
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Władysław Lasoń | -- | 4673 | 2022-11-25 12:24:08 | | | |
| 2 | Rita Xu | -1 word(s) | 4672 | 2022-11-28 02:31:27 | | |
Video Upload Options
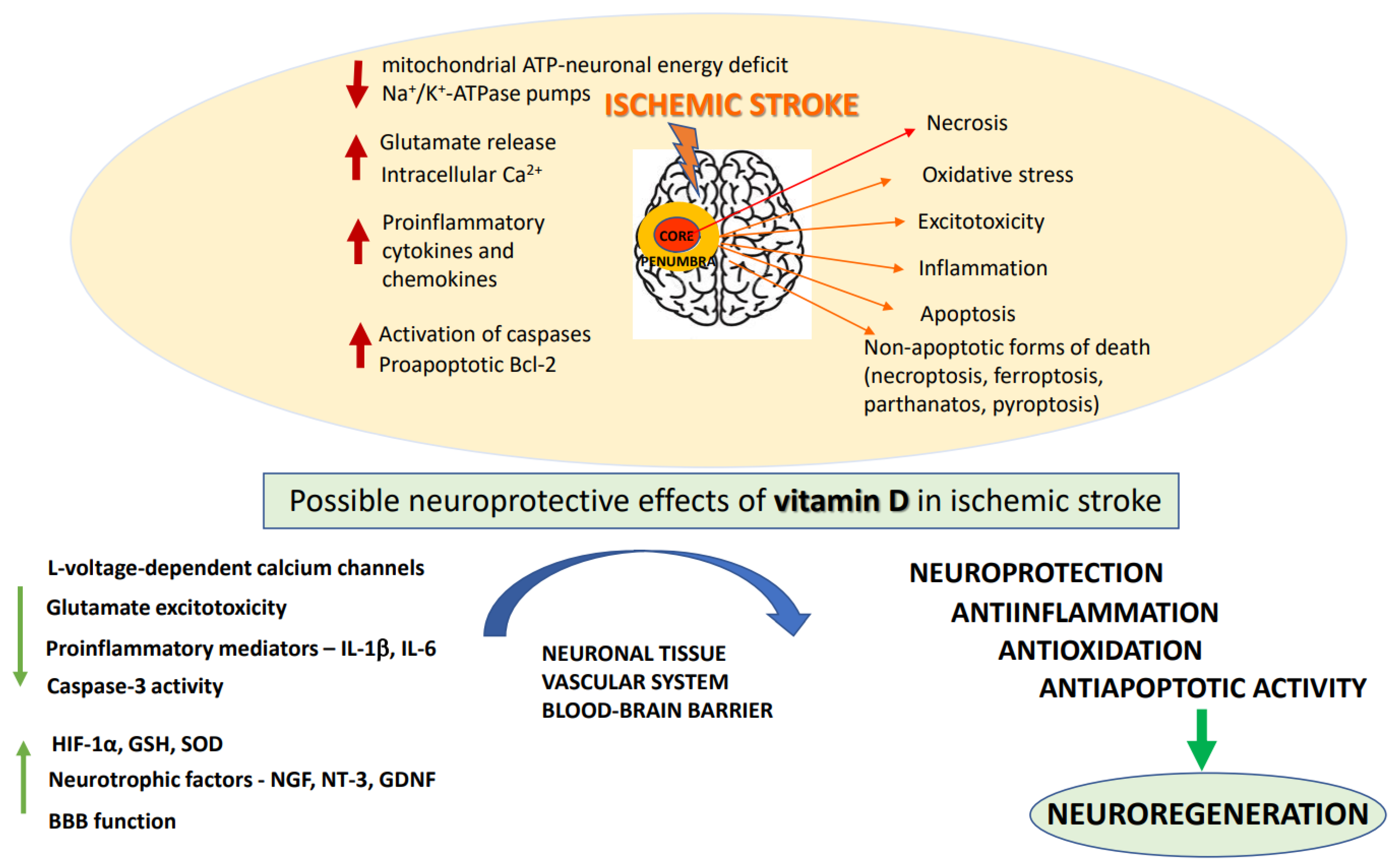
Ischemic stroke is one of the major causes of death and permanent disability worldwide. The only efficient treatment to date is anticoagulant therapy and thrombectomy, which enable restitution of blood flow to ischemic tissues. Numerous promising neuroprotectants have failed in clinical trials. Given the complex pathomechanism of stroke, a multitarget pharmacotherapy seems a more rational approach in stroke prevention and treatment than drugs acting on single molecular targets. Vitamin D3 has emerged as a potential treatment adjunct for ischemic stroke, as it interferes with the key prosurvival pathways and shows neuroprotective, anti-inflammatory, regenerative and anti-aging properties in both neuronal and vascular tissue.
1. Introduction
2. Pathomechanisms of Ischemic Stroke
2.1. Pathomorphological Features of Ischemic Stroke
2.2. Biochemical Basis of Ischemic Stroke and Neuroprotective Strategies of Its Treatment
3. The Basics of Vitamin D
3.1. Sources, Biosynthesis and Metabolism
3.2. Genomic and Non-Genomic Mechanisms of Vitamin D Action
3.3. Vitamin D Analogues
4. The Effects of Vitamin D3 in the CNS
References
- Aho, K.; Harmsen, P.; Hatano, S.; Marquardsen, J.; Smirnov, V.E.; Strasser, T. Cerebrovascular Disease in the Community: Results of a WHO Collaborative Study. Bull. World Health Organ. 1980, 58, 113–130.
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.; Culebras, A.; Elkind, M.S.V.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An Updated Definition of Stroke for the 21st Century: A Statement for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 2064–2089.
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Bhutta, Z.A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coates, M.M.; et al. Global, Regional, and National Life Expectancy, All-Cause Mortality, and Cause-Specific Mortality for 249 Causes of Death, 1980–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544.
- Swanepoel, A.C.; Pretorius, E. Prevention and Follow-up in Thromboembolic Ischemic Stroke: Do We Need to Think out of the Box? Thromb. Res. 2015, 136, 1067–1073.
- Berger, J.R. COVID-19 and the Nervous System. J. Neurovirol. 2020, 26, 143–148.
- Higgins, V.; Sohaei, D.; Diamandis, E.P.; Prassas, I. COVID-19: From an Acute to Chronic Disease? Potential Long-Term Health Consequences. Crit. Rev. Clin. Lab. Sci. 2021, 58, 297–310.
- Montalvan, V.; Lee, J.; Bueso, T.; De Toledo, J.; Rivas, K. Neurological Manifestations of COVID-19 and Other Coronavirus Infections: A Systematic Review. Clin. Neurol. Neurosurg. 2020, 194, 105921.
- Nannoni, S.; de Groot, R.; Bell, S.; Markus, H.S. Stroke in COVID-19: A Systematic Review and Meta-Analysis. Int. J. Stroke 2021, 16, 137–149.
- Sagris, D.; Papanikolaou, A.; Kvernland, A.; Korompoki, E.; Frontera, J.A.; Troxel, A.B.; Gavriatopoulou, M.; Milionis, H.; Lip, G.Y.H.; Michel, P.; et al. COVID-19 and Ischemic Stroke. Eur. J. Neurol. 2021, 28, 3826–3836.
- Silva Andrade, B.; Siqueira, S.; de Assis Soares, W.R.; de Souza Rangel, F.; Santos, N.O.; dos Santos Freitas, A.; Ribeiro da Silveira, P.; Tiwari, S.; Alzahrani, K.J.; Góes-Neto, A.; et al. Long-COVID and Post-COVID Health Complications: An Up-to-Date Review on Clinical Conditions and Their Possible Molecular Mechanisms. Viruses 2021, 13, 700.
- Albers, G.W.; Goldstein, L.B.; Hess, D.C.; Wechsler, L.R.; Furie, K.L.; Gorelick, P.B.; Hurn, P.; Liebeskind, D.S.; Nogueira, R.G.; Saver, J.L. Stroke Treatment Academic Industry Roundtable (STAIR) Recommendations for Maximizing the Use of Intravenous Thrombolytics and Expanding Treatment Options with Intra-Arterial and Neuroprotective Therapies. Stroke 2011, 42, 2645–2650.
- Cui, X.; Gooch, H.; Petty, A.; McGrath, J.J.; Eyles, D. Vitamin D and the Brain: Genomic and Non-Genomic Actions. Mol. Cell. Endocrinol. 2017, 453, 131–143.
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-Endothelial Interactions at the Blood-Brain Barrier. Nat. Rev. Neurosci. 2006, 7, 41–53.
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42.
- Xu, Y.; Liang, L. Vitamin D3/Vitamin D Receptor Signaling Mitigates Symptoms of Post-Stroke Depression in Mice by Upregulating Hippocampal BDNF Expression. Neurosci. Res. 2021, 170, 306–313.
- Torrisi, M.; Bonanno, L.; Formica, C.; Arcadi, F.A.; Cardile, D.; Cimino, V.; Bramanti, P.; Morini, E. The Role of Rehabilitation and Vitamin D Supplementation on Motor and Psychological Outcomes in Poststroke Patients. Medicine 2021, 100, e27747.
- Marek, K.; Cicho, N. The Role of Vitamin D in Stroke Prevention and the Effects of Its Supplementation for Post-Stroke Rehabilitation. Nutrients 2022, 14, 2761.
- Detante, O.; Jaillard, A.; Moisan, A.; Barbieux, M.; Favre, I.M.; Garambois, K.; Hommel, M.; Remy, C. Biotherapies in Stroke. Rev. Neurol. 2014, 170, 779–798.
- Hossmann, K.-A. Viability Thresholds and the Penumbra of Focal Ischemia. Ann. Neurol. 1994, 36, 557–565.
- Sakai, S.; Shichita, T. Inflammation and Neural Repair after Ischemic Brain Injury. Neurochem. Int. 2019, 130, 104316.
- Doyle, K.; Simon, R.; Stenzel-poore, M. Mechanisms of Ischemic Brain Damage—Review Article. Neuropharmacology 2008, 55, 310–318.
- Sekerdag, E.; Solaroglu, I.; Gursoy-Ozdemir, Y. Cell Death Mechanisms in Stroke and Novel Molecular and Cellular Treatment Options. Curr. Neuropharmacol. 2018, 16, 1396–1415.
- Radak, D.; Katsiki, N.; Resanovic, I.; Jovanovic, A.; Sudar-Milovanovic, E.; Zafirovic, S.; Mousad, S.A.; Isenovic, E.R. Apoptosis and Acute Brain Ischemia in Ischemic Stroke. Curr. Vasc. Pharmacol. 2016, 15, 115–122.
- Rossi, D.J.; Oshima, T.; Attwell, D. Glutamate Release in Severe Brain Ischaemia Is Mainly by Reversed Uptake. Nature 2000, 403, 316–321.
- Krzyzanowska, W.; Pomierny, B.; Filip, M.; Pera, J. Glutamate Transporters in Brain Ischemia: To Modulate or Not? Acta Pharmacol. Sin. 2014, 35, 444–462.
- Choi, D.W. Excitotoxicity: Still Hammering the Ischemic Brain in 2020. Front. Neurosci. 2020, 14, 579953.
- Bruno, V.; Battaglia, G.; Copani, A.; D’Onofrio, M.; Di Iorio, P.; De Blasi, A.; Melchiorri, D.; Flor, P.J.; Nicoletti, F. Metabotropic Glutamate Receptor Subtypes as Targets for Neuroprotective Drugs. J. Cereb. Blood Flow Metab. 2001, 21, 1013–1033.
- Ogden, K.K.; Traynelis, S.F. New Advances in NMDA Receptor Pharmacology. Trends Pharmacol. Sci. 2011, 32, 726–733.
- Hardingham, G.E.; Fukunaga, Y.; Bading, H. Extrasynaptic NMDARs Oppose Synaptic NMDARs by Triggering CREB Shut-off and Cell Death Pathways. Nat. Neurosci. 2002, 5, 405–414.
- Parsons, M.P.; Raymond, L.A. Extrasynaptic NMDA Receptor Involvement in Central Nervous System Disorders. Neuron 2014, 82, 279–293.
- Yan, J.; Peter Bengtson, C.; Buchthal, B.; Hagenston, A.M.; Bading, H. Coupling of NMDA Receptors and TRPM4 Guides Discovery of Unconventional Neuroprotectants. Science 2020, 370, eaay3302.
- Szydlowska, K.; Tymianski, M. Calcium, Ischemia and Excitotoxicity. Cell Calcium 2010, 47, 122–129.
- Crack, P.J.; Taylor, J.M. Reactive Oxygen Species and the Modulation of Stroke. Free Radic. Biol. Med. 2005, 38, 1433–1444.
- Gürsoy-Özdemir, Y.; Can, A.; Dalkara, T. Reperfusion-Induced Oxidative/Nitrativie Injury to Neurovascular Unit after Focal Cerebral Ischemia. Stroke 2004, 35, 1449–1453.
- Datta, A.; Sarmah, D.; Mounica, L.; Kaur, H.; Kesharwani, R.; Verma, G.; Veeresh, P.; Kotian, V.; Kalia, K.; Borah, A.; et al. Cell Death Pathways in Ischemic Stroke and Targeted Pharmacotherapy. Transl. Stroke Res. 2020, 11, 1185–1202.
- Tuo, Q.Z.; Zhang, S.T.; Lei, P. Mechanisms of Neuronal Cell Death in Ischemic Stroke and Their Therapeutic Implications. Med. Res. Rev. 2022, 42, 259–305.
- Zhou, Y.; Liao, J.; Mei, Z.; Liu, X.; Ge, J. Insight into Crosstalk between Ferroptosis and Necroptosis: Novel Therapeutics in Ischemic Stroke. Oxid. Med. Cell. Longev. 2021, 2021, 9991001.
- Kono, H.; Rock, K.L. How Dying Cells Alert the Immune System to Danger. Nat. Rev. Immunol. 2008, 8, 279–289.
- Bohacek, I.; Cordeau, P.; Lalancette-Hébert, M.; Gorup, D.; Weng, Y.C.; Gajovic, S.; Kriz, J. Toll-like Receptor 2 Deficiency Leads to Delayed Exacerbation of Ischemic Injury. J. Neuroinflamm. 2012, 9, 1–17.
- Linnik, M.D.; Zobrist, R.H.; Hatfield, M.D. Evidence Supporting a Role for Programmed Cell Death in Focal Cerebral Ischemia in Rats. Stroke 1993, 24, 2002–2008.
- Alonso De Leciñana, M.; Díez-Tejedor, E.; Gutierrez, M.; Guerrero, S.; Carceller, F.; Roda, J.M. New Goals in Ischemic Stroke Therapy: The Experimental Approach—Harmonizing Science with Practice. Cerebrovasc. Dis. 2005, 20, 159–168.
- Uzdensky, A.B. Apoptosis Regulation in the Penumbra after Ischemic Stroke: Expression of pro- and Antiapoptotic Proteins. Apoptosis 2019, 24, 687–702.
- Charriaut-Marlangue, C.; Margaill, I.; Represa, A.; Popovici, T.; Plotkine, M.; Ben-Ari, Y. Apoptosis and Necrosis after Reversible Focal Ischemia: An in Situ DNA Fragmentation Analysis. J. Cereb. Blood Flow Metab. 1996, 16, 186–194.
- MacManus, J.P.; Hill, I.E.; Huang, Z.-G.; Rasquinha, I.; Xue, D.; Buchan, A.M. DNA Damage Consistent with Apoptosis in Transient Focal Ischaemic Neocortex. Neuroreport 1994, 5, 493–496.
- Yilmaz, G.; Granger, D.N. Cell Adhesion Molecules and Ischemic Stroke. Neurol. Res. 2008, 30, 783–793.
- Kassis, H.; Shehadah, A.; Chopp, M.; Zhang, Z.G. Epigenetics in Stroke Recovery. Genes 2017, 8, 89.
- Hu, Z.; Zhong, B.; Tan, J.; Chen, C.; Lei, Q.; Zeng, L. The Emerging Role of Epigenetics in Cerebral Ischemia. Mol. Neurobiol. 2017, 54, 1887–1905.
- Bendik, I.; Friedel, A.; Roos, F.F.; Weber, P.; Eggersdorfer, M. Vitamin D: A Critical and Essential Micronutrient for Human Health. Front. Physiol. 2014, 5, 248.
- Norman, A.W. From Vitamin D to Hormone D: Fundamentals of the Vitamin D Endocrine System Essential for Good Health. Am. J. Clin. Nutr. 2008, 88, 491S–499S.
- Bikle, D.D. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem. Biol. 2014, 21, 319–329.
- Gil, Á.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and Novel Actions. Ann. Nutr. Metab. 2018, 72, 87–95.
- Carlberg, C. Nutrigenomics of Vitamin D. Nutrients 2019, 11, 676.
- Norlin, M. Effects of Vitamin D in the Nervous System: Special Focus on Interaction with Steroid Hormone Signalling and a Possible Role in the Treatment of Brain Cancer. J. Neuroendocrinol. 2020, 32, e12799.
- Bivona, G.; Gambino, C.M.; Iacolino, G.; Ciaccio, M. Vitamin D and the Nervous System. Neurol. Res. 2019, 41, 827–835.
- Slominski, A.T.; Janjetovic, Z.; Fuller, B.E.; Zmijewski, M.A.; Tuckey, R.C.; Nguyen, M.N.; Sweatman, T.; Li, W.; Zjawiony, J.; Miller, D.; et al. Products of Vitamin D3 or 7-Dehydrocholesterol Metabolism by Cytochrome P450scc Show Anti-Leukemia Effects, Having Low or Absent Calcemic Activity. PLoS ONE 2010, 5, 7–10.
- Slominski, A.T.; Kim, T.; Hobrath, J.V.; Oak, A.S.W.; Edith, K.Y.; Tieu, E.W.; Li, W.; Tuckey, R.C.; Jetten, A.M. Endogenously Produced Nonclassical Vitamin D Hydroxy-Metabolites Act as “Biased” Agonists on Vdr and Inverse Agonists on Rorα and Rorγ. J. Steroid Biochem. Mol. Biol. 2017, 173, 42–56.
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2015, 96, 365–408.
- Bikle, D.D.; Schwartz, J. Vitamin D Binding Protein, Total and Free Vitamin D Levels in Different Physiological and Pathophysiological Conditions. Front. Endocrinol. 2019, 10, 317.
- Wimalawansa, S.J. Vitamin D deficiency: Effects on oxidative stress, epigenetics, gene regulation, and aging. Biology 2019, 8, 30.
- Garcion, E.; Wion-Barbot, N.; Montero-Menei, C.N.; Berger, F.; Wion, D. New Clues about Vitamin D Functions in the Nervous System. Trends Endocrinol. Metab. 2002, 13, 100–105.
- Zmijewski, M.A.; Carlberg, C. Vitamin D Receptor(s): In the Nucleus but Also at Membranes? Exp. Dermatol. 2020, 29, 876–884.
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D Receptor (VDR)-Mediated Actions of 1α,25(OH) 2 Vitamin D 3: Genomic and Non-Genomic Mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559.
- Norman, A.W. Minireview: Vitamin D Receptor: New Assignments for an Already Busy Receptor. Endocrinology 2006, 147, 5542–5548.
- Shaffer, P.L.; Gewirth, D.T. Structural Basis of VDR-DNA Interactions on Direct Repeat Response Elements. EMBO J. 2002, 21, 2242–2252.
- Pertile, R.A.N.; Cui, X.; Eyles, D.W. Vitamin D Signaling and the Differentiation of Developing Dopamine Systems. Neuroscience 2016, 333, 193–203.
- Alroy, I.; Towers, T.L.; Freedman, L.P. Transcriptional Repression of the Interleukin-2 Gene by Vitamin D3: Direct Inhibition of NFATp/AP-1 Complex Formation by a Nuclear Hormone Receptor. Mol. Cell. Biol. 1995, 15, 5789–5799.
- Gurlek, A.; Pittelkow, M.R.; Kumar, R. Modulation of Growth Factor/Cytokine Synthesis and Signaling by 1α,25-Dihydroxyvitamin D3: Implications in Cell Growth and Differentiation. Endocr. Rev. 2002, 23, 763–786.
- Snegarova, V.; Naydenova, D. Vitamin D: A Review of Its Effects on Epigenetics and Gene Regulation. Folia Med. 2020, 62, 662–669.
- Ricca, C.; Aillon, A.; Bergandi, L.; Alotto, D.; Castagnoli, C.; Silvagno, F. Vitamin D Receptor Is Necessary for Mitochondrial Function and Cell Health. Int. J. Mol. Sci. 2018, 19, 1672.
- Chen, J.; Tang, Z.; Slominski, A.T.; Li, W.; Żmijewski, M.A.; Liu, Y.; Chen, J. Vitamin D and Its Analogs as Anticancer and Anti-Inflammatory Agents. Eur. J. Med. Chem. 2020, 207, 112738.
- Leyssens, C.; Verlinden, L.; Verstuyf, A. The Future of Vitamin D Analogs. Front. Physiol. 2014, 5, 122.
- Maestro, M.A.; Molnár, F.; Carlberg, C. Vitamin D and Its Synthetic Analogs. J. Med. Chem. 2019, 62, 6854–6875.
- Regulska, M.; Leśkiewicz, M.; Budziszewska, B.; Kutner, A.; Jantas, D.; Basta-Kaim, A.; Kubera, M.; Jaworska-Feil, L.; Lasoń, W. Inhibitory Effects of 1,25-Dihydroxyvitamin D3 and Its Low-Calcemic Analogues on Staurosporine-Induced Apoptosis. Pharmacol. Rep. 2007, 59, 393–401.
- Regulska, M.; Leśkiewicz, M.; Budziszewska, B.; Kutner, A.; Basta-Kaim, A.; Kubera, M.; Jaworska-Feil, L.; Lasoń, W. Involvement of P13-K in Neuroprotective Effects of the 1,25-Dihydroxyvitamin D3 Analogue—PRI-2191. Pharmacol. Rep. 2006, 58, 900–907.
- Pierucci, F.; Garcia-Gil, M.; Frati, A.; Bini, F.; Martinesi, M.; Vannini, E.; Mainardi, M.; Luzzati, F.; Peretto, P.; Caleo, M.; et al. Vitamin D3 Protects against Aβ Peptide Cytotoxicity in Differentiated Human Neuroblastoma SH-SY5Y Cells: A Role for S1P1/P38MAPK/ATF4 Axis. Neuropharmacology 2017, 116, 328–342.
- Grimm, M.O.W.; Thiel, A.; Lauer, A.A.; Winkler, J.; Lehmann, J.; Regner, L.; Nelke, C.; Janitschke, D.; Benoist, C.; Streidenberger, O.; et al. Vitamin D and Its Analogues Decrease Amyloid-β (Aβ) Formation and Increase Aβ-Degradation. Int. J. Mol. Sci. 2017, 18, 2764.
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the Vitamin D Receptor and 1α-Hydroxylase in Human Brain. J. Chem. Neuroanat. 2005, 29, 21–30.
- Cui, X.; Gooch, H.; Groves, N.J.; Sah, P.; Burne, T.H.; Eyles, D.W.; McGrath, J.J. Vitamin D and the Brain: Key Questions for Future Research. J. Steroid Biochem. Mol. Biol. 2015, 148, 305–309.
- Lang, F.; Ma, K.; Leibrock, C.B. 1,25(OH)2D3 in Brain Function and Neuropsychiatric Disease. Neurosignals 2019, 27, 40–49.
- Lowe, D.W.; Hollis, B.W.; Wagner, C.L.; Bass, T.; Kaufman, D.A.; Horgan, M.J.; Givelichian, L.M.; Sankaran, K.; Yager, J.Y.; Katikaneni, L.D.; et al. Vitamin D Insufficiency in Neonatal Hypoxic–ischemic Encephalopathy. Pediatr. Res. 2017, 82, 55–62.
- Chen, K.B.; Lin, A.M.Y.; Chiu, T.H. Systemic Vitamin D3 Attenuated Oxidative Injuries in the Locus Coeruleus of Rat Brain. Ann. NY Acad. Sci. 2003, 993, 313–324.
- Landel, V.; Stephan, D.; Cui, X.; Eyles, D.; Feron, F. Differential Expression of Vitamin D-Associated Enzymes and Receptors in Brain Cell Subtypes. J. Steroid Biochem. Mol. Biol. 2018, 177, 129–134.
- Neveu, I.; Naveilhan, P.; Jehan, F.; Baudet, C.; Wion, D.; De Luca, H.F.; Brachet, P. 1,25-Dihydroxyvitamin D3 Regulates the Synthesis of Nerve Growth Factor in Primary Cultures of Glial Cells. Mol. Brain Res. 1994, 24, 70–76.
- Neveu, I.; Naveilhan, P.; Baudet, C.; Brachet, P.; Metsis, M. 1,25-Dihydroxyvitamin D3 Regulates NT-3, NT-4 but Not BDNF MRNA in Astrocytes. Neuroreport 1994, 6, 124–126.
- Pertile, R.A.N.; Cui, X.; Hammond, L.; Eyles, D.W. Vitamin D Regulation of GDNF/Ret Signaling in Dopaminergic Neurons. FASEB J. 2018, 32, 819–828.
- Lin, C.-I.; Chang, Y.; Kao, N.; Lee, W.; Cross, T.; Lin, S. 1,25(OH)2D3 Alleviates Aβ(25-35)-Induced Tau Hyperphosphorylation, Excessive Reactive Oxygen Species, and Apoptosis Through Interplay with Glial Cell Line-Derived Neurotrophic Factor Signaling in SH-SY5Y Cells. Int. J. Mol. Sci. 2020, 21, 4215.
- McGrath, J.J.; Féron, F.P.; Burne, T.H.J.; MacKay-Sim, A.; Eyles, D.W. Vitamin D3—Implications for Brain Development. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 557–560.
- Ilie, P.C.; Stefanescu, S.; Smith, L. The Role of Vitamin D in the Prevention of Coronavirus Disease 2019 Infection and Mortality. Aging Clin. Exp. Res. 2020, 32, 1195–1198.
- Eyles, D.; Almeras, L.; Benech, P.; Patatian, A.; Mackay-Sim, A.; McGrath, J.; Féron, F. Developmental Vitamin D Deficiency Alters the Expression of Genes Encoding Mitochondrial, Cytoskeletal and Synaptic Proteins in the Adult Rat Brain. J. Steroid Biochem. Mol. Biol. 2007, 103, 538–545.
- Levenson, C.W.; Figueirôa, S.M. Gestational Vitamin D Deficiency: Long-Term Effects on the Brain. Nutr. Rev. 2008, 66, 726–729.
- Monteggia, L.M.; Björkholm, C. BDNF—A Key Transducer of Antidepressant Effects. Neuropharmacology 2016, 102, 72–79.
- Duman, R.S.; Deyama, S.; Fogaça, M.V. Role of BDNF in the Pathophysiology and Treatment of Depression: Activity-dependent Effects Distinguish Rapid-acting Antidepressants. Eur. J. Neurosci. 2021, 53, 126–139.
- Kouba, B.R.; Camargo, A.; Gil-Mohapel, J.; Rodrigues, A.L.S. Molecular Basis Underlying the Therapeutic Potential of Vitamin D for the Treatment of Depression and Anxiety. Int. J. Mol. Sci. 2022, 23, 7077.
- Marazziti, D.; Parra, E.; Palermo, S.; Barberi, F.M.; Buccianelli, B.; Ricciardulli, S.; Cappelli, A.; Mucci, F.; Dell’Osso, L. Vitamin D: A Pleiotropic Hormone with Possible Psychotropic Activities. Curr. Med. Chem. 2021, 28, 3843–3864.




