Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zeyu Ma | -- | 2023 | 2022-11-22 06:06:44 | | | |
| 2 | Conner Chen | -2 word(s) | 2021 | 2022-11-22 08:26:59 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ma, Z.; Wang, B.; Tao, L. Bioactive Polymers via the Biginelli Reaction. Encyclopedia. Available online: https://encyclopedia.pub/entry/35653 (accessed on 13 January 2026).
Ma Z, Wang B, Tao L. Bioactive Polymers via the Biginelli Reaction. Encyclopedia. Available at: https://encyclopedia.pub/entry/35653. Accessed January 13, 2026.
Ma, Zeyu, Bo Wang, Lei Tao. "Bioactive Polymers via the Biginelli Reaction" Encyclopedia, https://encyclopedia.pub/entry/35653 (accessed January 13, 2026).
Ma, Z., Wang, B., & Tao, L. (2022, November 22). Bioactive Polymers via the Biginelli Reaction. In Encyclopedia. https://encyclopedia.pub/entry/35653
Ma, Zeyu, et al. "Bioactive Polymers via the Biginelli Reaction." Encyclopedia. Web. 22 November, 2022.
Copy Citation
Multicomponent reactions (MCRs) have been used to prepare polymers with appealing functions. The Biginelli reaction, one of the oldest and most famous MCRs, has sparked new scientific discoveries in polymer chemistry since 2013. The applications of the Biginelli reaction in developing functional polymers are mainly focusing on polymers that can be used in the biomedical area as antioxidants, anticancer agents, and bioimaging probes.
the Biginelli reaction
functional polymer
bioactive polymer
1. Introduction
Multicomponent reactions (MCRs) use three or more reactants to generate one single complex product in a one-pot manner. Thanks to pioneering studies, many MCRs have been used for polymer preparation. These MCRs includes the Passerini [1][2][3][4][5][6][7], Ugi [8][9][10][11][12][13][14][15][16][17], Biginelli [18][19][20][21][22][23][24][25][26], Hantzsch [27][28][29][30][31], and Kabachnik-Fields reactions [32][33][34][35][36], as well as alkyne-based MCRs [37][38][39] and metal-catalyzed MCRs [40][41][42][43]. Nowadays, MCRs have been widely acknowledged as handy tools for generating polymers with intriguing main-chain and side-chain structures.
Functions are the core of polymer research; synthesis strategies aiming to create new polymer structures may spark the appearance of new functional polymers. Recently, some polymers developed through MCRs have been applied in environmental science, materials science, and biomedical science [7][16][17][26][28][29][31][34][44][45][46][47][48][49][50]. In these studies, the use of MCRs to introduce various functional groups was demonstrated to be a major pathway to developing multi-functional polymers. Besides their use as efficient coupling tools, MCRs themselves generate functional structures, which open new opportunities to develop new functional polymers.
Among MCRs, the Biginelli reaction is one of the most famous, which was first reported by Italian chemist Pietro Biginelli in 1891 [51]. It is a tri-component reaction involving an aldehyde, a β-keto ester, and a (thio)urea to produce a 3,4-dihydropyrimidin-2(1H)-(thi)ones (DHPM(T)) heterocycle (Figure 1, down). The first step of the Biginelli reaction is the condensation between an aldehyde and a (thio)urea; then, the product reacts with a β-keto ester to furnish an additive product, which lastly cyclizes to form DHPM(T) under acid conditions. The Biginelli reaction offers robustness, easily accessible substrates, and mild conditions, with water as the only byproduct. Meanwhile, DHPM(T) derivatives have been documented as potential calcium antagonists, mitotic inhibitors, bacterial inhibitors, and antioxidants [52][53][54]. Therefore, the Biginelli reaction has been highly valued by chemists to effectively construct heterocycles and identify new drugs since its emergence.
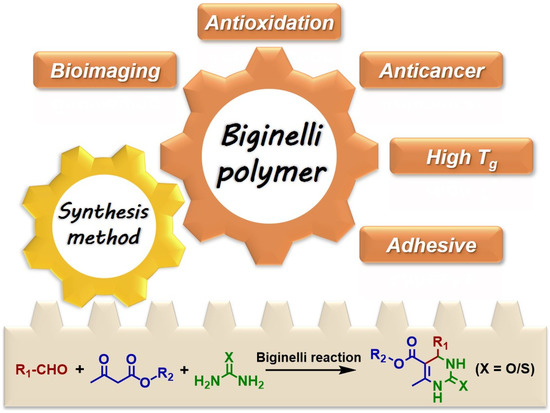
Figure 1. The Biginelli reaction in polymer chemistry.
The Biginelli reaction was introduced into polymer chemistry in 2009 [18], but it was not widely studied until 2013 [19]. In the early stage (2013–2016), researchers mainly focused on using the Biginelli reaction to develop polymer synthesis methodologies (Figure 1) [19][20][21][23][27][55]. Due to the deep understanding of this reaction in polymer chemistry, the bioactivities of DHPM(T) have been gradually exploited for developing new functional polymers; some unique properties and functions of Biginelli-type polymers have also been identified based on the synthesis methods (Figure 1) [21][22][25][26][56][57][58][59][60][61][62][63][64][65][66][67][68][69].
2. Bioactive Polymers
Oxidative stress occurs when excess reactive oxygen/nitrogen species (ROS/NOS) exist in the biological system [70]. These species attack biomacromolecules such as DNA, RNA, and proteins, thereby causing severe health issues. Oxidative stress has been recognized as being related to Alzheimer’s disease [71], cardiovascular disease [72], cancer [73], diabetes [74], and other diseases [75][76][77]. Antioxidants have been used to prevent or treat these diseases by regulating the uncontrolled redox system [78][79]. Compared to organic small molecule antioxidants that have limited clinical effects for the treatment of oxidative stress [80][81], polymeric antioxidants have enhanced in vivo stability, good water solubility, and increased bioavailability [82][83][84][85][86][87]. However, the direct introduction of small molecule antioxidants into polymers may cause a sharp loss of antioxidant capacity, resulting in an insufficient method for generating polymeric antioxidants. Thus, the development of new methodologies to efficiently produce polymeric antioxidants is important for both fundamental research and practical use.
DHPM(T) derivatives have been documented as radical scavengers since 2006 [88]. Moreover, owing to the modular nature of the Biginelli reaction, other antioxidant moieties can be facilely included to improve the antioxidation of the product. Therefore, the Biginelli reaction is a potential tool for exploring polymeric antioxidants. In 2017, Tao et al. developed a new strategy to obtain antioxidant polymers by introducing thiourea moieties and/or phenol moieties into polymers [25]. By combining ultra-fast reversible addition–fragmentation chain-transfer (RAFT) polymerization and the Biginelli reaction, they developed a high-throughput (HTP) strategy for preparing a library of 60 polymers containing different DHPM(T) moieties in the side chains.
A 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical-quenching experiment was conducted to test the radical-scavenging ability of the polymers. The ability of polymers to fade the purple color of DPPH reflected their antioxidant capacity. The visual results and the quantitative data obtained by measuring the UV absorbance at 495 nm demonstrated that the polymers containing thiourea moieties or/and phenol moieties efficiently quenched the DPPH radical.
Ultraviolet radiation (UVR) is a source of oxidative stress; radical scavengers may effectively control oxidative stress and protect biological systems from UVR damage [89]. Inspired by their previous work, in 2018, Tao et al. reported new antioxidant polymers developed by the Biginelli reaction that can protect cells from UVR [26]. They prepared 25 monomers using essential oil extracts, (thio)urea derivatives, and commercially available β-keto ester (2-acetoacetoxy)ethyl methacrylate (AEMA) in a mini-HTP manner; a library of 25 Biginelli-type polymers were facilely prepared through free radical polymerization. The radical-scavenging ability of the polymers was evaluated; three monomers of polymers with the fastest DPPH-quenching rates were selected and copolymerized with poly(ethylene glycol) methyl ether methacrylate (PEGMA) to develop potential water-soluble UVR protectors.
The as-prepared polymers were screened according to different criteria to obtain a single copolymer with low toxicity and that effectively protects cells from fatal UVR. The cytoprotective ability of the selected polymer was compared with superoxide dismutase (SOD) against UVR. L929 cells cultured with the selected copolymer (10 mg/mL) or SOD (10 mg/mL) were exposed to a UV lamp (∼254 nm, 40 W). The Biginelli-type polymer excellently protected the cells after 15 min of UVR (300 ± 20 μW/cm2), while the SOD hardly protected the cells. The mechanistic result revealed that the polymeric antioxidant may play an important role in preventing the DNA damage caused by UVR. These results demonstrate the utility of using the Biginelli reaction to develop polymeric UV protectors with practical use value. As the first facile preparation of a monomer/polymer library via the HTP-MCR strategy, the work also offers a new pathway for developing bioactive polymers.
To prompt the in-depth research of antioxidant polymers, in 2019, Tao et al. reported a ferrocene-containing antioxidant polymer prepared via the Biginelli reaction used to treat in vivo oxidative stress damage [58]. Ferrocene is a well-known reductant and free radical scavenger [90][91]. Owing to their low toxicity and versatile biological activities, ferrocene derivatives have been widely used as antimalarial and anticancer drugs [92][93]. The ferrocene group has been identified as capable of apparently enhancing the antioxidant ability of DHPMT derivatives [94]. In their study, Tao et al. used ferrocenecarboxaldehyde, thiourea, and AEMA as the substrates of the Biginelli reaction to obtain a DHPMT–ferrocene monomer, which was copolymerized with PEGMA to facilely produce a water-soluble polymer. This DHPMT–ferrocene polymer had better radical-scavenging ability than the polymers containing only ferrocene or DHPMT groups.
P0 was then evaluated in comparison to clinical drugs. Compared with small molecule antioxidants such as glutathione, P0 exhibited lower toxicity and offered much better protection to cells against tert-butyl hydroperoxide (t-BHP)-induced oxidative stress. Moreover, in an in vivo experiment using mice as model animals, P0 was superior to silymarin, an active pharmaceutical ingredient in clinically prescribed medicines in the treatment of CCl4-induced acute liver damage. Firstly, this research combines ferrocene and DHPMT groups into one polymer to achieve synergism for antioxidation, showing the value of the Biginelli reaction in developing new functional polymers for biomedical applications.
The antioxidant property of DHPMT moiety has also been incorporated in multi-functional biomaterials. In 2020, Tao et al. developed an antioxidant self-healing hydrogel via the Biginelli reaction [63]. They prepared a monomer containing both phenylboronic acid (PBA) and DHPMT groups, which was copolymerized with PEGMA to produce a PBA–DHPMT polymer. The resulting polymer quickly cross-linked poly(vinyl alcohol) (PVA) to form a hydrogel under mild conditions (pH = 7.4, 25 °C). Since the borate ester bonds between PBA and the diol groups in PVA are dynamic, the resulting hydrogel was self-healable. Besides antioxidant and self-healing properties, this hydrogel showed low cytotoxicity and was successfully used as a 3D cell culture matrix. Moreover, it demonstrated excellent bio-safety in a living mouse model. These results suggest that this newly-developed hydrogel may be a potential implant material and demonstrate the advantages of the Biginelli reaction in exploiting multi-functional biomaterials.
The PBA–DHPMT-containing polymer has also been exploited as a multi-functional bio-platform. Bacterial keratitis (BK) is a rapidly progressive and highly aggressive infectious corneal disease [95][96][97]. The inflammatory process generates ROS, which may further deteriorate the wound and impede wound tissue regeneration [98]. Thus, ROS scavenging is important in the treatment of BK. In 2022, as a new combined strategy to treat BK, Lin et al. used a PBA–DHPMT-containing glycopolymer to simultaneously kill bacteria, heal wounds, and scavenge ROS [69]. They prepared an amphipathic polymer through the random copolymerization of the PBA–DHPMT monomer and 2-lactobionamidoethyl methacrylate.
The resulting polymer self-assembled to form well-defined nanoparticles in the aqueous solution; the nanoparticles can conjugate with the bacteria and aggregate them into clusters due to the borate ester bonds formed by PBA groups and diols on the bacterial cell wall. Lin et al. further encapsulated levofloxacin (LEV, an antimicrobial drug) and chondroitin sulfate (CS, an anti-inflammatory drug) into the nanoparticles to realize the synergistic treatment of BK. The drug-carried nanoparticles were almost nontoxic to mammalian cells and can be effectively internalized by human corneal epithelial cells, indicating their ability to penetrate the cornea. With S. aureus as a model pathogen, the IC50 of the drug-carried nanoparticles was 17.5 μg/mL, which was half the value of the LEV (35 μg/mL). In the following in vivo experiment, the drug-carried nanoparticles showed the best efficacy on the BK model rats compared to LEV and CS. Due to the synergistic effects of the bactericidal activity of LEV, the wound-healing effect of CS, and the antioxidant capacity of DHPMT, the drug-carried nanoparticles can significantly decrease the intensity of inflammatory factors and relieve the extent of lesions in BK.
Besides antioxidation, DHPMT derivatives have been studied as potential antitumor molecules due to their mitosis inhibition activities [99][100][101]. For example, Monastrol, which can be facilely prepared from m-hydroxy benzaldehyde through the Biginelli reaction, specifically perturbs the Eg5 kinesin required for spindle bipolarity [99]. However, the poor water solubility and instability of organic small molecules in the bio-system lead to their poor bioavailability [102]. To overcome these problems, in 2020, Tao et al. used the Biginelli reaction to develop anticancer polymers based on DHPMT structures [61]. They prepared four different monomers containing DHPMT moieties, and then copolymerized these monomers with PEGMA to obtain water-soluble polymers, namely, P1–P4.
The results of molecular dynamics simulations revealed that these polymers may have inhibitory abilities similar to Monastrol. Tao et al. further studied the anticancer mechanism of these polymers at a cellular level. The normal cells (with the L929 cell as the model) and cancer cells (with the SMMC-7721 cell as the model) were parallelly cultured with the optimized polymer P4. P4 exhibited good cytosafety towards the L929 cells, while the mitotic activity was much like that of the blank control group in the culture medium only. On the contrary, for the SMMC-7721 cancer cells, P4 showed an inhibitory effect without killing them. The chromatins of SMMC-7721 did not move to the poles but were evenly radially distributed around the microtube protein, suggesting the specific inhibitory ability of P4 towards the mitosis of cancer cells.
As an efficient and robust coupling tool, the Biginelli reaction has also been used to develop biocompatible polymers with aggregation-induced emission (AIE) properties, which can be applied for bioimaging. In 2018, by combining RAFT polymerization and the Biginelli reaction, Zhang et al. prepared new cross-linked AIE nanoparticles for cell imaging [57]. They copolymerized PEGMA and AEMA by RAFT polymerization. An AIE-active dye 4′,4″-(1,2-diphenylethene-1,2-diyl)bis([1,10-biphenyl]-4-carbaldehyde) (CHO-TPE-CHO) and urea were then used to cross-link the polymer through the Biginelli reaction. The resulting amphipathic polymer had a relatively low critical micelle concentration (0.016 mg/mL). The polymer self-assembled in the aqueous solution to form spherical nanoparticles with high dispersity and strong green fluorescence at 510 nm (excitation wavelength = 379 nm).
References
- Kreye, O.; Toth, T.; Meier, M.A. Introducing multicomponent reactions to polymer science: Passerini reactions of renewable monomers. J. Am. Chem. Soc. 2011, 133, 1790–1792.
- Deng, X.X.; Li, L.; Li, Z.L.; Lv, A.; Du, F.S.; Li, Z.C. Sequence regulated Poly(ester-amide)s based on passerini reaction. ACS Macro Lett. 2012, 1, 1300–1303.
- Solleder, S.C.; Meier, M.A.R. Sequence control in polymer chemistry through the passerini three-component reaction. Angew. Chem. Int. Ed. 2014, 53, 711–714.
- Sehlinger, A.; Meier, M.A.R. Passerini and Ugi multicomponent reactions in polymer science. In Multi-Component and Sequential Reactions in Polymer Synthesis; Theato, P., Ed.; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2015; Volume 269, pp. 61–86.
- Zhang, J.; Zhang, M.; Du, F.S.; Li, Z.C. Synthesis of functional polycaprolactones via passerini multicomponent polymerization of 6-oxohexanoic acid and isocyanides. Macromolecules 2016, 49, 2592–2600.
- Zhang, J.; Wu, Y.H.; Wang, J.C.; Du, F.S.; Li, Z.C. Functional Poly(ester-amide)s with tertiary ester linkages via the passerini multicomponent polymerization of a dicarboxylic acid and a diisocyanide with different electron-deficient ketones. Macromolecules 2018, 51, 5842–5851.
- Oelmann, S.; Travanut, A.; Barther, D.; Romero, M.; Howdle, S.M.; Alexander, C.; Meier, M.A.R. Biocompatible unimolecular micelles obtained via the passerini reaction as versatile nanocarriers for potential medical applications. Biomacromolecules 2019, 20, 90–101.
- Kreye, O.; Tueruenc, O.; Sehlinger, A.; Rackwitz, J.; Meier, M.A.R. Structurally diverse polyamides obtained from monomers derived via the Ugi multicomponent reaction. Chem. Eur. J. 2012, 18, 5767–5776.
- Wang, R.; Liu, Z.-Q. Ugi multicomponent reaction product: The inhibitive effect on DNA oxidation depends upon the isocyanide moiety. J. Org. Chem. 2013, 78, 8696–8704.
- Sehlinger, A.; Dannecker, P.-K.; Kreye, O.; Meier, M.A.R. Diversely substituted polyamides: Macromolecular design using the Ugi four-component reaction. Macromolecules 2014, 47, 2774–2783.
- Yang, B.; Zhao, Y.; Fu, C.; Zhu, C.; Zhang, Y.; Wang, S.; Wei, Y.; Tao, L. Introducing the Ugi reaction into polymer chemistry as a green click reaction to prepare middle-functional block copolymers. Polym. Chem. 2014, 5, 2704–2708.
- Yang, B.; Zhao, Y.; Wei, Y.; Fu, C.; Tao, L. The Ugi reaction in polymer chemistry: Syntheses, applications and perspectives. Polym. Chem. 2015, 6, 8233–8239.
- Zhang, X.; Wang, S.; Liu, J.; Xie, Z.; Luan, S.; Xiao, C.; Tao, Y.; Wang, X. Ugi reaction of natural amino acids: A general route toward facile synthesis of polypeptoids for bioapplications. ACS Macro Lett. 2016, 5, 1049–1054.
- Dannecker, P.-K.; Sehlinger, A.; Meier, M.A.R. Polymacrocycles derived via Ugi multi-component reactions. Macromol. Rapid Commun. 2019, 40, 1800748.
- Tao, Y.; Wang, Z.; Tao, Y. Polypeptoids synthesis based on Ugi reaction: Advances and perspectives. Biopolymers 2019, 110, e23288.
- Zeng, Y.; Li, Y.; Liu, G.; Wei, Y.; Wu, Y.; Tao, L. Antibacterial self-healing hydrogel via the Ugi reaction. ACS Appl. Polym. Mater. 2020, 2, 404–410.
- Zeng, Y.; Liu, G.; Lv, T.; He, X.; Wei, Y.; Pan, R.; Yang, L.; Tao, L. Antioxidant polymers via the Ugi reaction for in vivo protection of UV-induced oxidative stress. Chem. Mater. 2022, 34, 2645–2654.
- Wu, G.M.; Sun, W.L.; Shen, Z.Q. Synthesis and properties of two Poly(phenyl methacylate)s functionalized with pedent Dihydropyrimid(thi)one groups. Chin. J. Polym. Sci. 2009, 27, 293–296.
- Zhu, C.; Yang, B.; Zhao, Y.; Fu, C.; Tao, L.; Wei, Y. A new insight into the biginelli reaction: The dawn of multicomponent click chemistry? Polym. Chem. 2013, 4, 5395–5400.
- Zhao, Y.; Wu, H.; Zhang, Y.; Wang, X.; Yang, B.; Zhang, Q.; Ren, X.; Fu, C.; Wei, Y.; Wang, Z.; et al. Postpolymerization modification of Poly(dihydropyrimidin-2(1H)-thione)s via the thiourea-haloalkane reaction to prepare functional polymers. ACS Macro Lett. 2015, 4, 843–847.
- Zhao, Y.; Yu, Y.; Zhang, Y.; Wang, X.; Yang, B.; Zhang, Y.; Zhang, Q.; Fu, C.; Wei, Y.; Tao, L. From drug to adhesive: A new application of Poly(dihydropyrimidin-2(1H)-one)s via the biginelli polycondensation. Polym. Chem. 2015, 6, 4940–4945.
- Boukis, A.C.; Llevot, A.; Meier, M.A. High glass transition temperature renewable polymers via biginelli multicomponent polymerization. Macromol. Rapid Commun. 2016, 37, 643–649.
- Xue, H.; Zhao, Y.; Wu, H.; Wang, Z.; Yang, B.; Wei, Y.; Wang, Z.; Tao, L. Multicomponent combinatorial polymerization via the biginelli reaction. J. Am. Chem. Soc. 2016, 138, 8690–8693.
- Zhao, Y.; Wu, H.; Wang, Z.; Wei, Y.; Wang, Z.; Tao, L. Training the old dog new tricks: The applications of the biginelli reaction in polymer chemistry. Sci. China Chem. 2016, 59, 1541–1547.
- Wu, H.; Yang, L.; Tao, L. Polymer synthesis by mimicking nature’s strategy: The combination of ultra-fast RAFT and the biginelli reaction. Polym. Chem. 2017, 8, 5679–5687.
- Mao, T.; Liu, G.; Wu, H.; Wei, Y.; Gou, Y.; Wang, J.; Tao, L. High throughput preparation of UV-protective polymers from essential oil extracts via the biginelli reaction. J. Am. Chem. Soc. 2018, 140, 6865–6872.
- Wu, H.; Fu, C.; Zhao, Y.; Yang, B.; Wei, Y.; Wang, Z.; Tao, L. Multicomponent copolycondensates via the simultaneous hantzsch and biginelli reactions. ACS Macro Lett. 2015, 4, 1189–1193.
- Liu, G.; Zhang, Q.; Li, Y.; Wang, X.; Wu, H.; Wei, Y.; Zeng, Y.; Tao, L. High-throughput preparation of antibacterial polymers from natural product derivatives via the Hantzsch reaction. iScience 2020, 23, 100754.
- Liu, G.; Zeng, Y.; Lv, T.; Mao, T.; Wei, Y.; Jia, S.; Gou, Y.; Tao, L. High-throughput preparation of radioprotective polymers via Hantzsch’s reaction for in vivo x-ray damage determination. Nat. Commun. 2020, 11, 6214.
- Liu, G.; Pan, R.; Wei, Y.; Tao, L. The Hantzsch reaction in polymer chemistry: From synthetic methods to applications. Macromol. Rapid Commun. 2021, 42, e2000459.
- Liu, G.; Xu, Z.; Dai, X.; Zeng, Y.; Wei, Y.; He, X.; Yan, L.T.; Tao, L. De novo design of entropy-driven polymers resistant to bacterial attachment via multicomponent reactions. J. Am. Chem. Soc. 2021, 143, 17250–17260.
- Kakuchi, R.; Theato, P. Efficient multicomponent postpolymerization modification based on Kabachnik-fields reaction. ACS Macro Lett. 2014, 3, 329–332.
- Zhang, Y.; Zhao, Y.; Yang, B.; Zhu, C.; Wei, Y.; Tao, L. ‘One pot’ synthesis of well-defined Poly(aminophosphonate)s: Time for the Kabachnik–fields reaction on the stage of polymer chemistry. Polym. Chem. 2014, 5, 1857–1862.
- He, X.; Liu, G.; Tian, Y.; Mao, T.; Wu, H.; Wei, Y.; Tao, L. Antioxidant polymers via the Kabachnik-fields reaction to control cellular oxidative stress. Macromol. Biosci. 2020, 20, e1900419.
- He, X.; Zeng, Y.; Liu, G.; Tian, Y.; Wei, Y.; Zhao, L.; Yang, L.; Tao, L. Magnetic self-healing hydrogel from difunctional polymers prepared via the Kabachnik-fields reaction. ACS Macro Lett. 2022, 11, 39–45.
- He, X.; Yang, L.; Liu, G.; Wei, Y.; Zeng, Y.; Tao, L. Polymer chelator prepared via the Kabachnik–fields reaction for the in vivo prevention of heavy-metal damage. Chem. Mater. 2022, 34, 9558–9568.
- Hassan, S.; Müller, T.J.J. Multicomponent syntheses based upon copper-catalyzed Alkyne-Azide cycloaddition. Adv. Synth. Catal. 2015, 357, 617–666.
- Kayser, L.V.; Vollmer, M.; Welnhofer, M.; Krikcziokat, H.; Meerholz, K.; Arndtsen, B.A. Metal-free, multicomponent synthesis of pyrrole-based π-conjugated polymers from imines, acid chlorides, and alkynes. J. Am. Chem. Soc. 2016, 138, 10516–10521.
- Wei, B.; Li, W.; Zhao, Z.; Qin, A.; Hu, R.; Tang, B.Z. Metal-free multicomponent tandem polymerizations of alkynes, amines, and formaldehyde toward structure- and sequence-controlled luminescent polyheterocycles. J. Am. Chem. Soc. 2017, 139, 5075–5084.
- Lee, I.H.; Kim, H.; Choi, T.L. Cu-catalyzed multicomponent polymerization to synthesize a library of Poly(N-sulfonylamidines). J. Am. Chem. Soc. 2013, 135, 3760–3763.
- Leitch, D.C.; Kayser, L.V.; Han, Z.Y.; Siamaki, A.R.; Keyzer, E.N.; Gefen, A.; Arndtsen, B.A. A palladium-catalysed multicomponent coupling approach to conjugated Poly(1,3-dipoles) and polyheterocycles. Nat. Commun. 2015, 6, 7411.
- Kim, H.; Bang, K.T.; Choi, I.; Lee, J.K.; Choi, T.L. Diversity-oriented polymerization: One-shot synthesis of library of graft and dendronized polymers by Cu-catalyzed multicomponent polymerization. J. Am. Chem. Soc. 2016, 138, 8612–8622.
- Lee, I.H.; Bang, K.T.; Yang, H.S.; Choi, T.L. Recent advances in diversity-oriented polymerization using Cu-catalyzed multicomponent reactions. Macromol. Rapid Commun. 2022, 43, e2100642.
- Blasco, E.; Sims, M.B.; Goldmann, A.S.; Sumerlin, B.S.; Barner-Kowollik, C. 50th anniversary perspective: Polymer functionalization. Macromolecules 2017, 50, 5215–5252.
- Afshari, R.; Shaabani, A. Materials functionalization with multicomponent reactions: State of the art. ACS Comb. Sci. 2018, 20, 499–528.
- Tian, T.; Hu, R.; Tang, B.Z. Room temperature one-step conversion from elemental sulfur to functional polythioureas through catalyst-free multicomponent polymerizations. J. Am. Chem. Soc. 2018, 140, 6156–6163.
- Javanbakht, S.; Shaabani, A. Multicomponent reactions-based modified/functionalized materials in the biomedical platforms. ACS Appl. Bio Mater. 2020, 3, 156–174.
- Pan, R.; Liu, G.; Zeng, Y.; He, X.; Ma, Z.; Wei, Y.; Chen, S.; Yang, L.; Tao, L. A multi-responsive self-healing hydrogel for controlled release of curcumin. Polym. Chem. 2021, 12, 2457–2463.
- Zhang, J.; Zang, Q.; Yang, F.; Zhang, H.; Sun, J.Z.; Tang, B.Z. Sulfur conversion to multifunctional Poly(O-thiocarbamate)s through multicomponent polymerizations of sulfur, diols, and diisocyanides. J. Am. Chem. Soc. 2021, 143, 3944–3950.
- Peng, F.; Liu, H.; Hu, S.; Yue, F.; Xiao, D.; Guo, L.; Qi, H. High throughput preparation of antioxidant polysaccharide-based polymers with UV-resistant and antibacterial performance. Food Hydrocoll. 2022, 133, 107936.
- Biginelli, P. Ueber Aldehyduramide des Acetessigäthers. Ber. Dtsch. Chem. Ges. 1891, 24, 1317–1319.
- Nagarajaiah, H.; Mukhopadhyay, A.; Moorthy, J.N. Biginelli reaction: An overview. Tetrahedron Lett. 2016, 57, 5135–5149.
- Kaur, R.; Chaudhary, S.; Kumar, K.; Gupta, M.K.; Rawal, R.K. Recent synthetic and medicinal perspectives of dihydropyrimidinones: A review. Eur. J. Med. Chem. 2017, 132, 108–134.
- Matos, L.H.S.; Masson, F.T.; Simeoni, L.A.; Homem-de-Mello, M. Biological Activity of Dihydropyrimidinone (DHPM) derivatives: A systematic review. Eur. J. Med. Chem. 2018, 143, 1779–1789.
- Ren, X.; Yang, B.; Zhao, Y.; Zhang, X.; Wang, X.; Wei, Y.; Tao, L. One-pot polymer conjugation on carbon nanotubes through simultaneous π–π stacking and the biginelli reaction. Polymer 2015, 64, 210–215.
- Wang, Z.; Yu, Y.; Li, Y.; Yang, L.; Zhao, Y.; Liu, G.; Wei, Y.; Wang, X.; Tao, L. Post-polymerization modification via the biginelli reaction to prepare water-soluble polymer adhesives. Polym. Chem. 2017, 8, 5490–5495.
- Dong, J.; Liu, M.; Jiang, R.; Huang, H.; Wan, Q.; Wen, Y.; Tian, J.; Dai, Y.; Zhang, X.; Wei, Y. Synthesis and biological imaging of cross-linked fluorescent polymeric nanoparticles with aggregation-induced emission characteristics based on the combination of RAFT polymerization and the biginelli reaction. J. Colloid. Interface Sci. 2018, 528, 192–199.
- Mao, T.; Yang, L.; Liu, G.; Wei, Y.; Gou, Y.; Wang, J.; Tao, L. Ferrocene-containing polymer via the biginelli reaction for in vivo treatment of oxidative stress damage. ACS Macro Lett. 2019, 8, 639–645.
- Rong, L.; Zeng, M.; Liu, H.; Wang, B.; Mao, Z.; Xu, H.; Zhang, L.; Zhong, Y.; Yuan, J.; Sui, X. Biginelli reaction on cellulose acetoacetate: A new approach for versatile cellulose derivatives. Carbohydr. Polym. 2019, 209, 223–229.
- Esen, E.; Meier, M.A.R. Modification of starch via the biginelli multicomponent reaction. Macromol. Rapid Commun. 2020, 41, e1900375.
- Li, Y.; Tan, T.; Zhao, Y.; Wei, Y.; Wang, D.; Chen, R.; Tao, L. Anticancer polymers via the biginelli reaction. ACS Macro Lett. 2020, 9, 1249–1254.
- Windbiel, J.T.; Meier, M.A.R. Synthesis of new biginelli polycondensates: Renewable materials with tunable high glass transition temperatures. Polym. Int. 2020, 70, 506–513.
- Yang, L.; Zeng, Y.; Wu, H.; Zhou, C.; Tao, L. An antioxidant self-healing hydrogel for 3D cell cultures. J. Mater. Chem. B 2020, 8, 1383–1388.
- Mao, T.; He, X.; Liu, G.; Wei, Y.; Gou, Y.; Zhou, X.; Tao, L. Fluorescent polymers via post-polymerization modification of biginelli-type polymers for cellular protection against UV damage. Polym. Chem. 2021, 12, 852–857.
- Windbiel, J.T.; Meier, M.A.R. RAFT polymerization of a renewable ricinoleic acid-derived monomer and subsequent post-polymerization modification via the biginelli-3-component reaction. Macromol. Chem. Phys. 2021, 223, 2100360.
- Zeng, Y.; Zhu, C.; Tao, L. Stimuli-responsive multifunctional phenylboronic acid polymers via multicomponent reactions: From synthesis to application. Macromol. Rapid Commun. 2021, 42, e2100022.
- Zhang, D.; Zheng, J.; Zhang, P.; Zhao, R.; Chen, Z.; Wang, M.; Deng, K. Polyurea modified with 4-dihydropyrimidone-2-ketone rings by biginelli reaction and its boostered AIE characteristic. Macromol. Chem. Phys. 2021, 222, 2100284.
- Zhou, M.; Li, L.; Xie, W.; He, Z.; Li, J. Synthesis of a thermal-responsive dual-modal supramolecular probe for magnetic resonance imaging and fluorescence imaging. Macromol. Rapid Commun. 2021, 42, e2100248.
- Zhang, Y.; Li, G.; Zhang, X.; Lin, L. ROS-Scavenging glyco-nanoplatform for synergistic antibacterial and wound-healing therapy of bacterial keratitis. J. Mater. Chem. B 2022, 10, 4575–4587.
- Sies, H. Biochemistry of oxidative stress. Angew. Chem. Int. Ed. Engl. 1986, 25, 1058–1071.
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160.
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative stress in cardiovascular diseases. Antioxidants 2020, 9, 864.
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197.
- Song, Q.X.; Sun, Y.; Deng, K.; Mei, J.Y.; Chermansky, C.J.; Damaser, M.S. Potential role of oxidative stress in the pathogenesis of diabetic bladder dysfunction. Nat. Rev. Urol. 2022, 19, 581–596.
- Halliwell, B. Reactive oxygen species and the central nervous system. J. Neurochem. 1992, 59, 1609–1623.
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922.
- Lima, D.D.; Cyrino, L.A.R.; Ferreira, G.K.; Magro, D.D.D.; Calegari, C.R.; Cabral, H.; Cavichioli, N.; Ramos, S.A.; Ullmann, O.M.; Mayer, Y.; et al. Neuroinflammation and neuroprogression produced by oxidative stress in euthymic bipolar patients with different onset disease times. Sci. Rep. 2022, 12, 16742.
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74.
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709.
- Slatore, C.G.; Littman, A.J.; Au, D.H.; Satia, J.A.; White, E. Long-term use of supplemental multivitamins, vitamin C, vitamin E, and folate does not reduce the risk of lung cancer. Am. J. Respir. Crit. Care. Med. 2008, 177, 524–530.
- Halliwell, B. The antioxidant paradox: Less paradoxical now? Br. J. Clin. Pharmacol. 2013, 75, 637–644.
- Wang, Y.; Singh, A.; Xu, P.; Pindrus, M.A.; Blasioli, D.J.; Kaplan, D.L. Expansion and osteogenic differentiation of bone marrow-derived mesenchymal stem cells on a vitamin C functionalized polymer. Biomaterials 2006, 27, 3265–3273.
- Wattamwar, P.P.; Mo, Y.; Wan, R.; Palli, R.; Zhang, Q.; Dziubla, T.D. Antioxidant activity of degradable polymer Poly(trolox ester) to suppress oxidative stress injury in the cells. Adv. Funct. Mater. 2010, 20, 147–154.
- Hlushko, R.; Hlushko, H.; Sukhishvili, S.A. A family of linear phenolic polymers with controlled hydrophobicity, adsorption and antioxidant properties. Polym. Chem. 2018, 9, 506–516.
- Nagarajan, S.; Nagarajan, R.; Kumar, J.; Salemme, A.; Togna, A.R.; Saso, L.; Bruno, F. Antioxidant activity of synthetic polymers of phenolic compounds. Polymers 2020, 12, 1646.
- Brito, J.; Hlushko, H.; Abbott, A.; Aliakseyeu, A.; Hlushko, R.; Sukhishvili, S.A. Integrating antioxidant functionality into polymer materials: Fundamentals, strategies, and applications. ACS Appl. Mater. Interfaces 2021, 13, 41372–41395.
- Maraveas, C.; Bayer, I.S.; Bartzanas, T. Recent advances in antioxidant polymers: From sustainable and natural monomers to synthesis and applications. Polymers 2021, 13, 2465.
- Stefani, H.A.; Oliveira, C.B.; Almeida, R.B.; Pereira, C.M.; Braga, R.C.; Cella, R.; Borges, V.C.; Savegnago, L.; Nogueira, C.W. Dihydropyrimidin-(2H)-ones obtained by ultrasound irradiation: A new class of potential antioxidant agents. Eur. J. Med. Chem. 2006, 41, 513–518.
- Jager, T.L.d.; Cockrell, A.E.; Plessis, S.S.D. Ultraviolet light induced generation of reactive oxygen species. In Ultraviolet Light in Human Health, Diseases and Environment; Ahmad, S.I., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; Volume 996, pp. 15–23.
- Liu, Z.Q. Enhancing antioxidant effect against peroxyl radical-induced oxidation of DNA: Linking with ferrocene moiety! Chem. Rec. 2019, 19, 2385–2397.
- Liu, Z.Q. Multicomponent reactions for integrating multiple functional groups into an antioxidant. Chem. Rec. 2020, 20, 1516–1529.
- Staveren, D.R.v.; Metzler-Nolte, N. Bioorganometallic chemistry of ferrocene. Chem. Rev. 2004, 104, 5931–5985.
- Patra, M.; Gasser, G. The medicinal chemistry of ferrocene and its derivatives. Nat. Rev. Chem. 2017, 1, 0066.
- Wang, R.; Liu, Z.-Q. Ferrocene as a functional group enhances the inhibitive effect of dihydropyrimidine on radical-induced oxidation of DNA. Org. Chem. Front. 2014, 1, 792–797.
- Ung, L.; Bispo, P.J.M.; Shanbhag, S.S.; Gilmore, M.S.; Chodosh, J. The persistent dilemma of microbial kratitis: Global burden, diagnosis, and antimicrobial resistance. Surv. Ophthalmol. 2019, 64, 255–271.
- Hilliam, Y.; Kaye, S.; Winstanley, C. Pseudomonas aeruginosa and microbial keratitis. J. Med. Microbiol. 2020, 69, 3–13.
- Ting, D.S.J.; Ho, C.S.; Deshmukh, R.; Said, D.G.; Dua, H.S. Infectious keratitis: An update on epidemiology, causative microorganisms, risk factors, and antimicrobial resistance. Eye 2021, 35, 1084–1101.
- Forbes, S.J.; Rosenthal, N. Preparing the ground for tissue regeneration: From mechanism to therapy. Nat. Med. 2014, 20, 857–869.
- Mayer, T.U.; Kapoor, T.M.; Haggarty, S.J.; King, R.W.; Schreiber, S.L.; Mitchison, T.J. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science 1999, 186, 971–974.
- Guido, B.C.; Ramos, L.M.; Nolasco, D.O.; Nobrega, C.C.; Andrade, B.Y.; Pic-Taylor, A.; Neto, B.A.; Correa, J.R. Impact of kinesin Eg5 Inhibition by 3,4-Dihydropyrimidin-2(1H)-one derivatives on various breast cancer cell features. BMC Cancer 2015, 15, 283.
- Bhat, M.A.; Al-Dhfyan, A.; Al-Omar, M.A. Targeting cancer stem cells with novel 4-(4-Substituted phenyl)-5-(3,4,5-trimethoxy/3,4-dimethoxy)-benzoyl-3,4-dihydropyrimidine-2(1H)-one/thiones. Molecules 2016, 21, 1746.
- Ringsdorf, H. Structure and properties of pharmacologically active polymers. J. Polym. Sci. Polym. Symp. 1975, 51, 135–153.
More
Information
Subjects:
Polymer Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
951
Revisions:
2 times
(View History)
Update Date:
22 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




