The incidence of Japanese cedar pollinosis is increasing significantly in Japan, and a recent survey suggested that about 40% of the population will develop this disease. However, spontaneous remission is rare. The increased incident rate of Japanese cedar pollinosis is a huge issue in Japan. Allergen immunotherapy is the only fundamental treatment that modifies the natural course of allergic rhinitis and provides long-term remission that cannot be induced by general drug therapy. Sublingual immunotherapy for Japanese cedar pollinosis has been developed and has been covered by health insurance since 2014 in Japan. The indication for children was expanded in 2018. Clinical trials of sublingual immunotherapy for Japanese cedar pollinosis have demonstrated its long-term efficacy and safety. It is recommended for patients who wish to undergo fundamental treatment regardless of the severity of the practical guidelines for the management of allergic rhinitis in Japan. For sublingual immunotherapy, a long-term treatment period of 3 years or longer is recommended to obtain stable therapeutic effects.
1. Introduction
Japanese cedar (JC) (
Cryptomeria japonica) pollinosis is the most prevalent allergic rhinitis (AR) in Japan. The number of patients with JC pollinosis has markedly increased over the last two decades; however, spontaneous remission is rare
[1][2][3][4][5]. Therefore, once JC pollinosis develops, many patients may suffer from nasal and ocular allergic symptoms for a long time. The scattering period of JC pollen is long, and the amount of JC pollen scattered has increased in recent years; thus, the symptoms tend to be robust and exacerbated. Symptomatic drug therapy is the main treatment for AR in Japan and no effective preventive treatment for AR has been established to date. However, allergen immunotherapy (AIT) is the only fundamental treatment expected to modify the natural course of AR. Sublingual immunotherapy (SLIT) for patients with AR induced by JC pollen and house dust mites (HDM) has been started as a medical treatment and is covered by health insurance in Japan. SLIT for JC pollinosis was recently demonstrated to be effective and tolerable in clinical trials with a high level of evidence. Although AIT requires long-term treatment of 3 years or more to obtain a stable therapeutic effect, it lacks efficacy in some patients. Recently, immunological changes induced by AIT have been demonstrated in clinical trials or basic research. However, biomarkers that objectively predict and judge therapeutic effects have not been established to date.
2. Characteristics of JC Pollinosis in Japan
JC pollinosis, AR induced by JC pollen (
Figure 1), which is frequently accompanied by conjunctivitis, was first reported in the 1960s by Saito et al.
[6]. Since the 1950s, JC trees have been planted across Japan in areas other than Hokkaido and Okinawa because of the rising demand for cedar wood. Many of the planted JC trees are now over 30 years old and have high pollen-producing ability, leading to increasing amounts of scattered JC pollen. JC trees disperse a large amount of pollen, over a long period, mainly from February to March, reaching distances over 100 km, and therefore, many patients with JC pollinosis can be found in urban areas such as Tokyo (1). When a large amount of JC pollen scatters it causes severe allergic nasal symptoms, and that these symptoms are exacerbated with repeated exposure has been confirmed by experiments using pollen exposure chambers in Japan
[7].
Figure 1. Photomicrograph image of Japanese cedar pollen collected on the rooftop of the University of Yamanashi.
Of note, many cases of JC pollinosis and cypress pollinosis coexist. The major allergens of JC pollen and cypress pollen are highly homologous, and about 70% of JC pollinosis cases are also sensitized to cypress pollen
[8][9]. Cypress has been planted in a wide area that stretches between east and west Japan.
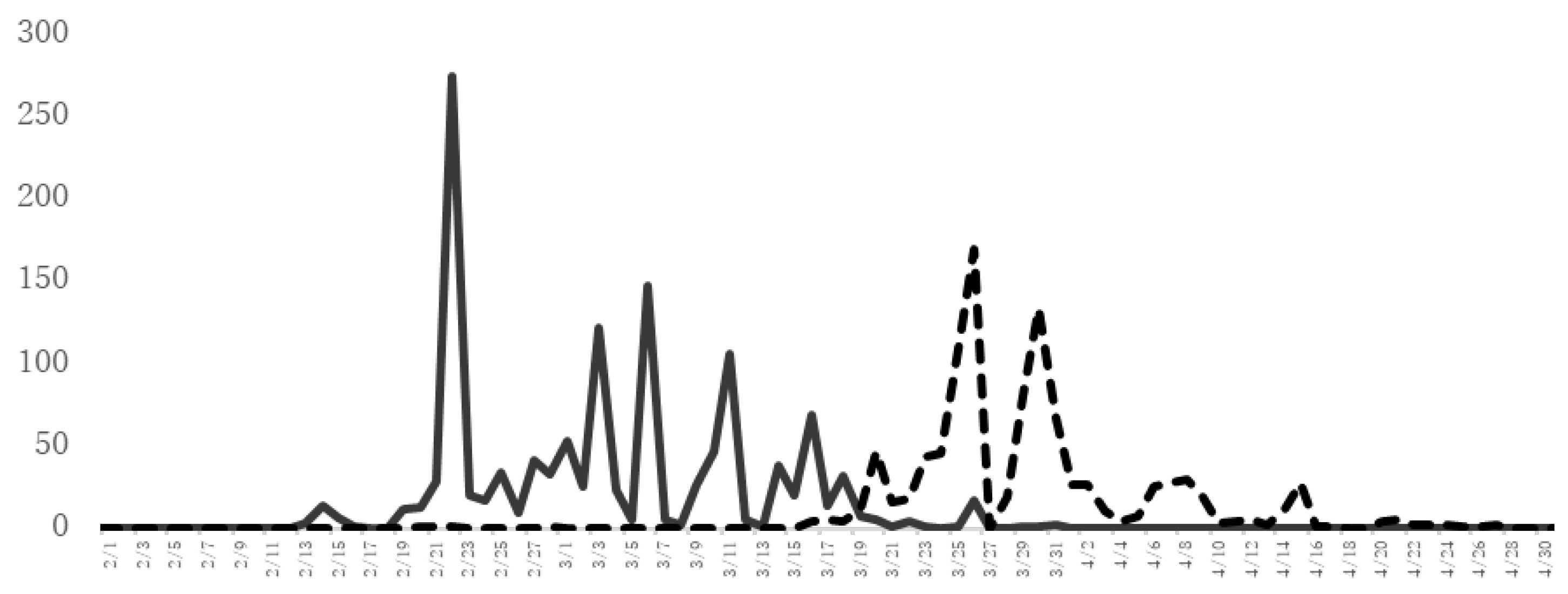
Figure 2 shows the JC and Cypress pollen counts in Yamanashi Prefecture, which has one of the highest frequencies of patients with JC pollinosis in Japan. The cypress pollen dispersal season continues after the cedar pollen dispersal season. Therefore, many patients with coexisting JC pollinosis and cypress pollinosis have allergic symptoms for a long period from February to April or May.
Figure 2. The amount of JC pollen and cypress pollen scattered. The number (grains/cm2) of JC pollen (solid line) and cypress pollen (dotted line) scattered as assessed by the Durham method at the University of Yamanashi, School of Medicine (Yamanashi Prefecture) from February to April 2021.
A questionnaire survey for otolaryngologists and their families in Japan conducted in 2019 reported that the overall prevalence of AR was 49.2%. Thus, approximately one in two Japanese people have AR to an allergen. Compared with similar surveys, the prevalence of JC pollinosis has increased rapidly from 16.2% in 1998 to 26.5% in 2008 and 38.8% in 2019. In a 2019 survey, 30.1% of those aged 5 to 9 years and 49.5% of those aged 10 to 19 years had JC pollinosis, indicating the age of onset is decreasing
[1][2][3]. The sensitization rate of JC pollen and the prevalence of JC pollinosis are associated with the amount of JC pollen dispersal
[10]. Although the symptoms of JC pollinosis disappear in some cases, their frequency is low in long-term observation studies
[4][5].
3. General Drug Treatment for JC Pollinosis
Treatments for the symptoms of pollinosis are provided according to the Nasal Allergy Guidelines in Japan. Pharmacotherapy such as second-generation antihistamines are used for patients with mild and moderate symptoms consisting of sneezing and a runny nose. Leukotriene receptor antagonists or prostaglandin D2/thromboxane A2 receptor antagonists are used for moderate symptoms with nasal congestion, and a combination of nasal topical steroids and chemical mediator receptor antagonist such as second-generation antihistamines are recommended for severe cases in Japan
[1]. Additionally, early primary pharmacotherapy when pollen scattering starts and symptoms appear is recommended for cases with annual severe symptoms of AR caused by pollen. In Japan, omalizumab, an anti-IgE therapy, has been approved for use in patients aged over 12 years with severe nasal allergic symptoms induced by JC pollinosis that are uncontrolled even with the above drug combinations. The efficacy of add-on omalizumab in patients treated with nasal steroids and antihistamines during the JC pollen season was confirmed in a clinical trial of patients with JC pollinosis
[11].
4. AIT for JC Pollinosis
Unlike general drug therapies, SLIT, together with conventional subcutaneous immunotherapy (SCIT), is a therapeutic method that is expected to induce long-term remission. Because there is no established method to prevent AR, AIT is in a significant position as a fundamental treatment
[1]. SCIT was started in the 1960s in Japan, and SLIT for JC pollinosis as a liquid formulation, Cedartolen
®, was approved in 2014 as the first SLIT medication in Japan. The liquid formulation of JC pollen SLIT contains a maintenance dose of 2000 JAU of JC pollen extract. Then, a SLIT tablet, Cedarcure
®, for JC pollinosis with an increased effective concentration was developed and approved in 2018. The JC pollen SLIT tablets used as a maintenance dose contain 5000 JAU of JC pollen extract (10,000 JAU/mL contains 7.3–21 μg/mL of Cry j 1, the major allergen of JC pollen). To date, only one company has developed SLIT for JC pollinosis. The liquid formulation Cedartolen
® is now discontinued and only the tablet Cedarcure
® is available. There have been no reports of clinical trials of other SLIT preparations for JC pollinosis. Cedarcure
® is used at 2000 JAU for one week as a dose-up period, followed by a daily maintenance dose of 5000 JAU.
A tablet of JC pollen SLIT maintained in the oral cavity for one minute disintegrates quickly and is absorbed through the oral floor mucosa. Mild side effects such as local itching and edema are often observed during the dose increase period or the first month after administration. Systemic adverse effects such as anaphylaxis are very rare, and its safety profile is higher than that of conventional SCIT. Currently, a treatment period of 3 years or longer is recommended to obtain a stable and long-term therapeutic effect according to the Nasal Allergy Guidelines of Japan
[1]. As for the treatment costs, it is covered by health insurance in Japan; however, in the case of adults, it costs about JPY 100,000 for three years of treatment.
Regarding the indications of AIT for JC pollinosis, AIT modifies the natural course of AR and is a treatment that is expected to lead to remission; thus, it is recommended for patients who desire a fundamental improvement. The Nasal Allergy Guidelines of Japan recommend AIT for AR of any severity, from mild to severe. It is especially recommended for patients whose symptoms are poorly controlled by general drug therapy, patients who experience side effects from drug therapy, and patients who want to reduce drug use
[1]. The indication for JC pollen SLIT tablets was expanded to children in 2018, and it is a good indication for children who take it adequately.
Because of the recent increase in JC pollinosis in Japan, the number of patients with concurrent AR caused by JC pollen and HDM has increased. Although there have been no reports on the appropriate method of the concomitant use of sublingual immunotherapy, a clinical trial was conducted on the combined use of JC pollen SLIT and HDM SLIT. There was no difference in safety between the combined treatment and single treatments when they were started to be administered 4 weeks apart
[12][13]. Combined therapy is recommended for patients with AR who have symptoms induced by JC pollen and HDM allergens and who wish to be treated simultaneously.