Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Stavroula Chaloulakou | -- | 1831 | 2022-11-27 23:09:22 | | | |
| 2 | Camila Xu | Meta information modification | 1831 | 2022-11-28 04:02:23 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chaloulakou, S.; Poulia, K.A.; Karayiannis, D. Nutritional Considerations during Spaceflights. Encyclopedia. Available online: https://encyclopedia.pub/entry/36678 (accessed on 07 February 2026).
Chaloulakou S, Poulia KA, Karayiannis D. Nutritional Considerations during Spaceflights. Encyclopedia. Available at: https://encyclopedia.pub/entry/36678. Accessed February 07, 2026.
Chaloulakou, Stavroula, Kalliopi Anna Poulia, Dimitrios Karayiannis. "Nutritional Considerations during Spaceflights" Encyclopedia, https://encyclopedia.pub/entry/36678 (accessed February 07, 2026).
Chaloulakou, S., Poulia, K.A., & Karayiannis, D. (2022, November 27). Nutritional Considerations during Spaceflights. In Encyclopedia. https://encyclopedia.pub/entry/36678
Chaloulakou, Stavroula, et al. "Nutritional Considerations during Spaceflights." Encyclopedia. Web. 27 November, 2022.
Copy Citation
The main nutritional concerns during a spaceflight include the sufficient provision of energy to counteract the negative energy balance, which is often experienced by astronauts, the prevention of a deficiency in micronutrients, and fluid and sodium management. Furthermore, of utmost importance is the provision of specific nutrients to face spaceflight-induced pathophysiological events.
space flight
muscle loss
muscle mass
sarcopenia
1. Introduction
The space environment causes a plethora of stressors on the human body and has the potential to affect astronauts’ physiological and metabolic functions. Space stressors include space radiation, microgravity, isolation, and confinement [1][2][3]. These factors pose a challenge to the human body, which needs to adapt in order to cope with this hostile environment [4]. The future goals of human space exploration include missions to the Moon and Mars, but also space tourism [5][6]. Therefore, investigating the impact of spaceflight on the human body is of great significance, not only for astronauts, but also for the people who will travel to space as tourists. Furthermore, it will serve as a point of reference for future, long-term space missions—although current evidence is based on short-term missions so it should be carefully interpreted [1][7].
Optimal nutrition is of paramount importance for the crew’s health and the success of the mission, and an increasing number of studies have focused on the role of nutrition as a countermeasure to spaceflight-induced pathophysiological events [8][9]. Therefore, the aim of this research was to identify the effects of space stressors on astronauts’ physiology and to investigate the role of nutrition on human health under spaceflight conditions.
2. The Hostile Space Environment: Space Radiation, Microgravity, and Isolation
Space radiation is a primary risk factor for astronauts [10]. Exposure to ionizing radiation increases the production of reactive oxygen species (ROS) and leads to an imbalance between the production of oxygen free radicals and the body’s defensive antioxidant capacity, thus resulting in oxidative stress [8][11]. Microgravity is also a main risk factor for space travelers, as it provokes body fluid redistribution ‘’upwards’’ from the legs and abdomen to the heart and head. This causes multiple systems and organ deconditioning, including the vestibular, cardiovascular, and musculoskeletal system, as well as ophthalmic and endocrine changes, among others [4][12][13].
Other, less studied, yet principal factors include isolation and confinement. Living in an isolated and confined environment can cause social tensions, sleep disturbances, anxiety, and depression [14]. Furthermore, given space crews’ multinationalism, cultural differences among crew members on the International Space Station could also be a cause of psychosocial stress [15]. Moreover, the crew are not able to leave the spacecraft if they feel they need to abandon the mission, although this would be a rare event in the highly trained crews. These factors can lead to the appearance of pathophysiological signs and symptoms, from neurocognitive changes, and abnormal stress hormone levels to fatigue, sleep disturbances, and immunomodulatory changes [16][17]. As astronauts face abnormal day lengths and schedules, sleep disturbances may also result from a dysregulation of the 24 h cycle and consecutive changes in astronauts’ circadian rhythms [18]. Furthermore, anorexia and decreased energy intake have been observed during space missions, partially due to psychological factors [8]. Moreover, isolation, combined with exposure to microgravity and space radiation can induce significant alterations in the human body’s immune function [16].
The aforementioned effects vary greatly among individuals. Emotional instability, genetic predisposition, and personality differences can increase the susceptibility of the subjects to psychosocial, physiological, and sentimental changes [19][20]. Additionally, an astronaut’s experience has a significant impact on the solidarity of their behavior and on their overall adaptability to the new environment. For example, an experienced astronaut is less likely to face problems, compared to an astronaut on their first space mission [8][17].
Finally, contact with the natural environment on Earth, which has an undoubted effect on people’s mentality and quality of life, is not feasible in space. Neilson et al. [21] suggested ways to integrate a ‘’virtual’’ natural landscape into the International Space Station, using digital media, vegetation, virtual reality, and artificial windows in order to provide a virtual substitute to satisfy the astronauts’ instinctive need to be in contact with their natural environment.
The effect of spaceflight stressors on human health has gained scientific interest and multiple review papers have attempted to illuminate the metabolic, physiological, and mental disturbances experienced by astronauts due to the effects of the space environment, including radiation exposure, and sociological alterations resulting from their confinement and isolation [22][23]. In this narrative research, the physiological changes and the role of nutrition and nutritional status have been examined.
3. Spaceflight-Induced Physiological Changes
During the first days of spaceflight, space adaptation syndrome or space motion sickness is observed in astronauts. The main symptoms of the syndrome include nausea, dizziness, fatigue, anorexia, and headaches and they are attributed to the pathophysiological impairments in the vestibular system [8][24].
Moreover, the effects of the space environment on bone health has also been of scientific concern. During exposure to microgravity, bone resorption increases significantly, while bone formation remains unchanged or decreases. This imbalance leads to 1–1.5% bone mass loss per month [25]. This loss rate is similar to the bone loss rate observed in postmenopausal individuals per year [12]. Spaceflight-induced bone loss varies between skeletal sites. For example, bone tissue is better preserved in the non-weight-bearing upper limbs, compared to the weight-bearing lower limbs [26]. The increased level of bone resorption is associated with increased levels of calcium and other minerals in the blood circulation and the increased excretion of it in the urine, a process that enhances the astronauts’ risk of developing kidney stones during and after the spaceflight [27][28]. According to Gabel et al. [26], in a sample of seventeen astronauts (14 men and 3 women), spaceflight-induced bone loss was related to the duration of the flight; preflight bone turnover markers, which can be used to identify astronauts at risk; and pre-flight physical activity levels. Further research is needed to investigate whether this loss is recuperated upon their return to Earth. Gabel et al. [29] observed an incomplete bone loss reversal in astronauts’ tibia 1 year from the end of their spaceflight. It is believed that flight duration is proportional to the reverse of bone loss. The longer the space flight, the greater the loss and the more difficult the recovery.
Apart from bone loss, muscle loss and a rapid atrophy of skeletal muscle is also observed in astronauts, as, under microgravity conditions, movements require minimal muscle effort and force, which causes muscle mass and volume loss, especially in the lower limbs [30][31]. This decrease in muscle mass leads to weakness and diminished functional capacity, which is particularly noticeable in astronauts upon their return to a gravity condition [32]. A recent review of the factors directly contributing to this phenomenon include a change in the levels of cortisol, vitamin D, antioxidants, growth hormones, thyroid hormones, and testosterone, which occur during space flights [33]. Studies have shown that rapid volumetric skeletal muscle loss, as revealed by magnetic resonance imaging (MRI), occurs in the quadriceps (−6%); gastrocnemius (−6%); and posterior back muscles (−10%), following 6–9 days of spaceflight [34][35].
The cardiovascular system is also influenced by spaceflight. Body fluid redistribution due to microgravity leads to approximately 2 L of fluid shifting to the upper limbs and head, thus increasing cardiac output (CO) by 18–26% [15][36]. The aforementioned fluid shift causes a decrease in the circulating blood volume (10–15%), aerobic capacity, and heart size (cardiac atrophy) [37][38]. These changes are associated with orthostatic hypotension, a phenomenon that most astronauts experience after a spaceflight [15][39]. Moreover, according to a prospective cohort study of 11 astronauts during long-duration missions on the International Space Station, microgravity was associated with a cessation of blood flow in the internal jugular vein, which can, in turn, lead to thrombosis in otherwise healthy astronauts [40]. Thrombosis is a phenomenon that needs further study, as there is evidence of microgravity-induced changes in hemostasis mechanisms, such as decreased blood velocity, endothelial dysfunction, and increased fibrinogen synthesis [41][42].
Another major consequence of space travel is the dysregulation of the immune system. It is well-established that the immune system is influenced by various factors, including environment, diet, microbiome, and psychological stressors. During short-term six-month flights, astronauts are exposed to space radiation, face circadian rhythm dysregulations, and micronutrient deficiencies, among others, which are all to blame for astronauts’ altered immunity [40]. Prolonged isolation and confinement may also lead to reduced immunity [16]. Adequate nutritional intake is crucial for an optimal immune function, but no specific nutrient has proved to be effective for this purpose.
The ocular system and vision are also negatively affected during space travel. Previously published data suggest that astronauts experience vision changes; distance and near visual acuity deterioration; and the so-called spaceflight associated neuro-ocular syndrome (SANS), for which is mainly characterised by optic disc edema [42].
Finally, other factors include insulin resistance, impaired wound healing, and alterations in the respiratory and the central nervous system [42][43][44].
4. Nutritional Concerns during Spaceflights
The main nutritional concerns during a spaceflight include the sufficient provision of energy to counteract the negative energy balance, which is often experienced by astronauts, the prevention of a deficiency in micronutrients, and fluid and sodium management [5][9]. Weight loss, which has been observed since the early space exploration missions, is a major health concern for astronauts and a main factor leading to accelerated muscle mass loss [45].
Low energy intake is negatively associated with cardiovascular deconditioning, according to bed rest studies that have investigated the effect of hypocaloric diets on astronauts’ physiology [46][47]. During future long-term missions, negative energy balance will possibly be a cause of greater implications [48]. Factors that may cause reduced dietary intake include altered taste and smell due to the redistribution of body fluids, limited food variety and palatability, isolation, menu fatigue, altered microbiome, and increased physical activity levels [48][49][50]. These factors are depicted in Figure 1.
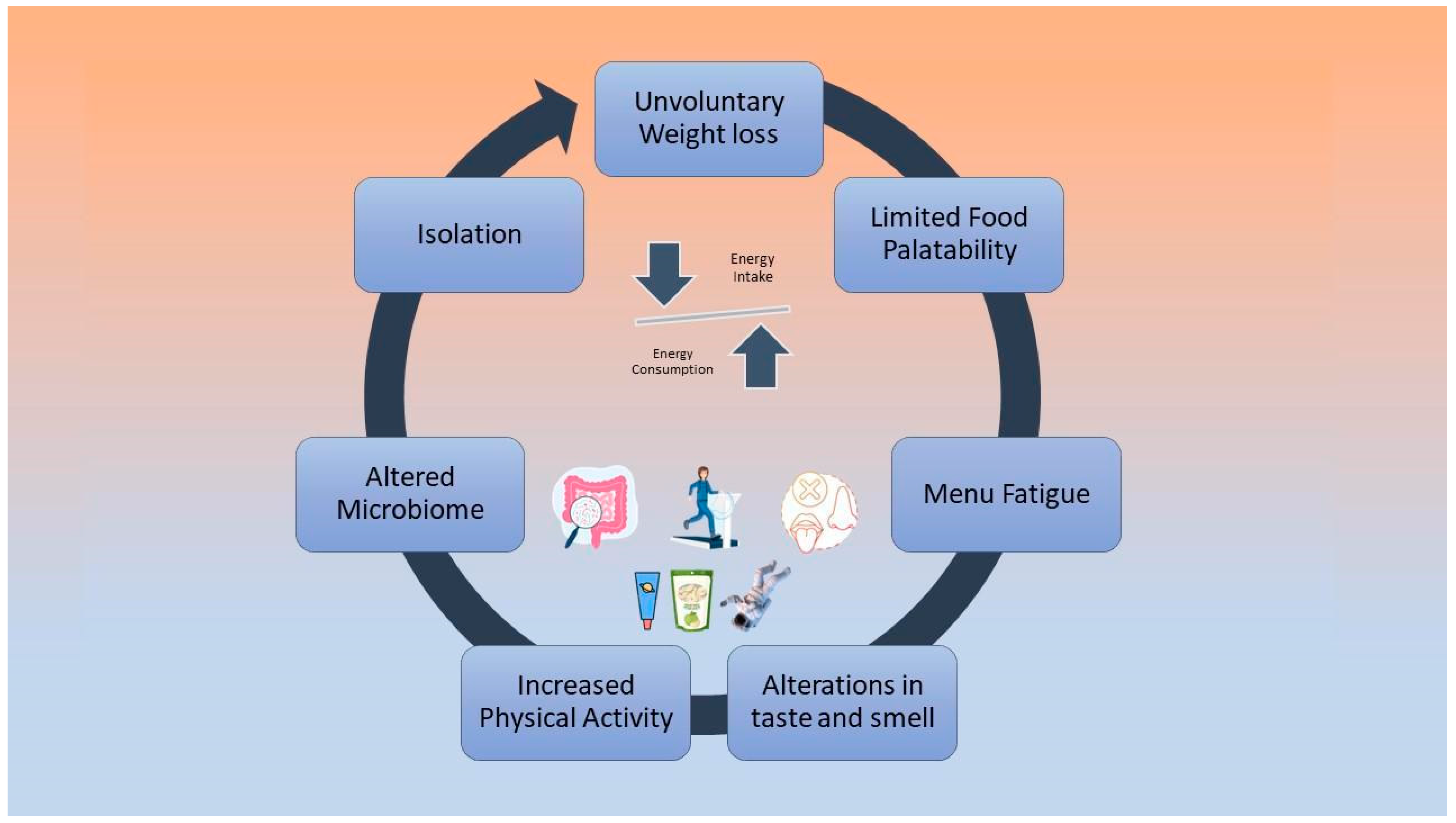
Figure 1. Causes of negative energy balance during spaceflights.
Furthermore, according to a recent systematic review by Tang et al. [5], the high concentration of carbon dioxide on the space stations affects energy intake by reducing food consumption. However, the easy preparation of food, i.e., the simple addition of hot water, is positively linked to the food consumption of astronauts [45]. Strategies to mitigate this problem are the addition of fresh, tasty, calorie-dense foods to the menu, considering food culture and cultural habits and the promotion of joint eating activities among astronauts. The environmental and physiological factors that affect the smell and taste of astronauts during a space flight need further study [5][45][49].
Regarding the deficiency of micronutrients, copper and zinc require extra attention, as a 21-day bed rest study showed increased fecal excretion of these micronutrients [51]. Regarding sodium levels, most space foods are high in salt due to the processing and the need for long-term preservation. This high sodium intake can have several negative pathophysiological effects, such as elevated urinary calcium excretion and an increased risk of kidney stones. Despite efforts to limit added in food items consumed by the participants in a spaceflight, current food on the ISS stillcontains high amounts of salt. A recent review by Tang et al. [5] supported the replacement of high sodium processed food items with fresh ones, which should be preserved in appropriate conditions to ensure their palatability and the preservation of their organoleptic properties.
References
- Mhatre, S.D.; Iyer, J.; Puukila, S.; Paul, A.M.; Tahimic, C.G.; Rubinstein, L.; Lowe, M.; Alwood, J.S.; Sowa, M.B.; Bhattacharya, S.; et al. Neuro-consequences of the spaceflight environment. Neurosci. Biobehav. Rev. 2022, 132, 908–935.
- Willey, J.S.; Britten, R.A.; Blaber, E.; Tahimic, C.G.; Chancellor, J.; Mortreux, M.; Sanford, L.D.; Kubik, A.J.; Delp, M.D.; Mao, X.W. The individual and combined effects of spaceflight radiation and microgravity on biologic systems and functional outcomes. J. Environ. Sci. Health Part C 2021, 39, 129–179.
- Van Ombergen, A.; Demertzi, A.; Tomilovskaya, E.; Jeurissen, B.; Sijbers, J.; Kozlovskaya, I.B.; Parizel, P.M.; Van de Heyning, P.H.; Sunaert, S.; Laureys, S.; et al. The effect of spaceflight and microgravity on the human brain. J. Neurol. 2017, 264, 18–22.
- Demontis, G.C.; Germani, M.M.; Caiani, E.G.; Barravecchia, I.; Passino, C.; Angeloni, D. Human Pathophysiological Adaptations to the Space Environment. Front. Physiol. 2017, 8, 547.
- Tang, H.; Rising, H.H.; Majji, M.; Brown, R.D. Long-Term Space Nutrition: A Scoping Review. Nutrients 2021, 14, 194.
- Mammarella, N. Can Space Tourism Boost Sustainable Behavior? Front. Psychol. 2021, 12, 771936.
- Aubert, E.A.; Larina, I.; Momken, I.; Blanc, S.; White, O.; Prisk, G.K.; Linnarsson, D. Towards human exploration of space: The THESEUS review series on cardiovascular, respiratory, and renal research priorities. npj Microgravity 2016, 2, 16031.
- Costa, F.; Ambesi-Impiombato, F.S.; Beccari, T.; Conte, C.; Cataldi, S.; Curcio, F.; Albi, E. Spaceflight Induced Disorders: Potential Nutritional Countermeasures. Front. Bioeng. Biotechnol. 2021, 9, 666683.
- Bergouignan, A.; Stein, T.P.; Habold, C.; Coxam, V.; O’Gorman, D.; Blanc, S. Towards human exploration of space: The THESEUS review series on nutrition and metabolism research priorities. npj Microgravity 2016, 2, 16029.
- Montesinos, C.A.; Khalid, R.; Cristea, O.; Greenberger, J.S.; Epperly, M.W.; Lemon, J.A.; Boreham, D.R.; Popov, D.; Gorthi, G.; Ramkumar, N.; et al. Space Radiation Protection Countermeasures in Microgravity and Planetary Exploration. Life 2021, 11, 829.
- Goodwin, T.J.; Christofidou-Solomidou, M. Oxidative Stress and Space Biology: An Organ-Based Approach. Int. J. Mol. Sci. 2018, 19, 959.
- Man, J.; Graham, T.; Squires-Donelly, G.; Laslett, A.L. The effects of microgravity on bone structure and function. npj Microgravity 2022, 8, 9.
- Bychkov, A.; Reshetnikova, P.; Bychkova, E.; Podgorbunskikh, E.; Koptev, V. The current state and future trends of space nutrition from a perspective of astronauts’ physiology. Int. J. Gastron. Food Sci. 2021, 24, 100324.
- Anderson, A.P.; Fellows, A.M.; Binsted, K.A.; Hegel, M.T.; Buckey, J.C. Autonomous, Computer-Based Behavioral Health Countermeasure Evaluation at HI-SEAS Mars Analog. Aerosp. Med. Hum. Perform. 2016, 87, 912–920.
- Baran, R.; Marchal, S.; Campos, S.G.; Rehnberg, E.; Tabury, K.; Baselet, B.; Wehland, M.; Grimm, D.; Baatout, S. The Cardiovascular System in Space: Focus on In Vivo and In Vitro Studies. Biomedicines 2021, 10, 59.
- Ponomarev, S.; Kalinin, S.; Sadova, A.; Rykova, M.; Orlova, K.; Crucian, B. Immunological Aspects of Isolation and Confinement. Front. Immunol. 2021, 12, 697435.
- Pagel, J.I.; Choukèr, A. Effects of isolation and confinement on humans-implications for manned space explorations. J. Appl. Physiol. 2016, 120, 1449–1457.
- Guo, J.H.; Qu, W.M.; Chen, S.G.; Chen, X.P.; Lv, K.; Huang, Z.L.; Wu, Y.L. Keeping the right time in space: Importance of circadian clock and sleep for physiology and performance of astronauts. Mil Med Res 2014, 1, 23.
- Loriè, E.P.; Baatout, S.; Choukér, A.; Buchheim, J.-I.; Baselet, B.; Russo, C.D.; Wotring, V.; Monici, M.; Morbidelli, L.; Gagliardi, D.; et al. The Future of Personalized Medicine in Space: From Observations to Countermeasures. Front. Bioeng. Biotechnol. 2021, 9, 739747.
- Zwart, S.R.; Gibson, C.R.; Gregory, J.F.; Mader, T.H.; Stover, P.J.; Zeisel, S.H.; Smith, S.M. Astronaut ophthalmic syndrome. FASEB J. 2017, 31, 3746–3756.
- Neilson, B.N.; Craig, C.M.; Altman, G.C.; Travis, A.T.; Vance, J.A.; Klein, M.I. Can the Biophilia Hypothesis Be Applied to Long-Duration Human Space Flight? A Mini-Review. Front. Psychol. 2021, 12, 703766.
- Yatagai, F.; Honma, M.; Dohmae, N.; Ishioka, N. Biological effects of space environmental factors: A possible interaction between space radiation and microgravity. Life Sci. Space Res. 2019, 20, 113–123.
- Furukawa, S.; Nagamatsu, A.; Nenoi, M.; Fujimori, A.; Kakinuma, S.; Katsube, T.; Wang, B.; Tsuruoka, C.; Shirai, T.; Nakamura, A.J.; et al. Space Radiation Biology for “Living in Space”. BioMed Res. Int. 2020, 2020, 4703286.
- Carriot, J.; Mackrous, I.; Cullen, K.E. Challenges to the Vestibular System in Space: How the Brain Responds and Adapts to Microgravity. Front. Neural Circuits 2021, 15, 760313.
- Stavnichuk, M.; Mikolajewicz, N.; Corlett, T.; Morris, M.; Komarova, S.V. A systematic review and meta-analysis of bone loss in space travelers. npj Microgravity 2020, 6, 13.
- Gabel, L.; Liphardt, A.-M.; Hulme, A.P.; Heer, M.; Zwart, S.R.; Sibonga, J.D.; Smith, S.M.; Boyd, S.K. Pre-flight exercise and bone metabolism predict unloading-induced bone loss due to spaceflight. Br. J. Sports Med. 2022, 56, 196–203.
- Smith, S.M.; Heer, M.; Shackelford, L.C.; Sibonga, J.D.; Spatz, J.; Pietrzyk, R.A.; Hudson, E.K.; Zwart, S.R. Bone metabolism and renal stone risk during International Space Station missions. Bone 2015, 81, 712–720.
- Liakopoulos, V.; Leivaditis, K.; Eleftheriadis, T.; Dombros, N. The kidney in space. Int. Urol. Nephrol. 2012, 44, 1893–1901.
- Gabel, L.; Liphardt, A.-M.; Hulme, P.A.; Heer, M.; Zwart, S.R.; Sibonga, J.D.; Smith, S.M.; Boyd, S.K. Incomplete recovery of bone strength and trabecular microarchitecture at the distal tibia 1 year after return from long duration spaceflight. Sci. Rep. 2022, 12, 9446.
- Comfort, P.; McMahon, J.J.; Jones, P.A.; Cuthbert, M.; Kendall, K.; Lake, J.P.; Haff, G.G. Effects of Spaceflight on Musculoskeletal Health: A Systematic Review and Meta-analysis, Considerations for Interplanetary Travel. Sports Med. 2021, 51, 2097–2114.
- Lang, T.; Leblanc, A.; Evans, H.; Lu, Y.; Genant, H.; Yu, A. Cortical and Trabecular Bone Mineral Loss from the Spine and Hip in Long-Duration Spaceflight. J. Bone Miner. Res. 2004, 19, 1006–1012.
- Vico, L.; van Rietbergen, B.; Vilayphiou, N.; Linossier, M.-T.; Locrelle, H.; Normand, M.; Zouch, M.; Gerbaix, M.; Bonnet, N.; Novikov, V.; et al. Cortical and Trabecular Bone Microstructure Did Not Recover at Weight-Bearing Skeletal Sites and Progressively Deteriorated at Non-Weight-Bearing Sites During the Year Following International Space Station Missions. J. Bone Miner. Res. 2017, 32, 2010–2021.
- Lee, P.H.U.; Chung, M.; Ren, Z.; Mair, D.B.; Kim, D.-H. Factors mediating spaceflight-induced skeletal muscle atrophy. Am. J. Cell Physiol. Physiol. 2022, 322, C567–C580.
- Leblanc, A.; Lin, C.; Shackelford, L.; Sinitsyn, V.; Evans, H.; Belichenko, O.; Schenkman, B.; Kozlovskaya, I.; Oganov, V.; Bakulin, A.; et al. Muscle volume, MRI relaxation times (T2), and body composition after spaceflight. J. Appl. Physiol. 2000, 89, 2158–2164.
- Leblanc, A.; Schneider, V.; Shackelford, L.; West, S.; Oganov, V.; Bakulin, A.; Voronin, L. Bone mineral and lean tissue loss after long duration space flight. J. Musculoskelet. Neuronal Interact. 2000, 1, 157–160.
- Sandal, P.H.; Kim, D.; Fiebig, L.; Winnard, A.; Caplan, N.; Green, D.A.; Weber, T. Effectiveness of nutritional countermeasures in microgravity and its ground-based analogues to ameliorate musculoskeletal and cardiopulmonary deconditioning—A Systematic Review. PLoS ONE 2020, 15, e0234412.
- Patel, S. The effects of microgravity and space radiation on cardiovascular health: From low-Earth orbit and beyond. IJC Heart Vasc. 2020, 30, 100595.
- Moore, A.D.; Downs, M.E.; Lee, S.; Feiveson, A.H.; Knudsen, P.; Ploutz-Snyder, L. Peak exercise oxygen uptake during and following long-duration spaceflight. J. Appl. Physiol. 2014, 117, 231–238.
- Mulavara, A.P.; Peters, B.T.; Miller, C.A.; Kofman, I.S.; Reschke, M.F.; Taylor, L.C.; Lawrence, E.L.; Wood, S.J.; Laurie, S.S.; Lee, S.M.C.; et al. Physiological and Functional Alterations after Spaceflight and Bed Rest. Med. Sci. Sports Exerc. 2018, 50, 1961–1980.
- Marshall-Goebel, K.; Laurie, S.S.; Alferova, I.V.; Arbeille, P.; Auñón-Chancellor, S.M.; Ebert, D.J.; Lee, S.M.C.; Macias, B.R.; Martin, D.S.; Pattarini, J.M.; et al. Assessment of Jugular Venous Blood Flow Stasis and Thrombosis During Spaceflight. JAMA Netw. Open 2019, 2, e1915011.
- Kim, D.S.; Vaquer, S.; Mazzolai, L.; Roberts, L.N.; Pavela, J.; Watanabe, M.; Weerts, G.; Green, D.A. The effect of microgravity on the human venous system and blood coagulation: A systematic review. Exp. Physiol. 2021, 106, 1149–1158.
- Strollo, F.; Gentile, S.; Strollo, G.; Mambro, A.; Vernikos, J. Recent Progress in Space Physiology and Aging. Front. Physiol. 2018, 9, 1551.
- Crucian, B.E.; Chouker, A.; Simpson, R.J.; Mehta, S.; Marshall, G.; Smith, S.M.; Zwart, S.R.; Heer, M.; Ponomarev, S.; Whitmire, A.; et al. Immune System Dysregulation During Spaceflight: Potential Countermeasures for Deep Space Exploration Missions. Front. Immunol. 2018, 9, 1437.
- Strollo, F.; Gentile, S.; Pipicelli, A.M.V.; Mambro, A.; Monici, M.; Magni, P. Space Flight-Promoted Insulin Resistance as a Possible Disruptor of Wound Healing. Front. Bioeng. Biotechnol. 2022, 10, 868999.
- Douglas, G.L.; Wheeler, R.M.; Fritsche, R.F. Sustaining Astronauts: Resource Limitations, Technology Needs, and Parallels between Spaceflight Food Systems and those on Earth. Sustainability 2021, 13, 9424.
- Bosutti, A.; Malaponte, G.; Zanetti, M.; Castellino, P.; Heer, M.; Guarnieri, G.; Biolo, G. Calorie Restriction Modulates Inactivity-Induced Changes in the Inflammatory Markers C-Reactive Protein and Pentraxin-3. J. Clin. Endocrinol. Metab. 2008, 93, 3226–3229.
- Biolo, G.; Ciocchi, B.; Stulle, M.; Bosutti, A.; Barazzoni, R.; Zanetti, M.; Antonione, R.; Lebenstedt, M.; Platen, P.; Heer, M.; et al. Calorie restriction accelerates the catabolism of lean body mass during 2 wk of bed rest. Am. J. Clin. Nutr. 2007, 86, 366–372.
- Taylor, A.J.; Beauchamp, J.D.; Briand, L.; Heer, M.; Hummel, T.; Margot, C.; McGrane, S.; Pieters, S.; Pittia, P.; Spence, C. Factors affecting flavor perception in space: Does the spacecraft environment influence food intake by astronauts? Compr. Rev. Food Sci. Food Saf. 2020, 19, 3439–3475.
- Sirmons, T.A.; Roma, P.G.; Whitmire, A.M.; Smith, S.M.; Zwart, S.R.; Young, M.; Douglas, G.L. Meal replacement in isolated and confined mission environments: Consumption, acceptability, and implications for physical and behavioral health. Physiol. Behav. 2020, 219, 112829.
- Laurens, C.; Simon, C.; Vernikos, J.; Gauquelin-Koch, G.; Blanc, S.; Bergouignan, A. Revisiting the Role of Exercise Countermeasure on the Regulation of Energy Balance During Space Flight. Front. Physiol. 2019, 10, 321.
- Heacox, H.N.; Gillman, P.L.; Zwart, S.R.; Smith, S.M. Excretion of Zinc and Copper Increases in Men during 3 Weeks of Bed Rest, with or without Artificial Gravity. J. Nutr. 2017, 147, 1113–1120.
More
Information
Subjects:
Others
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
28 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




