Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Juan García-Guzmán | -- | 6254 | 2022-11-21 09:31:18 | | | |
| 2 | Conner Chen | -6 word(s) | 6248 | 2022-11-22 08:06:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
García-Guzmán, J.J.; Sierra-Padilla, A.; Palacios-Santander, J.M.; Fernández-Alba, J.J.; Macías, C.G.; Cubillana-Aguilera, L. Electrochemical Lactate (Bio)Sensor Transduction Approaches. Encyclopedia. Available online: https://encyclopedia.pub/entry/35512 (accessed on 08 February 2026).
García-Guzmán JJ, Sierra-Padilla A, Palacios-Santander JM, Fernández-Alba JJ, Macías CG, Cubillana-Aguilera L. Electrochemical Lactate (Bio)Sensor Transduction Approaches. Encyclopedia. Available at: https://encyclopedia.pub/entry/35512. Accessed February 08, 2026.
García-Guzmán, Juan José, Alfonso Sierra-Padilla, José María Palacios-Santander, Juan Jesús Fernández-Alba, Carmen González Macías, Laura Cubillana-Aguilera. "Electrochemical Lactate (Bio)Sensor Transduction Approaches" Encyclopedia, https://encyclopedia.pub/entry/35512 (accessed February 08, 2026).
García-Guzmán, J.J., Sierra-Padilla, A., Palacios-Santander, J.M., Fernández-Alba, J.J., Macías, C.G., & Cubillana-Aguilera, L. (2022, November 21). Electrochemical Lactate (Bio)Sensor Transduction Approaches. In Encyclopedia. https://encyclopedia.pub/entry/35512
García-Guzmán, Juan José, et al. "Electrochemical Lactate (Bio)Sensor Transduction Approaches." Encyclopedia. Web. 21 November, 2022.
Copy Citation
Monitoring of lactate is spreading from the evident clinical environment, where its role as a biomarker is notorious, to the agrifood ambit as well. In the former, lactate concentration can serve as a useful indicator of several diseases (e.g., tumour development and lactic acidosis) and a relevant value in sports performance for athletes, among others. In the latter, the spotlight is placed on the food control, bringing to the table meaningful information such as decaying product detection and stress monitoring of species. No matter what purpose is involved, electrochemical (bio)sensors stand as a solid and suitable choice.
lactate monitoring
electrochemical (bio)sensors
agrifood control
1. Introduction
Within the spectrum of relevant biomarkers, L-lactate is rising as one of the most significant due to its intrinsic role in diverse real-life scenarios. As is well-known, lactate is a metabolite that originates from anaerobic metabolism. This metabolic route is triggered when the regular aerobic metabolism is not enough to satisfy the energy requirements. Typically, an extra energy demand occurs during physical training, increasing the lactate level in blood from 0.5–2.0 to 25 mM or even more in intense exercise. Interestingly, the generation of lactate also involves a proton concentration increment and the consequent pH diminishment, leading to cell acidosis. Moreover, the accumulation of lactate in working muscles during the exercise is conducive to diverse health issues from slight fatigue to severe pain. This is why real lactate monitoring is frequently pursued in sport medicine [1][2]. Particularly, the term lactate threshold was coined in this ambit to draw the highest effort limit for an athlete with no risk of lactate accumulation complications. However, the most common practices to determine lactate pass through blood extraction, which limits its continuous determination and hinders an easy and comfortable assessment [3]. For these reasons, a reliable and robust lactate (bio)sensor would provide a game changer tool for physicians. Optimal trainings may be designed in order to minimise the risk for the athlete avoiding injuries and granting the maximum reward.
Nevertheless, the importance of lactate in the agrifood field is also vast and very essential to the educated observer. In the first place, lactate is frequently used as an acidulant additive (E270) due to its nonvolatile properties. More importantly, derived from its anaerobic natural origin, lactate can be found in fermented products such as milk, yoghurt, cheese, etc. Additionally, other more exotic foodstuffs (sauerkraut and kimchi) can contain it as well. It is not difficult to reason that lactate concentration may be a reliable indicator of the fermentation process and the quality of the prepared foodstuff. It is also very significant in the assessment of beverage spoilage. For instance, juice degradation can be evaluated by employing lactate concentration to estimate lactate-consumer bacteria present in the drink. The uncontrolled proliferation of microorganisms will clearly impoverish the quality of the juice, precluding its regular consumption. Remarkably, lactate has an essential role in the wine industry due to its inverse relationship with malic acid during wine fermentation, being also an indicator of its organoleptic properties. Nonetheless, it is noteworthy to mention that in this scenario, D-lactate isomer generation is considered as a symptom of beverage spoilage. Furthermore, D-lactate is also recurrent evidence of decomposition in solid food such as meat or egg [4]. In addition, lactate determination is relevant in other very raw food, namely farmed fish used in aquaculture. Frequently, fish tanks are overpopulated with specimens, provoking high levels of stress, which results in an increase in lactate and diseases. Therefore, real lactate monitoring would assist in paving the way for more rightful breeding practices in this flourishing industry [5].
Obviously not only is lactate interest found in fish diseases, but also in human wellbeing. Firstly, several diseases increase regular blood lactate leading to ischemic situation [6]. Therefore, the inverse is also true; it is demonstrates that lactate is a potential biomarker in the early diagnosis and monitoring of several diseases (e.g., diabetes or cardiac issues) [7]. In the same vein, lactate assessment is highly appreciated in intensive care units (ICUs), where an abnormal level is a beacon of meaningful information of the current and future status of the patient. In this sense, it is possible to relate it with situations such as haemorrhagic shock, pulmonary embolism, cardiogenic shock, respiratory poisoning and renal failure, among others [8][9][10][11][12][13]. Moreover, alternative studies pointed out the existence of abnormal lactate concentration in cancer cells during metastases [14]. Moreover, lactate is also relevant in neuroscience, being a sleep biomarker and an indicator of brain metabolism [15][16]. In addition, as has been previously mentioned, lactate is inversely related with pH; hence, a high increase of lactate causes lactic acidosis, altering the acid-base homeostasis and negatively affecting the entire organism if the pancreas and liver are not able to excrete it properly [17]. These two parameters, lactate and pH, can be extremely important in certain scenarios where the person is weakened or under a specific risk. For instance, during delivery, it is highly recommended to monitor it in order to distinguish between respiratory acidosis and metabolic acidosis, thus helping to prevent the sequelae of lactic acidosis in the newborn. Unfortunately, this is not feasible yet, mainly due to the current methodology designed for this purpose, which is bloody and complex. The extraction of blood is, at the present time, unavoidable and is very limited during births extended in time, for evident reasons. Ironically, it is in the difficult births when the risk is higher and the lactate/pH monitoring is of paramount importance due to the dangers for the baby being critical (e.g., postnatal neurological problems, asphyxia, premature death, etc.) [18]. Furthermore, in the reproductive field, it has also been demonstrated that lactate is a relevant species for cell cultures needed in embryonic cell growth, especially in the first stages [19].
At this point, the reader is able now to assimilate the importance of lactate and the real need to determine it in both agrifood and biomedicine environments (Figure 1).
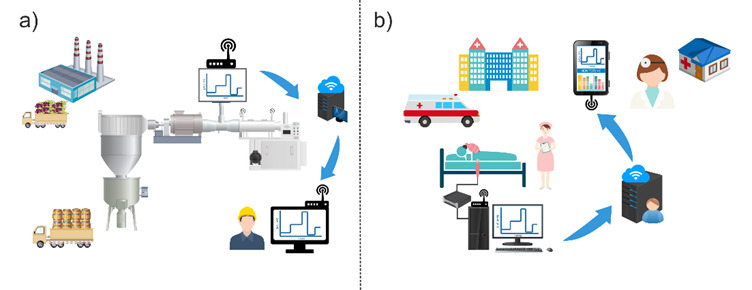
Figure 1. Scheme of relevant high-value situations for real and continuous lactate monitoring, (a) wine production and (b) intensive care units (ICUs).
In this sense, the scientific community has invested huge efforts and funds to achieve this. Time has not been wasted and the initial methodology proposed by Barker and Summerson in 1941 based on non-enzymatic colorimetric assays is now considered obsolete [20]. More specific, sensitive and reliable procedures have taken up the torch. Regarding the biomedicine ambit, high-performance liquid chromatography (HPLC) [21], fluorometry [22], chemiluminescence [23], microwave sensing [24], holography [25] and magnetic resonance spectroscopy [26] have been already employed to determine lactate. It is noteworthy to mention that lactate determination in ICUs and clinics is currently carried out by using an enzyme-based spectrophotometric and colorimetric method prior to blood sampling. Usually, lactate dehydrogenase is employed as biological recognition element to catalyse lactate–pyruvate conversion through NADH formation. Subsequently, NADH can be determined via spectrophotometric techniques and can find out the initial lactate concentration in the fluid [27]. Despite the suitable sensitivity and reliability supplied by this technique, it only allows for discrete measurements in time, requires the addition of an additional reagent (NAD+) and more importantly, it is not possible to have an unlimited blood sampling due to obvious reasons. On the other hand, spectrophotometry [28], electrophoresis [29], nuclear magnetic resonance spectroscopy [30], electrochemiluminescence [31], high-performance liquid chromatography [32] and liquid chromatography–mass spectrometry [33] have also been applied in food analysis. However, despite the excellent outputs that may be provided, all these methodologies also possess several important drawbacks such as complex sample treatments, highly trained personnel requirements, slow response time and in some cases, expensive instruments. Furthermore, other aspect should be also evaluated: (i) the possibility of online analyses, (ii) its performance in ICUs/clinical environment and (iii) feasible real-time monitoring.
Electrochemical devices have a wide range of advantages such as simplicity, rapid response, high sensitivity, low cost and direct measurement with low or no sample preparation. In addition, portability and the possibility of in situ and online analysis in a minimally invasive manner are also among their most important characteristics [17][34]. Particularly, selectivity can be greatly enhanced by using a biological recognition element within the body of the sensor. In the case of lactate, enzymes are frequently used due to their biocatalyst properties and high specificity. Nevertheless, electrochemical biosensors have some limitations as well (e.g., relatively short lifetime, storage limitations, environmental conditioning, etc.), which have led the researchers into an alternative train of thought. The replacement of the biological recognition element for a synthetic one, such as nanomaterials or molecularly imprinted polymers (MIPs), seeks the same electrocatalysis advantages and high selectivity, avoiding sensitive biological parts. Hence, the electrochemical lactate-sensing field has been greatly expanded during the last years due to the so-called non-enzymatic sensors [35].
2. Electrochemical (Bio)Sensor Transduction Approaches
2.1. Amperometric Lactate Biosensors
Among the most commonly employed sensors in lactate detection, amperometric (bio)sensors monitor the current between a working electrode (WE) and a counter electrode (CE); meanwhile a potential is being applied between WE and a reference electrode (RE).
Recorded current can be correlated with the bulk concentration of the electroactive species or its production/consumption in the studied sample. The reader may revise the deep and instructive review from Nikolaus et al. to find more fundamental information [36]. Amperometric sensors are considered as a direct and simple tool, where the main bottleneck is the design of a suitable WE. In these cases, it is necessary to evaluate several factors such as the electrode material and its consequently chemical modification, among others. However, amperometric lactate biosensors are not so straightforward in their fundamentals. Enzymes are frequently bonded to the electrode material to enhance the specificity of the resulting sensor [36]. In order to take advantage of the lock and key enzymatic mechanism, new electroactive species are assessed instead of lactate itself. For example, consider the two most employed enzymes for this purpose [5], namely L-lactate oxidase (LOx) and L-lactate dehydrogenase (LDH), and their catalytic reactions exposed below:
Both scenarios involve the transformation from L-lactate to pyruvate, using either oxygen or the corresponding cofactor (NAD+), and the production of a secondary electroactive specie during the enzymatic reaction, H2O2 and NADH (reduced form of nicotinamide dinucleotide) for L-LOx and LDH, respectively. Importantly, both species’ concentrations will be easily correlated with the initial lactate in the biosensor surroundings, and more importantly, their oxidation will provide a current that is only attributable to the target analyte. The trained reader may notice that the biosensor will inherit a certain dependency from the enzymatic choice. Oxygen or NAD+ must be available in the medium for the correct biosensor performance. This is not a negligible detail, and it should be properly studied. Nevertheless, this ideal free of interferent current also possesses a considerable flaw. The potential to re-oxidise both species is relatively high (ca. ≥ 0.6 V vs. Ag/AgCl reference electrode), even with suitable electrode materials (e.g., Au and Pt), and it may affect other common substances in the matrix studied (e.g., ascorbic acid, uric acid, dopamine, etc.).
Researchers have found many alternatives to minimise this issue. Simple but effective approaches rely on a Nafion layer onto the surface of the electrode that rejects anionic compounds prone to be oxidised in the WE. On top of that, a polymeric layer (e.g., polyurethane, chitosan, etc.) can be also drop-casted to hinder the diffusions of susceptible substances. Burmeister et al. claimed an expansion in the linear range of the resulting sensor and an interferent-free signal by using this concept [37]. In a more recent work, Lee et al. presented an amperometric biosensor based on the immobilisation of LOx through polypyrrole and polydopamine layers [38]. According to the authors, the preparation was performed in a one-pot pathway, and it was also possible to immobilise glucose oxidase by using this approach in a similar electrode. Lee et al. claimed that the employment of both polypyrrole and polydopamine greatly enhanced the sensitivity of the resulting device. Similar to other studies, a permselective layer made of polyphenol was placed onto the surface of the modified electrode to improve the selectivity of the sensor. Concerning the results obtained, a sensitivity of 3.30 µA mM−1 cm−2 and a linear range of 0–0.5 mM were found. Indeed, it seems that this sensitivity and stability are acceptable. Furthermore, the permselective layer dwarfs interferent issues, even using an oxidation potential of 0.7 V vs. Ag/AgCl. Nonetheless, its short linear range limits the direct and undiluted application, although the authors should be praised for developing such as fast and simple manufacturing methodology. Booth et al. considered growing platinum black in carbon-based conductive homemade fibres to build their amperometric biosensor [39]. The authors benefited from the catalytic effect of the deposited Pt towards H2O2. In addition, a layer of poly-m-phenylene diamine was also electrodeposited. Finally, a hydrogel of poly(ethylene glycol) diglycidyl ether loaded with LOx was integrated in the sensor by dip coating. These polymers act as a protective layer towards interferents such as serotonin, ascorbic acid and dopamine, among others. It is noteworthy to mention that a similar fibre was modified with iridium oxide (IrOx) to serve as a pH probe. Booth et al. stated that the sensor was able to measure concentration in the physiologically relevant range and a limit of detection of 19 µM was obtained from the resulting device. Remarkably, the amperometric biosensor was used to evaluate damaged tissue in mice brains, and local lactate changes were recognised and monitored. Thus, the authors proposed this device as possible implantable biosensors.
Other authors have also explored the electrocatalytic effect of nanomaterials. Istrate et al. employed a ternary composite based on gold nanoparticles, reduced graphene oxide and polyallylamine to immobilise LDH onto a screen-printed carbon electrode [40]. Certainly, gold nanoparticles have been stated in other works as an excellent material to build lactate biosensors, which is even more convenient in tandem with carbon nanomaterials [41]. Istrate et al. reported a resulting device with an extensive linear range (4–16 mM), a low limit of detection (1 µM) and high sensitivity (0.28 µA mM−1 cm−2). With the aim of food application, several specific interferents were assayed, such as acetic acid, ethanol, glucose, ascorbic acid and glutamic acid. Only ascorbic acid was considered an interferent, and only while it was in the same concentration range than lactate. Finally, the sensor was applied to real samples of yoghurt and wine, obtaining excellent outputs. However, it should be noticed that reported operational range cannot be considered as a real linear range. An issue of enzyme saturation is likely to be found in this case. Moreover, the error bars found in the calibration measurements obstruct an accurate real value obtention. Nonetheless, the convenience of the device in applications of foodstuffs such as dairy and wine products can be still considered.
In all previous scenarios, the oxidation potential employed to carry out the measurement is high due to the H2O2 oxidation requirements (even with the electrocatalytic effect of Pt or similar electrode materials). Current trends are based in diminishing the potential required to perform the chemical reaction. In this regard, redox mediators have left a relevant footprint in the field of lactate detection, and a wide range of possibilities can be found around this topic. Concerning which feasible redox mediator to employ, it is possible to classify them according to their nature: (i) conducting polymer-based mediators (e.g., poly(aniline)–poly(vinyl sulphonate) [42]), (ii) organic dye-based mediators (e.g., Meldola blue [43], tetrathiafulvalene [44]) and (iii) transition metal compound-based mediators. Within this last group, Prussian blue (PB) stands out as the most extensively applied in amperometric lactate biosensors [45][46]. Remarkably, PB’s electrocatalytic property towards H2O2 oxidation is three orders of magnitude higher than that of regular Pt materials [47]. Thus, it makes the biosensor performance possible by applying a near-zero potential. This is translated in a narrow working potential window where interferences are greatly avoided. Recently, PB has been successfully implemented in devices with a high technological readiness level (TRL). In this sense, Gao et al. presented fully integrated wearable sensor arrays for sweat metabolite monitoring [48]. Glucose, lactate, Na+ and K+ were evaluated in the multianalyte platform. Regarding the lactate sensor, a thicker PB layer was electrodeposited onto a Au electrode. Interestingly, the authors indicated that thicker PB layers contribute to expand the resulting linear response range of the sensor. In addition, LOx was placed between 2 layers composed of chitosan (permeable film) and carbon nanotubes. This combination produced a very extensive linear response range (5–30 mM) able to cover the physiological concentration in sweat, even during intense exercise. Furthermore, a negligible interference of other species (e.g., ascorbic acid, uric acid, etc.) was appreciated in the sensor, according to the authors. Importantly, the multiplatform was successfully applied in real-time physiological conditions in human subjects at different exercise intensities. The results obtained demonstrated the suitability of the developed sensors. Particularly, lactate amperometric sensor supplied coherent values in all cases, but it was noted that lactate concentration also depended on the lactate excretion and the sweat rate. In the same vein, Vinoth et al. reported a fully printed microfluidic wearable device to monitor lactate, pH, Na+ and K+ [49]. In this regard, a multianalyte platform is also presented to assess sweat ions and metabolites. Concerning which lactate amperometric biosensor PB nanoparticles were employed as redox mediator layer, the authors remarked that PB nanoparticles possessed a higher surface volume ratio, which entail improvement in the catalytic and sensing performance. Additionally, single-walled carbon nanotubes were deposited on the surface of the electrode to promote the catalytic effect and increase the surface. Similar to other authors, Vinoth et al. also used chitosan as a permeable layer [49]. However, in this case, chitosan was used as immobilisation matrix for PB nanoparticles as well as for LOx. Furthermore, an additional chitosan layer was placed on top of that to prevent enzyme/mediator leaching. A limit of detection (0.2 mM) and remarkable linear response range (1–25 mM) were obtained. In this case, the potential applied during chronoamperometry was −0.17 V vs. Ag/AgCl pseudoreference electrode. Furthermore, the resulting sensor was also evaluated in microfluidic channels. Sweat samples analysed by this methodology offered coherent values in all cases. Temperature and pH influence were also assessed, revealing an improvement margin by using a temperature and pH correction. It should be stressed that reliable measurements in real scenarios (e.g., factories, home, etc.) may require the assessment of environmental factors, especially temperature. This kind of correction approach steps closer to the final application of the device, and therefore, authors should be praised for that. On the other hand, interferents studied (uric and ascorbic acid and glucose) did not affect the lactate determination. Only a slight drift was noted during successive calibrations. According to the authors, this could be attributed to an active material leaching, an issue that may be solved by using a crosslinking methodology to immobilise the enzyme.
2.2. Potentiometric and Conductometric Lactate Biosensors
Although less common, it is also possible to determine lactate through potential monitoring instead of using an intensity current. Potentiometric sensors measure electrical potential difference between two different electrodes (WE and RE) both embedded in the same media. In this case, WE is usually based on a modified electrode with a certain ion-selective membrane (ISM), generating the so-called indicator electrode or ion-selective electrode (ISE).
ISEs allow people to correlate the activity of a certain species with the potential recorded, according to the Nernst equation. Particularly, this measurement is carried out under near-zero current. In this scenario, the transduction mechanism is based on the charge carriers free to move inside the ISM (target ions) and its conversion to electrons in the interface of the electrode. This process requires assistance of the RE acting as a contact. Classically, a Ag/AgCl wire immersed in a solution with a known amount of Cl− can be used as a liquid contact. Nonetheless, current potentiometry is ruled by all-solid-contact electrodes (e.g., conducting polymers or carbon nanotubes) due to their undeniable advantages such as lower detection limits and miniaturisation possibilities, among others [50]. The first attempt to apply potentiometry to lactate determination was carried out in 1979 by Shinbo et al. The authors immobilised LDH in a gelatine to catalyse the oxidation of lactate by ferricyanide; hence, the variation in the ferricyanide/ferrocyanide concentration ratio could be used to monitor lactate concentration with the resulting potentiometric biosensor [51]. More recently, Lupu et al. developed a more advanced lactate potentiometric sensor, also based on LDH. In this work, plasma-enhanced chemical vapour deposition and colloidal lithography were used to deposit nanostructured polyacrylic acid (PAA) onto the surface of the WE and build a suitable immobilisation environment. It is widely accepted that the immobilisation of an enzyme in a nanostructured material provides several advantages, mainly the possibility of a superior enzyme loading due to the nanomaterial surface increase. In this case, the resulting potentiometric sensor provided acceptable sensitivity (49.7 mV per decade) and limit of detection (2 × 10−7 M). However, the linear range obtained is very limited for real applications without considering diluting factors [52]. The authors pointed out possible applications to the brain environment where the lactate concentration can reach the nanomolar range, but other considerations, such as sampling methodology and possible interference species in that media, among others, must be addressed first. Interestingly, Ibupoto et al. developed a potentiometric sensor based on ZnO nanorods as immobilisation matrix for LOx. Interestingly, ZnO materials expose strong bonding with enzyme/ionophore membranes, improving the efficiency of the catalytic effect as well as enhancing the flow of the testing substance through the sensor; thus, the output signal is meaningfully magnified. This, altogether with the superior surface of ZnO nanorods, would stand as a suitable environment for immobilisation [52]. The resulting potentiometric sensor possessed adequate sensitivity (41.3 mV per decade) and a linear range between 1 × 10−4 and 1 mM of lactate. Despite these average results, the authors reported a superior lifetime (more than 3 weeks) in terms of enzymatic biosensors and good selectivity towards several common interferent species (e.g., ascorbic acid, urea and glucose).
Even less common, but still viable, is the possibility to determine lactate through a field-effect transistor device, where the sensing element—usually a lactate-related enzyme—modifies the gate of the transistor and an outer sensor interrogates the sample. For example, Minamiki et al. took advantage of this methodology to fabricate an organic field-effect transistor for lactate determination [53]. LOx and an osmium redox polymer composite were deposited as sensing membranes on the gate. The authors reported a negligible effect of usual interferences such as MgCl2, CaCl2, NaCl, p-cresol, urea and glucose. In addition, a relatively high linear response range was found (1–10 mM) due to the miniaturisation of the extended gate, according to the authors. Despite the fact that the authors examined interference species (e.g., p-cresol, urea, and glucose) in similar sweat concentrations, no real sample was assayed. Furthermore, it should be noticed that the linear range is not enough to cover lactate concentration during intense exercise (ca. 20 mM). Therefore, the device should be reconsidered in another biological fluid or real sample matrix (e.g., foodstuff), otherwise dilution is mandatory. In the same field, Schuck et al. presented a common-gate field by using a graphene-Nafion material to modify the gate of the transistor [54]. In this case, the sensing element was the LDH enzyme mixed with glutaraldehyde as the crosslinking element and covered by a chitosan layer. According to the authors, this configuration was chosen to maximise the binding stability as well as to hinder possible interferent species. Interestingly, the authors made a comparison with LOx and stated that LDH was more convenient for field-effect transistors, since the hydrogen peroxide production of LOx has no partial charges. The resulting device provided a linear response range of 0.25–10 mM and was also evaluated in human fluids such as human plasma and serum. On the one hand, plasma analysis presented higher sensitivity than serum, according to the authors. On the other hand, an acceptable correlation coefficient (R2 = 0.9787) was obtained, proving the suitability of the device. In addition, uric and ascorbic acid were evaluated as interferents, as well as glucose, due to its potential doping effect over the active layer, according to the authors. In any case, lactate determination was affected by these compounds, corroborating the suitability of the field-effect transistor as an accurate tool for clinics.
2.3. Enzyme Immobilisation Influence and Viable Approaches
The reader may have noticed that there is not a clear and straightforward methodology to immobilise the enzymes on the biosensors. The reality shows that the research community is still exploring the possibilities of this field because a clear improvement still exists in it. In fact, enzyme immobilisation should not be never considered as a meaningless factor. Long-term stability and sensitivity are definitely related with the immobilisation methodology. In addition, enzyme leaching and the decrease in enzyme activity by deactivation can be diminished using the proper approach. In the literature, immobilisation methods can be classified as follows: (i) physical adsorption, (ii) physical entrapment, (iii) covalent binding and (iv) crosslinking-based methods [34].
Each one of them possesses intrinsic pros and cons. For example, physical adsorption of the enzyme (Figure 2a) is the simplest methodology. It can be carried out by using the drop-casting method to dry a drop of enzymatic mixture or employing the dip-coating approach to put the enzyme and the electrode substrate (modified or not) in contact for a certain period of time. Only van der Waals forces are involved in the attachment of the enzyme. On the bright side, enzymes are not altered, so their enzymatic activity is maintained. However, it is possible to deduce the evident flaw of this approach: the retaining capacity. Interaction between the enzyme and the solution results in a slow but relentless dissociation of the enzyme from the modified electrode, leading to a decrease in the enzymatic activity, and hence to the sensitivity of the biosensor. Thus, physical adsorption is not frequently currently employed [55][56]. A viable option is to improve the retainment consist of the entrapment of the enzyme using a physical barrier (Figure 2b). Enzymes can be immobilised in a polymer or sol–gel placed on top of the substrate. Controlled polymerisation can be achieved by using electropolymerisation or photo-binding, among others. Contrarily to physical adsorption, the enzyme is perfectly confined in the matrix, which provides minimum leaching as well as excellent additional features such as mechanical, thermal and chemical stability. Nevertheless, the drawback resides here on how the membrane affects the diffusion of the analyte through the biosensor, resulting in a decrease in the sensitivity. Recently, Lee et al. employed this methodology to immobilise LOx in a polypyrrole/polydopamine polymer matrix, entrapping the enzyme within it [38]. It is also possible to enhance the retainment of the enzyme via chemical bonding. Covalent binding (Figure 2c,d) methodology consists of the creation of covalent bonding between the functional group and the substrate. Usually, amino acid residues are used for this purpose, and some reverse inhibitors can be employed as well in order to protect the enzyme active centre. In theory, this bond can be made in any substrate prior to its modification with functional groups [57]. The resulting enzyme–substrate union can be oriented in a specific manner (Figure 2c) or randomly (Figure 2d), but in both scenarios, a stable monolayer is formed. As an illustrative example, Lupu et al. described the covalent binding between LDH and a polyacrylic acid layer [52]. This layer was previously activated by using N-hydroxysuccinimide and 1-ethyl-(3-dimetylaminopropyl)carbodiimide to generate (COOH-) free spots where the covalent union can be performed. Nonetheless, the sensitivity of the sensor will depend on the enzyme loading, which in this case is related with the available surface to bind the enzyme. Enhancement of the surface is pursued in order to increase the number of enzymes bonded. The main option relies on the modification of the surface with nanomaterials, especially carbon nanomaterials (e.g., carbon nanotubes, graphene, etc). Otherwise, the increase in enzyme loading can be performed by constructing a three-dimensional grid using a bifunctional agent, which agglomerates a great number of enzymes. This process is known as the crosslinking method (Figure 2e). Due to the intense binding, an enhanced stability and higher enzyme loading are obtained. The resulting 3D grid depends on the bifunctional group (e.g., glutaraldehyde) and the initial protein used. Chan et al. described a crosslinking methodology using a combination of bovine serum albumin (BSA) and glutaraldehyde (GA) to immobilise simultaneously lactate dehydrogenase and pyruvate oxidase onto a carbon electrode [2]. Importantly, the sensitivity of the biosensor based on crosslinking may not be as high as expected. Even though the number of enzymes is higher, it should be noticed that the multiple unions of the enzyme with the bifunctional group can lead to an alteration of its structure, decreasing the enzymatic activity.
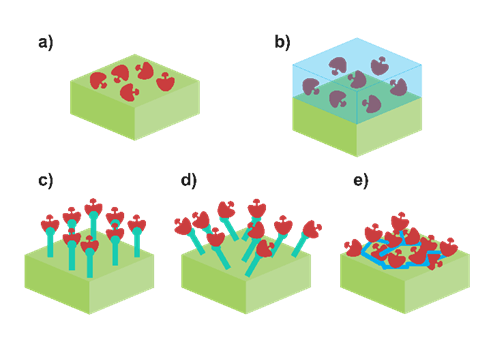
Figure 2. Scheme of different enzyme immobilisation approaches: (a) adsorption, (b) physical entrapment, (c) oriented covalent binding, (d) random covalent binding and (e) crosslinking methods.
2.4. Non-Enzymatic Sensors
The main reason to use enzymes in lactate biosensors is to provide a high specificity towards this species. However, as the core of the lactate biosensor, the biological recognition element possesses a critical role in its performing. Thus, the above-mentioned possibilities should be carefully assessed in order to obtain the most robust and sensitive device in each scenario. Moreover, enzymes will be always affected by other parameters such as pH and temperature, which may not be adequate in other applications. It is true that for medical applications, pH and temperature remain in physiological conditions, ensuring enzymatic stability. Nevertheless, this is not so convenient in sport medicine, where sweat pH can change dramatically; and is much more problematic in the agrifood field, which possesses a wider range of pH and temperature. This influence can be decreased by using membranes to make contact between the enzyme and the surroundings more difficult [58]. On the other hand, other authors have decided to explore an alternative path and have tried to propose a lactate sensor without this biological recognition element in order to avoid these dependences. The resulting devices are the so-called non-enzymatic sensors, sensors which employ other materials to provide selectivity to the system. In general terms, three different currents can be appreciated based on the materials used: (i) nanomaterials, (ii) metal–organic frameworks (MOFs) and (iii) molecularly imprinted polymer (MIP)-based sensors (Figure 3).
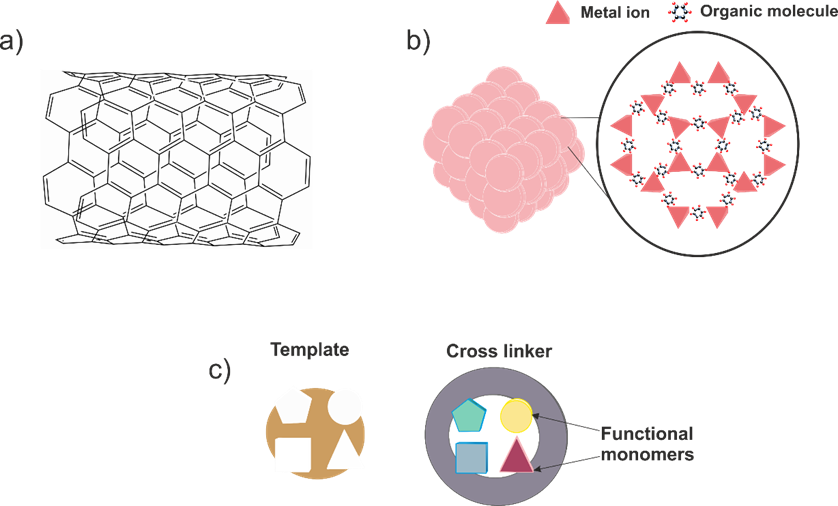
Figure 3. Main alternate approaches to enzyme-based lactate sensors: (a) nanomaterials, (b) metal–organic frameworks (MOFs) and (c) molecularly imprinted polymers (MIPs).
In the first place, specific features of nanomaterials are exploited to promote lactate oxidation mechanism over other species. For example, Change et al. proposed a non-enzymatic lactate sensor by using cobalt oxide nanostructures [59]. The authors indicated that the material possessed Co2+ and Co3+ centres while the material was embedded in solution. The presence of both centres oxidised lactic acid into pyruvic acid in a selective manner. They also pointed out that the electrochemical signal was directly related with the morphology of the catalyst. Concerning to the results obtained, a linear response range from 0.01 to 3 mM was reported, as well as a limit of detection of 6 µM. Regarding the influence of interferents, several species at relatively high concentrations were evaluated during lactate calibrations, such as citric acid, glucose, maleic acid, tartaric acid and urea. According to the authors, no significant interferences were detected. In the same vein, Kim et al. presented a porous nanostructured nickel oxide-based sensor to promote selective lactate oxidation [60]. In this case, the effect of the calcination temperature and the subsequent nanostructure generated was also studied. In this regard, a temperature of 250 °C was chosen as the optimum value to obtain the selective nanomaterials to integrate into the sensor. The device provided sensitivity of 62.35 μA mM−1 cm−2, a limit of detection of 27 μM and a linear response range of 0.01–7.75 mM. Interestingly, the calibration was carried out at a potential of +0.55 V. However, no influence of other interferent species (ascorbic acid, uric acid and dopamine) was noted. It is also possible to find in the literature examples of composite nanomaterials used to design the sensor architecture. Hussain et al. proposed a nanocomposite based on CuO and multiwalled carbon nanotubes with a Nafion layer [61]. The authors proposed a lactate oxidation mechanism similar to the one found in LOx. Briefly, lactate was oxidised to pyruvate in the presence of oxygen, and also produced hydrogen peroxide. This hydrogen peroxide is decomposed into oxygen in a two-step mechanism where two electrons are released. Hence, the current recorded is attributable to this electron flow. A relatively extensive linear range response (100 pM–10 mM) and an excellent limit of detection of 88.5 pM were reported. Moreover, lactic acid determination was satisfactorily achieved in the presence of other biological molecules such as cholesterol, dopamine or testosterone, among others. In contrast to other works, Hussain et al. used a current voltage monitoring instead of a classic amperometry. As can be appreciated, non-enzymatic lactate sensors based on nanomaterials can be considered as interesting and innovative alternatives in lactate sensing. Nonetheless, if the reader carefully examines the works in this pipeline, they could notice an important fact. In the majority of these works, lactate sensors should work in an alkaline media to promote the selective lactate oxidation, which is usually guaranteed due to a concentrated NaOH media. Therefore, there is still a pH sensor dependence similar to the one found in enzymatic sensors, which must not be overlooked.
Concerning the lactate sensors based on MOFs, these structures are also located in the nanomaterial kingdom, and they can be understood as a crystalline material built through the coordination of metal ions/clusters with organic linkers [62]. The trained reader may assume that there is an unusual freedom to construct the desired MOF by using different building blocks. In fact, this is the core of the application of these materials in sensing, their tuneable properties. Regarding their application in lactate determination, Wang et al. reported a 2D-oriented MOF of Cu3(btc) nanocubes modified with freestanding amino-functionalised graphene nanocomposite to prepare a lactate and glucose sensor [63]. According to the authors, the excellent features of the resulting sensor allowed for the simultaneous detection of both analytes by using cyclic voltammetry. Contrarily, for the determination of each species, a specific potential was selected for the amperometry assays, namely −0.10 and 0.65 V for lactate and glucose, respectively. A wide list of interferent species either ionic or organic molecules was evaluated, but no concerning effects were reported. For lactate sensing, a linear dynamic range of 0.05–22.6 mM and a limit of detection of 5 μM were found. In addition, enhanced lifetime stability of 50 days was also indicated. Finally, the sensor was applied to real sweat samples, obtaining acceptable outputs. Interestingly, the sensor also possessed excellent performance under flexibility tests, proving its feasible future applications in the wearable sensing field. However, it is noteworthy to mention that the application of this device to another field, such as clinical or agrifood environments, should involve a reassessment of the interferents. The reported studies employed a concentration of organic species of 1 μM, which is a relatively low concentration value for a real sample matrix in both ambits. The consequent results will state if this application is feasible or not.
The last alternative approach to avoid enzymes is based on MIPs. In brief, these materials are produced via polymerisation by using the target analyte as a template for the resulting polymer; later, the template is removed, leaving cavities with the exact shape to rebind a target analyte molecule, in a similar manner to how enzymes work (lock-and-key mechanism) [64][65]. In addition to the evident selectivity, molecularly imprinted polymers also bestow the sensor with high reliability and either mechanical or chemical stability. Despite these advantages, there are not many studies related with sensors based on MIPs for lactate-sensing applications. In this sense, the work developed by Pereira et al. should be praised. In this case, electropolymerisation was used to fabricate the MIP, granting better control during the polymerisation and better adhesion to the electrode material [66]. Prior to the polymerisation, the electrode material was modified with reduced graphene oxide and gold nanoparticles to improve the electrochemical performance of the sensor. Before the application of the developed sensor, an electrochemical and a morphological characterisation were performed. The electrochemical characterisation comprised classical studies in cyclic voltammetry as well as electrochemical impedance spectroscopy assays, obtaining in both cases the ideal behaviour for an electrochemical sensor. Moreover, the results obtained by atomic force microscopy revealed a roughness increase in the material when the lactate was inside the structure, but it was later demonstrated that this variation was reversible. Concerning the analytical performance of the sensor, differential pulse voltammetry was employed to determine lactate. A split linear range was reported; the first one comprised lactate concentration between 1 × 10−10 and 1 × 10−9 M, and the second one from 2 × 10−9 and 1.5 × 10−8 M. Unfortunately, no clear explanation for this fact was supplied by the authors. On the other hand, a useful rebinding study was provided, exposing the suitable stability of the sensor. Furthermore, the selectivity of the sensor was also evaluated by using diverse organic molecules (lactic acid, acetic acid and glucose, among others), but no relevant response was obtained towards these molecules comparing the molecularly imprinted polymer with a nonimprinted one. Finally, the resulting device was applied to enriched sugarcane vinasse samples. In all scenarios, near 100% recoveries were obtained, revealing the good accuracy of the sensor. Nonetheless, the “dual” and low linear response range and limit of detection (8.9 × 10−11 M) values obtained limit the application of the sensor to real samples without considering dilution factors. It is true that the outputs of selectivity are very promising, but they are still far from being considered a strong alternative to classic enzyme-based sensors.
References
- Brooks, G.A.; Arevalo, J.A.; Osmond, A.D.; Leija, R.G.; Curl, C.C.; Tovar, A.P. Lactate in contemporary biology: A phoenix risen. J. Physiol. 2021, 10, 1229–1251.
- Chan, D.; Barsan, M.M.; Korpan, Y.; Brett, C.M.A. L-lactate selective impedimetric bienzymatic biosensor based on lactate dehydrogenase and pyruvate oxidase. Electrochim. Acta 2017, 231, 209–215.
- Lamas-Ardisana, P.J.; Loaiza, O.A.; Añorga, L.; Jubete, E.; Borghei, M.; Ruiz, V.; Ochoteco, E.; Cabañero, G.; Grande, H.J. Disposable amperometric biosensor based on lactate oxidase immobilised on platinum nanoparticle-decorated carbon nanofiber and poly(diallyldimethylammonium chloride) films. Biosens. Bioelectron. 2014, 56, 345–351.
- Tsafrakidou, P.; Sameli, N.; Bosnea, L.; Chorianopoulos, N.; Samelis, J. Assessment of the spoilage microbiota in minced free-range chicken meat during storage at 4 C in retail modified atmosphere packages. Food Microbiol. 2021, 99, 103822.
- Rassaei, L.; Olthuis, W.; Tsujimura, S.; Sudhölter, E.J.R.; Van Den Berg, A. Lactate biosensors: Current status and outlook. Anal. Bioanal. Chem. 2014, 406, 123–137.
- Amin, S.; Tahira, A.; Solangi, A.; Mazzaro, R.; Ibupoto, Z.H.; Vomiero, A. A sensitive enzyme-free lactic acid sensor based on NiO nanoparticles for practical applications. Anal. Methods 2019, 11, 3578–3583.
- Fuernau, G. Lactate and other biomarkers as treatment target in cardiogenic shock. Curr. Opin. Crit. Care 2019, 25, 403–409.
- Seheult, J.; Fitzpatrick, G.; Boran, G. Lactic acidosis: An update. Clin. Chem. Lab. Med. 2017, 55, 322–333.
- Levitt, D.G.; Levitt, J.E.; Levitt, M.D. Quantitative Assessment of Blood Lactate in Shock: Measure of Hypoxia or Beneficial Energy Source. BioMed Res. Int. 2020, 2020, 2608318.
- Ebner, M.; Pagel, C.F.; Sentler, C.; Harjola, V.; Lerchbaumer, M.H.; Stangl, K.; Pieske, B.; Hasenfu, G.; Konstantinides, S.V.; Lankeit, M. Venous lactate improves the prediction of in-hospital adverse outcomes in normotensive pulmonary embolism. Eur. J. Intern. Med. 2021, 86, 25–31.
- Sauer, C.M.; Gómez, J.; Botella, M.R.; Ziehr, D.R.; Oldham, W.M.; Gavidia, G.; Rodríguez, A.; Elbers, P.; Girbes, A.; Bodi, M.; et al. Understanding critically ill sepsis patients with normal serum lactate levels: Results from U.S. and European ICU cohorts. Sci. Rep. 2021, 11, 20076.
- Nasu, T.; Ueda, K.; Kawashima, S.; Okishio, Y.; Kunitatsu, K. Prediction of early acute kidney injury after trauma using prehospital systolic blood pressure and lactate levels: A prospective validation study. Injury 2022, 53, 81–85.
- Sugimoto, M.; Takayama, W.; Murata, K.; Otomo, Y. The impact of lactate clearance on outcomes according to infection sites in patients with sepsis: A retrospective observational study. Sci. Rep. 2021, 11, 22394.
- García-Cañaveras, J.C.; Lahoz, A. Tumor Microenvironment-Derived Metabolites: A Guide to Find New Metabolic Therapeutic Targets and Biomarkers. Cancers 2021, 13, 3230.
- Powell, C.L.; Davidson, A.R.; Brown, A.M. Universal Glia to Neurone Lactate Transfer in the Nervous System: Physiological Functions and Pathological Consequences. Biosensors 2020, 10, 183.
- Naylor, E.; Aillon, D.V.; Barrett, B.S.; Wilson, G.S.; Johnson, D.A.; Johnson, D.A.; Harmon, H.P.; Gabbert, S.; Petillo, P.A. Lactate as a biomarker for sleep. Sleep 2012, 35, 1209–1222.
- Rathee, K.; Dhull, V.; Dhull, R.; Singh, S. Biosensors based on electrochemical lactate detection: A comprehensive review. Biochem. Biophys. Rep. 2016, 5, 35–54.
- Hashemzadeh, S.; Omidi, Y.; Rafii-Tabar, H.; Cummins, G.; Kremer, J.; Bernassau, A.; Brown, A.; Bridle, H.L.; Schulze, H.; Bachmann, T.T.; et al. Sensors for fetal hypoxia and metabolic acidosis: A review. Sensors 2018, 186, 2648.
- Hernández-Ibáñez, N.; García-Cruz, L.; Montiel, V.; Foster, C.W.; Banks, C.E.; Iniesta, J. Electrochemical lactate biosensor based upon chitosan/carbon nanotubes modified screen-printed graphite electrodes for the determination of lactate in embryonic cell cultures. Biosens. Bioelectron. 2016, 77, 1168–1174.
- Barker, S.B.; Summerson, W.H. the Colorimetric Determination of Lactic Acid in Biological Material. J. Biol. Chem. 1941, 138, 535–554.
- Alhusban, A.A.; Albustanji, S.; Hamadneh, L.A.; Shallan, A.I. High performance liquid chromatography–tandem mass spectrometry method for correlating the metabolic changes of lactate, pyruvate and L-Glutamine with induced tamoxifen resistant MCF-7 cell line potential molecular changes. Molecules 2021, 26, 4824.
- Briones, M.; Busó-Rogero, C.; Catalán-Gómez, S.; García-Mendiola, T.; Pariente, F.; Redondo-Cubero, A.; Lorenzo, M.E. ZnO nanowire-based fluorometric enzymatic assays for lactate and cholesterol. Microchim. Acta 2020, 187, 180.
- Roda, A.; Guardigli, M.; Calabria, D.; Calabretta, M.; Cevenini, L.; Michelini, E. A 3D-printed device for a smartphone-based chemiluminescence biosensor for lactate in oral fluid and sweat. Analyst 2014, 139, 6494–6501.
- Goh, J.H.; Mason, A.; Al-Shamma’a, A.I.; Field, M.; Shackcloth, M.; Browning, P. Non invasive microwave sensor for the detection of lactic acid in cerebrospinal fluid (CSF). J. Phys. Conf. Ser. 2011, 307, 012017.
- Sartain, F.K.; Yang, X.; Lowe, C.R. Holographic lactate sensor. Anal. Chem. 2006, 78, 5664–5670.
- Hvinden, I.C.; Berg, H.E.; Sachse, D.; Skaga, E.; Skottvoll, F.S.; Lundanes, E.; Sandberg, C.J.; Vik-mo, E.O.; Rise, F.; Wilson, S.R. Nuclear Magnetic Resonance Spectroscopy to Identify Metabolite Biomarkers of Nonresponsiveness to Targeted Therapy in Glioblastoma Tumor Stem Cells. J. Proteome Res. 2020, 18, 2012–2020.
- Gajovic, N.; Binyamin, G.; Warsinke, A.; Scheller, F.W.; Heller, A. Operation of a miniature redox hydrogel-based pyruvate sensor in undiluted deoxygenated calf serum. Anal. Chem. 2000, 72, 2963–2968.
- Borshchevskaya, L.N.; Gordeeva, T.L.; Kalinina, A.N.; Sineokii, S.P. Spectrophotometric determination of lactic acid. J. Anal. Chem. 2016, 71, 755–758.
- Pinheiro, K.M.P.; Duarte, L.M.; Duarte-Junior, G.F.; Coltro, W.K.T. Chip-based separation of organic and inorganic anions and multivariate analysis of wines according to grape varieties. Talanta 2021, 231, 122381.
- Teipel, J.C.; Hausler, T.; Sommerfeld, K.; Scharinger, A.; Walch, S.G.; Lachenmeier, D.W.; Kuballa, T. Application of 1H nuclear magnetic resonance spectroscopy as spirit drinks screener for quality and authenticity control. Foods 2020, 9, 1355.
- Martínez-Periñán, E.; Gutiérrez-Sánchez, C.; García-Mendiola, T.; Lorenzo, E. Electrochemiluminescence Biosensors Using Screen-Printed Electrodes. Biosensors 2020, 10, 118.
- Milagres, M.P.; Brandão, S.C.C.; Magalhães, M.A.; Minim, V.P.R.; Minim, L.A. Development and validation of the high performance liquid chromatography-ion exclusion method for detection of lactic acid in milk. Food Chem. 2012, 135, 1078–1082.
- Hird, S.J.; Lau, B.P.Y.; Schuhmacher, R.; Krska, R. Liquid chromatography-mass spectrometry for the determination of chemical contaminants in food. TrAC Trends Anal. Chem. 2014, 59, 59–72.
- Kucherenko, I.S.; Topolnikova, Y.V.; Soldatkin, O.O. Advances in the biosensors for lactate and pyruvate detection for medical applications: A review. TrAC Trends Anal. Chem. 2019, 110, 160–172.
- Zaryanov, N.V.; Nikitina, V.N.; Karpova, E.V.; Karyakina, E.E.; Karyakin, A.A. Nonenzymatic sensor for lactate detection in human sweat. Anal. Chem. 2017, 89, 11198–11202.
- Nikolaus, N.; Strehlitz, B. Amperometric lactate biosensors and their application in (sports) medicine, for life quality and wellbeing. Microchim. Acta 2008, 160, 15–55.
- Burmeister, J.J.; Palmer, M.; Gerhardt, G.A. L-lactate measures in brain tissue with ceramic-based multisite microelectrodes. Biosens. Bioelectron. 2005, 20, 1772–1779.
- Lee, M.; Kim, S.; Jang, M.; Park, H.S.; Lee, J.Y. One-Pot electrochemical fabrication of high performance amperometric enzymatic biosensors using polypyrrole and polydopamine. J. Ind. Eng. Chem. 2021, 97, 316–325.
- Booth, M.A.; Gowers, S.A.N.; Hersey, M.; Samper, I.C.; Park, S.; Anikeeva, P.; Hashemi, P.; Stevens, M.M.; Boutelle, M.G. Fiber-Based Electrochemical Biosensors for Monitoring pH and Transient Neurometabolic Lactate. Anal. Chem. 2021, 93, 6646–6655.
- Istrate, O.M.; Rotariu, L.; Bala, C. Amperometric L-Lactate biosensor based upon a gold nanoparticles/reduced graphene oxide/polyallylamine hydrochloride modified screen-printed graphite electrode. Chemosensors 2021, 9, 74.
- Hashemzadeh, S.; Omidi, Y.; Rafii-Tabar, H. Amperometric lactate nanobiosensor based on reduced graphene oxide, carbon nanotube and gold nanoparticle nanocomposite. Microchim. Acta 2019, 186, 680.
- Halliwell, C.M.; Simon, E.; Toh, C.S.; Bartlett, P.N.; Cass, A.E.G. Immobilisation of lactate dehydrogenase on poly(aniline)-poly(acrylate) and poly(aniline)-poly-(vinyl sulphonate) films for use in a lactate biosensor. Anal. Chim. Acta 2002, 453, 191–200.
- Pereira, A.C.; Kisner, A.; Tarley, C.R.T.; Kubota, L.T. Development of a carbon paste electrode for lactate detection based on Meldola’s Blue adsorbed on silica gel modified with niobium oxide and lactate oxidase. Electroanalysis 2011, 23, 1470–1477.
- Payne, M.E.; Zamarayeva, A.; Pister, V.I.; Yamamoto, N.A.D. Printed, flexible lactate sensors: Design considerations before performing on-body measurements. Sci. Rep. 2019, 9, 13720.
- Lin, Y.; Liu, K.; Yu, P.; Xiang, L.; Li, X.; Mao, L. A facile electrochemical method for simultaneous and on-line measurements of glucose and lactate in brain microdialysate with prussian blue as the electrocatalyst for reduction of hydrogen peroxide. Anal. Chem. 2007, 79, 9577–9583.
- Wang, L.; Tricard, S.; Yue, P.; Zhao, J.; Fang, J.; Shen, W. Polypyrrole and graphene quantum dots @Prussian Blue hybrid film on graphite felt electrodes: Application for amperometric determination of L-cysteine. Biosens. Bioelectron. 2016, 77, 1112–1118.
- Karyakin, A.A. Advances of Prussian blue and its analogues in (bio) sensors. Curr. Opin. Electrochem. 2017, 5, 92–98.
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514.
- Vinoth, R.; Nakagawa, T.; Mathiyarasu, J.; Mohan, A.M.V. Fully printed wearable microfluidic devices for high-throughput sweat sampling and multiplexed electrochemical analysis. ACS Sens. 2021, 6, 1174–1186.
- Bakker, E.; Pretsch, E. Modern potentiometry. Angew. Chem. Int. Ed. 2007, 46, 5660–5668.
- Sugiura, M.; Kamo, N.; Shinbo, T. Potentiometric enzyme electrode for lactate. Anal. Chem. 1979, 51, 100–104.
- Lupu, A.; Valsesia, A.; Bretagnol, F.; Colpo, P.; Rossi, F. Development of a potentiometric biosensor based on nanostructured surface for lactate determination. Sens. Actuators B Chem. 2007, 127, 606–612.
- Minamiki, T.; Tokito, S.; Minami, T. Fabrication of a flexible biosensor based on an organic field-effect transistor for lactate detection. Anal. Sci. 2019, 35, 103–106.
- Schuck, A.; Kim, H.E.; Moreira, J.K.; Lora, P.S.; Kim, Y.S. A graphene-based enzymatic biosensor using a common-gate field-effect transistor for l-lactic acid detection in blood plasma samples. Sensors 2021, 21, 1852.
- Shkotova, L.V.; Goriushkina, T.B.; Tran-Minh, C.; Chovelon, J.M.; Soldatkin, A.P.; Dzyadevych, S.V. Amperometric biosensor for lactate analysis in wine and must during fermentation. Mater. Sci. Eng. C 2008, 28, 943–948.
- Parra, A.; Casero, E.; Vázquez, L.; Pariente, F.; Lorenzo, E. Design and characterization of a lactate biosensor based on immobilized lactate oxidase onto gold surfaces. Anal. Chim. Acta 2006, 555, 308–315.
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205.
- Chen, C.; Cuartero, M.; Crespo, G.A.; Xuan, X.; Clara, P. Lactate biosensing for reliable on-body sweat analysis. ACS Sens. 2021, 6, 2763–2771.
- Chang, A.S.; Memon, N.N.; Amin, S.; Chang, F.; Aftab, U.; Abro, M.I.; Dad Chandio, A.; Shah, A.A.; Ibupoto, M.H.; Ansari, M.A.; et al. Facile non-enzymatic lactic acid sensor based on cobalt oxide nanostructures. Electroanalysis 2019, 31, 1296–1303.
- Kim, S.; Yang, W.S.; Kim, H.J.; Lee, H.N.; Park, T.J.; Seo, S.J.; Park, Y.M. Highly sensitive non-enzymatic lactate biosensor driven by porous nanostructured nickel oxide. Ceram. Int. 2019, 45, 23370–23376.
- Hussain, M.M.; Hussain, M.M.; Hussain, M.M.; Asiri, A.M.; Asiri, A.M.; Rahman, M.M.; Rahman, M.M.; Hussain, M.M. A non-enzymatic electrochemical approach for l-lactic acid sensor development based on CuO·MWCNT nanocomposites modified with a Nafion matrix. New J. Chem. 2020, 44, 9775–9787.
- Adil, K.; Belmabkhout, Y.; Pillai, R.S.; Cadiau, A.; Bhatt, P.M.; Assen, A.H.; Maurin, G.; Eddaoudi, M. Gas/vapour separation using ultra-microporous metal-organic frameworks: Insights into the structure/separation relationship. Chem. Soc. Rev. 2017, 46, 3402–3430.
- Wang, Z.; Gui, M.; Asif, M.; Yu, Y.; Dong, S.; Wang, H.; Wang, W.; Wang, F.; Xiao, F.; Liu, H. A facile modular approach to the 2D oriented assembly MOF electrode for non-enzymatic sweat biosensors. Nanoscale 2018, 10, 6629–6638.
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137.
- Lamaoui, A.; Palacios-Santander, J.M.; Amine, A.; Cubillana-Aguilera, L. Molecularly imprinted polymers based on polydopamine: Assessment of non-specific adsorption. Microchem. J. 2021, 164, 106043.
- Pereira, T.C.; Stradiotto, N.R. Electrochemical sensing of lactate by using an electrode modified with molecularly imprinted polymers, reduced graphene oxide and gold nanoparticles. Microchim. Acta 2019, 186, 764.
More
Information
Subjects:
Health Care Sciences & Services
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
22 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




