Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giancarlo Renella | -- | 1600 | 2022-11-22 10:24:55 | | | |
| 2 | Conner Chen | -5 word(s) | 1595 | 2022-11-23 07:31:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Giagnoni, L.; Renella, G. Biochar-Induced Temperature and Moisture Effects on Soil CUE. Encyclopedia. Available online: https://encyclopedia.pub/entry/35824 (accessed on 07 February 2026).
Giagnoni L, Renella G. Biochar-Induced Temperature and Moisture Effects on Soil CUE. Encyclopedia. Available at: https://encyclopedia.pub/entry/35824. Accessed February 07, 2026.
Giagnoni, Laura, Giancarlo Renella. "Biochar-Induced Temperature and Moisture Effects on Soil CUE" Encyclopedia, https://encyclopedia.pub/entry/35824 (accessed February 07, 2026).
Giagnoni, L., & Renella, G. (2022, November 22). Biochar-Induced Temperature and Moisture Effects on Soil CUE. In Encyclopedia. https://encyclopedia.pub/entry/35824
Giagnoni, Laura and Giancarlo Renella. "Biochar-Induced Temperature and Moisture Effects on Soil CUE." Encyclopedia. Web. 22 November, 2022.
Copy Citation
Biochar production and incorporation into soil is gaining momentum as a sustainable strategy for climate change mitigation, supported by ever increasing reports of significant carbon (C) sequestration in soil and reduction in greenhouse gas (GHG) emissions from the amended soils. With the progression in biochar testing and use, there is also emerging evidence that biochar induces C sequestration in soil, and that it may not be solely caused by its inherent chemical stability, but also by the complex microbially driven processes and an increase in C use efficiency (CUE) through soil microbial metabolism. Temperature and moisture are environmental factors that influence microbial CUE.
biochar
soil
soil microorganisms
carbon use efficiency
1. Biochar: Chemical Stability Influences the Carbon Use Efficiency (CUE) in Soil
Biochar is the product of thermochemical decomposition of biomass at temperatures ranging from 300 to 1000 °C in the absence of oxygen, either by pyrolysis or gasification, and can be used for extracting energy from biomass [1]. These processes decompose the organic biopolymers into small gaseous molecules such as methane (CH4), hydrogen (H2), carbon monoxide (CO), and carbon dioxide (CO2), condensable vapours (tars and oils), and a solid phase as a by-product termed biochar. Biochar production is maximized by pyrolysis at low temperatures (e.g., 300–450 °C) and slow heating rates and residence times (hours), whereas gasification is maximized at high temperatures (≥800 °C) and rapid heating rates (seconds), with the biochar production rates of slow and fast pyrolysis being 20–30% and 4–8%, respectively. In fact, gasification pyrolysis requires finely ground feed of specific biomasses with a narrow range of initial moisture content, a precisely controlled temperature and effective cooling of the vapour phase in order to achieve efficient recovery of the oily product. Therefore, it is a less flexible process mainly aimed at energy production, producing highly inert biochar with a sandy-dusty texture, whereas slow pyrolysis produces manipulable biochar which can be incorporated into soil. For this reason, biochar from fast pyrolysis has been used less than slow pyrolysis for the purposes of achieving agronomic and environmental goals such as climate change mitigation [2].
Based on the potential long mean residence time (MRT) of biochar in soil, the so-called charcoal vision [3] is nowadays considered as a strategy to offset a significant share of anthropogenic carbon (C) emissions through C sequestration in soil. In addition to its climate mitigation potential, incorporation of biochar into soil also brings significant improvement in soil fertility as it enhances the cation exchange capacity (CEC), neutralizes acidic pH values, changes the soil color and increases the soil thermal capacity, increases water retention, and immobilizes inorganic and organic pollutants [4]. Concurrence of these factors explains the frequently reported increases in crop yields [5][6][7], especially in highly degraded soils. Owing to its soil-improving and beneficial effects, biochar has been admitted into the new EU Regulation on Fertilizers (EU 1009/2019) under the product of pyrolysis component material category (CMC). The ever-increasing mass of information about the positive effects of biochar parallels to the historical evidence of the transformation of Amazonian dark earth soils (Terra Preta dos Indios), which maintain significantly higher pH values and fertility compared with the surrounding soils even after millennia or centuries from the charcoal burial [8].
Biochar-induced positive effects on soils and related ecosystem services are related to its stability. Initially, potential biochar stability in the environment was estimated through the process parameters such as pyrolysis temperature and feedstock types, but such parameters are currently no longer considered valid. Conversely, chemical properties such as O/C molar ratio [9], the H/Corg ratio value [10], and both the H/Corg and O/Corg ratio values are nowadays considered better descriptors of biochar stability [11][12]. In fact, these parameters reflect the labile C/recalcitrant C ratio value, and are well correlated to the results of thermal/chemical oxidation resistance tests. The molecular structure of biochar is predominantly aromatic, and its inherent stability depends upon this level molecular arrangement. Aromatic substances can form either amorphous phases, in which the aromatic substances are randomly organized, or crystalline phases, in which the aromatic structures form ordered condensed sheets, as observed in the pyrolysis and pyrogasification processes, respectively. Because more aromatic and more condensed molecular structures are supposed to be more resistant to chemical and biological degradation [2], the evaluation of both aromaticity and degree of aromatic condensation, for example by Nuclear Magnetic Resonance (NMR) spectroscopy, is increasingly used as a chemical indicator of biochar stability [13].
3. Biochar-Induced Temperature and Moisture Effects on Soil CUE
Temperature and moisture are environmental factors that influence microbial CUE [14]. Incorporation of biochar into soil significantly changes soil color and water retention properties. Reduced reflectance of biochar-amended soils increases soil temperature due to changes of soil albedo [15]. By definition, the albedo values range from 0 to 1, and the range is between 0.1–0.2 for dark soils and between 0.4–0.5 for light-colored soils [16], with either geographical, daily and seasonal variations. Beside the incident radiation, soil thermal capacity is also increased by soil moisture content, soil organic matter (SOM) content, and particle size distribution [16]. The biochar-related increase in water retention has a cooperative effect with albedo, especially in sandy soils that drain and dry out faster than clayey soils, as the specific heat of water in moist soil is ca. 5 times higher than in dry soil [17]. With few exceptions, long-term field trials show that biochar increases the water retention, and higher water retention in dry periods may reduce the accumulation of osmolytes [18] that generally increase the C:N ratio values of the microbial biomass and the apparent CUE values [19][20]. Microbial CUE is generally reduced upon an increase in soil temperature [21], mainly due to the faster acceleration of microbial respiration processes than microbial growth responses [14]. Although at the community level, microorganisms adapt to increased temperatures in terms of species composition, the link between biochar-induced changes in microbial community composition and thermal adaptation of microbial communities still needs to be assessed, and reliable information can be obtained only from the analysis of soils from long-term field trials.
Higher soil temperature can reduce the activation energy of SOM decomposition [22][23], though SOM activation energy depends on its molecular complexity [24] and increases upon the number of enzymatic steps required for substrate modification and decomposition [25]. Under different temperatures, changes of CUE values in the presence of molecular complex substrates are generally less pronounced than those recorded during the decomposition of low molecular weight organic compounds (LMWOCs) [26], and biochar generally has lower temperature sensitivity than native SOM [25]. In this regard, higher mean temperature and more constant moisture levels may facilitate microbial oxidative enzymes synthesis and release, and in cooperation with the nonspecific enzymatic mechanisms, they may reduce the activation energy for microbial respiration [27]. Overall, these mechanisms can make microbial oxidation of biochar in soil less dependent on its inherent thermal stability and more dependent on microbial enzymatic activity. However, no experiments aiming at determining the changes in the activation energy of SOM of biochar-amended soils have been conducted.
Biochar stability also depends on soil texture and its eventual association with soil minerals, or minerals deliberately associated with biomass feedstocks for producing different biochar types [28]. Because microbial activity occurs in hot spots mainly present in soil aggregates, the diffusion of biochar-borne substrates into the aggregates can possibly determine an ‘abiotic gate’ limiting their decomposition, at least shortly after soil amendment [29]. Limitations in the use of insoluble pools of biochar-borne C by soil microorganisms, due to physical and chemical protection in soil aggregates, can be alleviated when the decomposition process initiated by the synthesis of enzymes and sustained by the subsequent formation of more hydrophilic and soluble C pools, increase the microbial accessibility to organic substrates. Physico-chemical mechanisms occurring in soil such as sorption, diffusion, and occlusion into aggregates exert additional control on biochar stability in soil, because they increase the microbial energy investment in enzyme synthesis for C acquisition. These mechanisms, that depend on the properties of the soil solid phases and the soil structure complexity, along with surface hydrophobicity and molecular recalcitrance of biochar, control the biochar C transfer from stable to more labile pools. The biochar decomposition rate in soil is co-controlled by the diffusion of LMWOCs from the biochar particles surface towards soil aggregates driven by moisture (Figure 1), and from their sorption onto organic and inorganic soil solid phases [30].
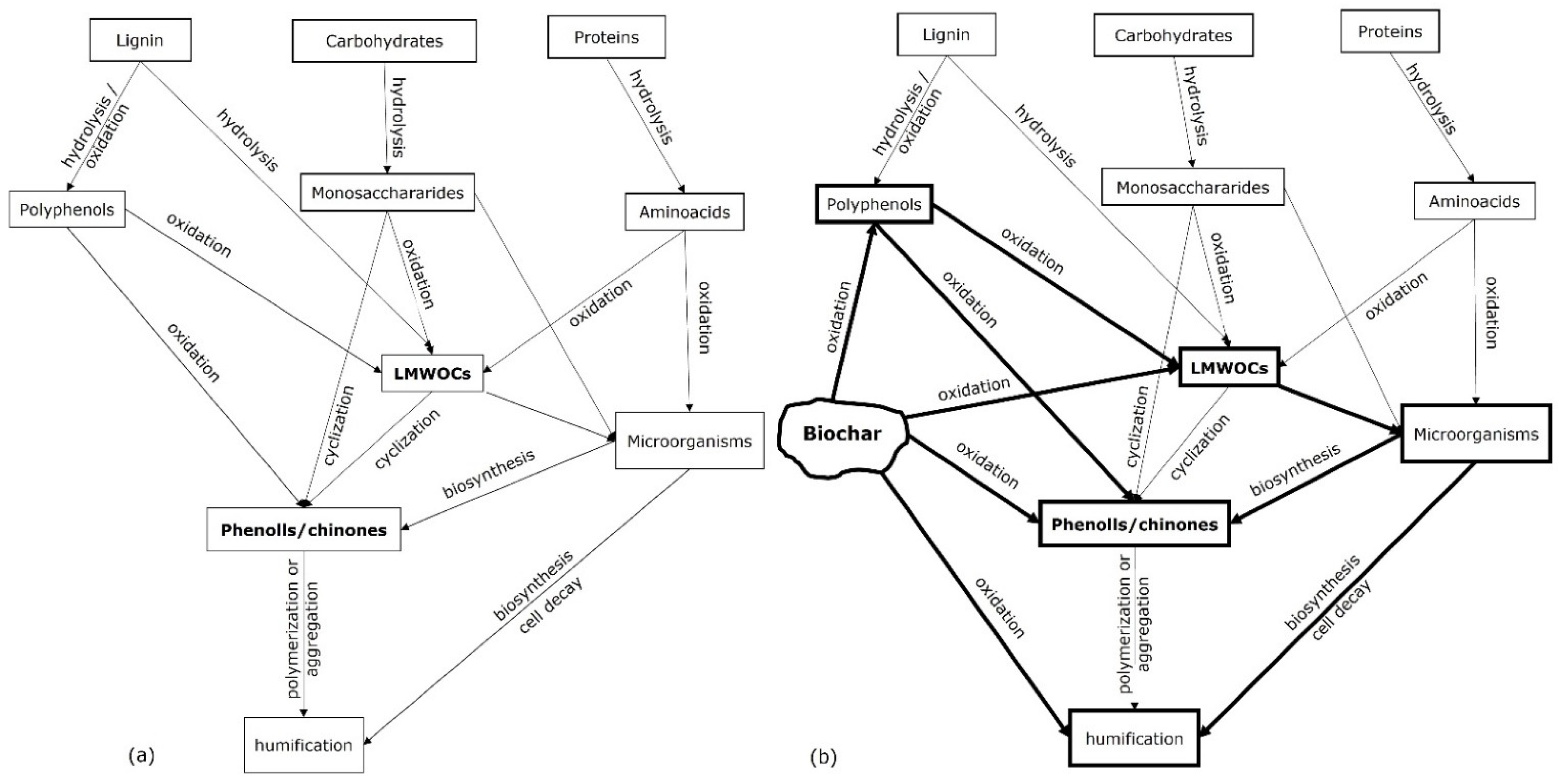
Figure 1. Organic matter decomposition in soil (a) and biochar decomposition in soil (b) processes mainly controlled by hydrolase and oxo-reductase enzymatic activities. The bold lines indicate the decomposition processes stimulated by the biochar amendment in soil. LMWCOs are the low molecular weight organic compounds.
These considerations also show that physical aspects are important for future formulation of biochar-based fertilizers, which are supposed to be more efficient for crop nutrition [31] but may not contribute to the maintenance of a porous soil structure as compared to organic amendments such as compost [32].
Overall, biochar confers resilience to soils allowing more constant microbial activity, attenuating the seasonal variations or eventual environmental drought stressful conditions, and enhancing the C stabilization through the ‘Microbial Carbon Pump’ mechanism [33][34]. Such fluctuations are particularly broad in agricultural soils, where biochar can be incorporated, because microbial CUE also decreases with soil depth due to energetic limitations [35], for example due to unfavourable C:N and C:P ratios. Biochar incorporation in the deeper horizons for an effective C storage may also increase the CUE in the long term owing to the release of LMWOCs (Figure 1) and let microorganisms living deeper soil layers reach CUE values similar to those of microorganisms of the surface horizons. There is no information on the effects of the biochar on the CUE in deeper soil horizons, and if proven, such a change may become an additional factor stabilizing organic C in the subsoil.
References
- Anca-Couce, A. Reaction mechanisms and multi-scale modelling of lignocellulosic biomass pyrolysis. Prog. Energy Combust. Sci. 2016, 53, 41–79.
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science and Technology, 2nd ed.; Earthscan: London, UK, 2015.
- Laird, A.D. The charcoal vision: A win-win-win scenario for simultaneously producing bioenergy, permanently sequestering carbon, while improving soil and water quality. Agron. J. 2008, 100, 178–181.
- Lehmann, J.; da Silva, J.P.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological anthrosol and a ferralsol of the central Amazon Basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357.
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187.
- Wong, J.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.; Bolan, N.; Wang, H.; Ok, Y. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22.
- Jaiswal, A.; Alkan, N.; Elad, Y.; Sela, N.; Philosoph, A.M.; Graber, E.R.; Frenkel, O. Molecular insights into biochar-mediated plant growth promotion and systemic resistance in tomato against Fusarium crown and root rot disease. Sci. Rep. 2020, 10, 13934.
- Sambroek, W.G. Amazonian soils: A reconnaissance of the soils of the Brazilian Amazon Valley. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, February 1966.
- Spokas, K.A. Review of the stability of biochar in soils: Predictability of O: C molar ratios. Carbon Manag. 2010, 1, 289–303.
- Budai, A.; Zimmerman, A.R.; Cowie, A.L.; Webber, J.B.W.; Singh, B.P.; Glaser, B.; Masiello, C.A.; Andersson, D.; Shields, F.; Lehmann, J.; et al. Biochar Carbon Stability Test Method: An Assessment of Methods to Determine Biochar Carbon Stability; Technical Report for International Biochar Initiative: Canandaigua, NY, USA, September 2013.
- ASTM D1762-84; Standard Test Method for Chemical Analysis of Wood Charcoal. ASTM International: West Conshohocken, PA, USA, 2021.
- EBC. European Biochar Foundation—European Biochar Certificate—Guidelines for a Sustainable Production of Biochar; European Biochar Foundation (EBC): Arbaz, Switzerland, 2012.
- Wiedemeier, D.B.; Brodowski, S.; Wiesenberg, G.L.B. Pyrogenic molecular markers: Linking PAH with BPCA analysis. Chemosphere 2015, 119, 432–437.
- Frey, S.D.; Lee, J.; Melillo, J.M.; Six, J. The temperature response of soil microbial efficiency and its feedback to climate. Nat. Clim. Change 2013, 3, 395–398.
- Genesio, L.; Miglietta, F.; Lugato, E.; Baronti, S.; Pieri, M.; Vaccari, F.P. Surface albedo following biochar application in durum wheat. Environ. Res. Lett. 2012, 70, 14025.
- Baumgardner, M.F.; Sylva, L.F.; Biehl, L.L.; Stoner, E.R. Reflectance Properties of Soils. Adv. Agron. 1985, 38, 1–44.
- Brutsaert, W. Evaporation into the Atmosphere; D. Reidel Publishing Company: Dordrecht, Holland, 1982.
- Boot, C.M.; Schaeffer, S.M.; Schimel, J.P. Static osmolyte concentrations in microbial biomass during seasonal drought in a California grassland. Soil Biol. Biochem. 2013, 57, 356–361.
- Uhlırova, E.; Elhottova, D.; Trıska, J.; Santruckova, H. Physiology and microbial community structure in soil at extreme water content. Folia Microbiol. 2005, 50, 161–166.
- Herron, P.M.; Stark, J.M.; Holt, C.; Hooker, T.; Cardon, Z.G. Microbial growth efficiencies across a soil moisture gradient assessed using 13C-acetic acid vapor and 15N-ammonia gas. Soil Biol. Biochem. 2009, 41, 1262–1269.
- Manzoni, S.; Taylor, P.; Richter, A.; Porporato, A.; Ågren, G.I. Soil carbon and nitrogen mineralization: Theory and models across scales. New Phytol. 2012, 196, 79–91.
- Wagai, R.; Kishimoto-Mo, A.W.; Yonemura, S.; Shirato, Y.; Hiradate, S.; Yagasaki, Y. Linking temperature sensitivity of soil organic matter decomposition to its molecular structure, accessibility, and microbial physiology. Glob. Change Biol. 2013, 19, 1114–1125.
- von Stockar, U.; Marison, I.W. The definition of energetic growth efficiencies for aerobic and anerobic microbial growth and their determination by calorimetry and other means. Thermochim. Acta 1993, 229, 157–172.
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173.
- Bosatta, E.; Ågren, G.I. Soil organic matter quality interpreted thermodynamically. Soil Biol. Biochem. 1999, 31, 1889–1891.
- Öquist, M.G.; Erhagen, B.; Haei, M.; Sparrman, T.; Ilstedt, U.; Schleuche, J.; Nilsson, M.B. The effect of temperature and substrate quality on the carbon use efficiency of saprotrophic decomposition. Plant Soil 2017, 414, 113–125.
- Ågren, G.I.; Wetterstedt, J.A.M. What determines the temperature response of soil organic matter decomposition? Soil Biol. Biochem. 2007, 39, 1794–1798.
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 1–9.
- Resat, H.; Bailey, V.; McCue, L.A.; Konopka, A. Modeling microbial dynamics in environments: Growth on soil carbon sources. Microb 2012, 63, 883–897.
- Müller, T.; Höper, H. Soil organic matter turnover as a function of the soil clay content: Consequences for model applications. Soil Biol. Biochem. 2004, 36, 877–888.
- Zheng, J.; Han, J.; Liu, Z.; Xia, W.; Zhang, X.; Li, L.; Liu, X.; Bian, R.; Cheng, K.; Zheng, J.; et al. Biochar compound fertilizer increases nitrogen productivity and economic benefits but decreases carbon emission of maize production. Agric. Ecosyst. Environ. 2017, 241, 70–78.
- Chen, K.; Peng, J.; Li, J.; Yang, Q.; Zhan, X.; Liu, N.; Han, X. Stabilization of soil aggregate and organic matter under the application of three organic resources and biochar-based compound fertilizer. J. Soils Sediments 2020, 20, 3633–3643.
- Miltner, A.; Bombach, P.; Schmidt-Brücken, B.; Kästner, M. SOM genesis: Microbial biomass as a significant source. Biogeochemistry 2012, 111, 45–55.
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105.
- Spohn, M.; Klaus, K.; Wanek, W.; Richter, A. Microbial carbon use efficiency and biomass turnover times depending on soil depth—implications for carbon cycling. Soil Biol. Biochem. 2016, 96, 74–81.
More
Information
Subjects:
Soil Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
23 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




