, BMS-742413) is under study for different types of routes, including subcutaneous and intranasal, due to the pharmacological properties of this molecule [28].
Researchers calculated the chemical and pharmacological properties of the second and third-generation gepants using the SwissADME tool (Figure 1). These properties can help identify compounds suitable for oral use [29]. All the parameters analyzed were within the normal range, except for the molecular weights that were slightly higher than those desired for oral molecules, which can reduce oral bioavailability. Refer to the Supplementary Materials in original context for a complete description of physicochemical descriptors and pharmacokinetic characteristics of rimegepant, ubrogepant, atogepant, and zavegepant. It is important to mention that some pharmacological features of this new group of medications still require further investigation.
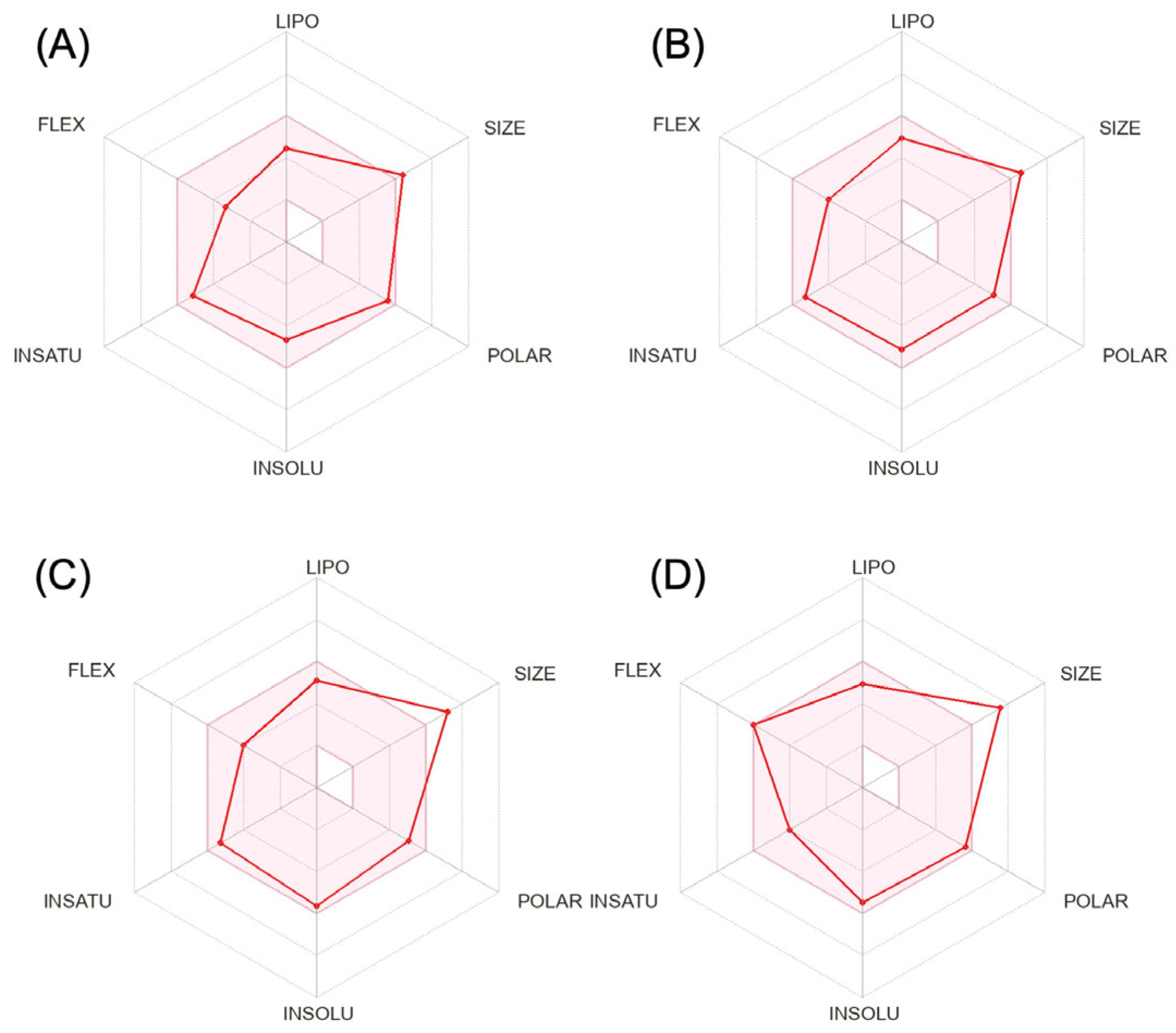
Figure 1. Physicochemical properties of rimegepant (A), ubrogepant (B), atogepant (C), and zavegepant (D).
References
- Stovner, L.; Hagen, K.; Jensen, R.; Katsarava, Z.; Lipton, R.; Scher, A.; Steiner, T.; Zwart, J.A. The global burden of headache: A documentation of headache prevalence and disability worldwide. Cephalalgia 2007, 27, 193–210.
- Feigin, V.L.; Vos, T.; Alahdab, F.; Amit, A.M.L.; Bärnighausen, T.W.; Beghi, E.; Beheshti, M.; Chavan, P.P.; Criqui, M.H.; Desai, R.; et al. Burden of Neurological Disorders Across the US From 1990-2017: A Global Burden of Disease Study. JAMA Neurol. 2021, 78, 165–176.
- Galvez-Sánchez, C.M.; Montoro Aguilar, C.I. Migraine and Neuroticism: A Scoping Review. Behav. Sci. 2022, 12, 30.
- Insinga, R.P.; Ng-Mak, D.S.; Hanson, M.E. Costs associated with outpatient, emergency room and inpatient care for migraine in the USA. Cephalalgia 2011, 31, 1570–1575.
- O’Hare, L.; Asher, J.M.; Hibbard, P.B. Migraine Visual Aura and Cortical Spreading Depression-Linking Mathematical Models to Empirical Evidence. Vision 2021, 5, 30.
- Scuteri, D.; Tonin, P.; Nicotera, P.; Bagetta, G.; Corasaniti, M.T. Real world considerations for newly approved CGRP receptor antagonists in migraine care. Expert Rev. Neurother. 2022, 22, 221–230.
- Guo, Y.; Cheng, Y.; An, J.; Qi, Y.; Luo, G. Neuropeptide changes in an improved migraine model with repeat stimulations. Transl. Neurosci. 2021, 12, 523–532.
- Descheemaeker, A.; Poras, H.; Wurm, M.; Luccarini, P.; Ouimet, T.; Dallel, R. Dual enkephalinase inhibitor PL37 as a potential novel treatment of migraine: Evidence from a rat model. Brain 2022, 145, 2664–2670.
- Mungoven, T.J.; Marciszewski, K.K.; Macefield, V.G.; Macey, P.M.; Henderson, L.A.; Meylakh, N. Alterations in pain processing circuitries in episodic migraine. J. Headache Pain 2022, 23, 9.
- Amara, S.G.; Jonas, V.; Rosenfeld, M.G.; Ong, E.S.; Evans, R.M. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature 1982, 298, 240–244.
- Rosenfeld, M.G.; Mermod, J.J.; Amara, S.G.; Swanson, L.W.; Sawchenko, P.E.; Rivier, J.; Vale, W.W.; Evans, R.M. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature 1983, 304, 129–135.
- Brain, S.D.; Williams, T.J.; Tippins, J.R.; Morris, H.R.; MacIntyre, I. Calcitonin gene-related peptide is a potent vasodilator. Nature 1985, 313, 54–56.
- Argunhan, F.; Brain, S.D. The Vascular-Dependent and -Independent Actions of Calcitonin Gene-Related Peptide in Cardiovascular Disease. Front. Physiol. 2022, 13, 833645.
- Goadsby, P.J.; Edvinsson, L.; Ekman, R. Release of vasoactive peptides in the extra-cerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann. Neurol. 1988, 23, 193–196.
- Goadsby, P.J.; Edvinsson, L.; Ekman, R. Vasoactive peptide release in the extra-cerebral circulation of humans during migraine headache. Ann. Neurol. 1990, 28, 183–187.
- Lassen, L.H.; Haderslev, P.A.; Jacobsen, V.B.; Iversen, H.K.; Sperling, B.; Olesen, J. CGRP may play a causative role in migraine. Cephalalgia Int. J. Headache 2002, 22, 54–61.
- Dos Santos, J.B.R.; da Silva, M.R.R. Small molecule CGRP receptor antagonists for the preventive treatment of migraine: A review. Eur. J. Pharmacol. 2022, 922, 174902.
- Wang, M.; Castonguay, W.C.; Duong, T.L.; Huebner, M.W.; Flinn, H.C.; Greenway, A.M.; Russo, A.F.; Sowers, L.P. Stimulation of CGRP-expressing neurons in the medial cerebellar nucleus induces light and touch sensitivity in mice. Neurobiol. Pain 2022, 12, 100098.
- Edvinsson, L.; Haanes, K.A.; Warfvinge, K.; Krause, D.N. CGRP as the target of new migraine therapies—Successful translation from bench to clinic. Nature reviews. Neurology 2018, 14, 338–350.
- Argyriou, A.A.; Mantovani, E.; Mitsikostas, D.D.; Vikelis, M.; Tamburin, S. A systematic review with expert opinion on the role of gepants for the preventive and abortive treatment of migraine. Expert Rev. Neurother. 2022, 22, 469–488.
- Connor, K.M.; Shapiro, R.E.; Diener, H.C.; Lucas, S.; Kost, J.; Fan, X.; Fei, K.; Assaid, C.; Lines, C.; Ho, T.W. Randomized, controlled trial of telcagepant for the acute treatment of migraine. Neurology 2009, 73, 970–977.
- Capi, M.; De Angelis, V.; De Bernardini, D.; De Luca, O.; Cipolla, F.; Lionetto, L.; Simmaco, M.; Martelletti, P. CGRP Receptor Antagonists and 5-HT1F Receptor Agonist in the Treatment of Migraine. J. Clin. Med. 2021, 10, 1429.
- Chiang, C.C.; VanderPluym, J.H. Ubrogepant in the Acute Management of Migraine: A Narrative Review. J. Pain Res. 2021, 14, 1185–1192.
- Garelja, M.L.; Walker, C.S.; Hay, D.L. CGRP receptor antagonists for migraine. Are they also AMY(1) receptor antagonists? Br. J. Pharmacol. 2022, 179, 454–459.
- Moreno-Ajona, D.; Villar-Martínez, M.D.; Goadsby, P.J. New Generation Gepants: Migraine Acute and Preventive Medications. J. Clin. Med. 2022, 11, 1656.
- DeFalco, A.P.; Lazim, R.; Cope, N.E. Rimegepant Orally Disintegrating Tablet for Acute Migraine Treatment: A Review. Ann. Pharmacother. 2021, 55, 650–657.
- Croop, R.; Goadsby, P.J.; Stock, D.A.; Conway, C.M.; Forshaw, M.; Stock, E.G.; Coric, V.; Lipton, R.B. Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: A randomised, phase 3, double-blind, placebo-controlled trial. Lancet 2019, 394, 737–745.
- Noor, N.; Angelette, A.; Lawson, A.; Patel, A.; Urits, I.; Viswanath, O.; Yazdi, C.; Kaye, A.D. A Comprehensive Review of Zavegepant as Abortive Treatment for Migraine. Health Psychol. Res. 2022, 10, 35506.
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
29 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




