Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sherif T.S. Hassan | -- | 3226 | 2022-11-20 17:50:39 | | | |
| 2 | Dean Liu | -3 word(s) | 3223 | 2022-11-21 03:38:50 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Hassan, S.T.S.; Šudomová, M.; Mazurakova, A.; Kubatka, P. Non-Flavonoid Polyphenols against Human Herpesviruses. Encyclopedia. Available online: https://encyclopedia.pub/entry/35356 (accessed on 07 February 2026).
Hassan STS, Šudomová M, Mazurakova A, Kubatka P. Non-Flavonoid Polyphenols against Human Herpesviruses. Encyclopedia. Available at: https://encyclopedia.pub/entry/35356. Accessed February 07, 2026.
Hassan, Sherif T. S., Miroslava Šudomová, Alena Mazurakova, Peter Kubatka. "Non-Flavonoid Polyphenols against Human Herpesviruses" Encyclopedia, https://encyclopedia.pub/entry/35356 (accessed February 07, 2026).
Hassan, S.T.S., Šudomová, M., Mazurakova, A., & Kubatka, P. (2022, November 20). Non-Flavonoid Polyphenols against Human Herpesviruses. In Encyclopedia. https://encyclopedia.pub/entry/35356
Hassan, Sherif T. S., et al. "Non-Flavonoid Polyphenols against Human Herpesviruses." Encyclopedia. Web. 20 November, 2022.
Copy Citation
Herpesviruses are one of the most contagious DNA viruses that threaten human health, causing severe diseases, including, but not limited to, certain types of cancer and neurological complications. The overuse and misuse of anti-herpesvirus drugs are key factors leading to drug resistance.
polyphenols
antiviral activity
herpes simplex virus
HSV-1
HSV-2
human cytomegalovirus (HCMV)
varicella-zoster virus (VZV)
1. Introduction
Human herpesviruses (HHVs) are infectious DNA viruses that belong to the family of Herpesviridae with the capacity to establish lifelong latent infections, which undergo periodic reactivation [1]. These viruses induce a broad spectrum of ailments, ranging from frequent cold sores to cancer, and remain a major cause of morbidity and mortality, particularly in immunocompromised patients [2]. The life cycle of HHVs comprises two critical phases: lytic infection and latent infection. During lytic infection, the virus replicates and produces several progeny virions, and then travels to the latent site (in this stage, the virus is inactive) to form latent infection [3][4]. Subsequently, the virus reactivates once the host’s immune system is weakened by diverse physiological and environmental factors that adversely affect the immune system. The reactivation process depends on the nature of the latently infected cell [5][6]. HHV infections are usually asymptomatic, and the symptoms occur once the immune system is compromised [7]. An overview of the herpesvirus life cycle is shown in Figure 1.
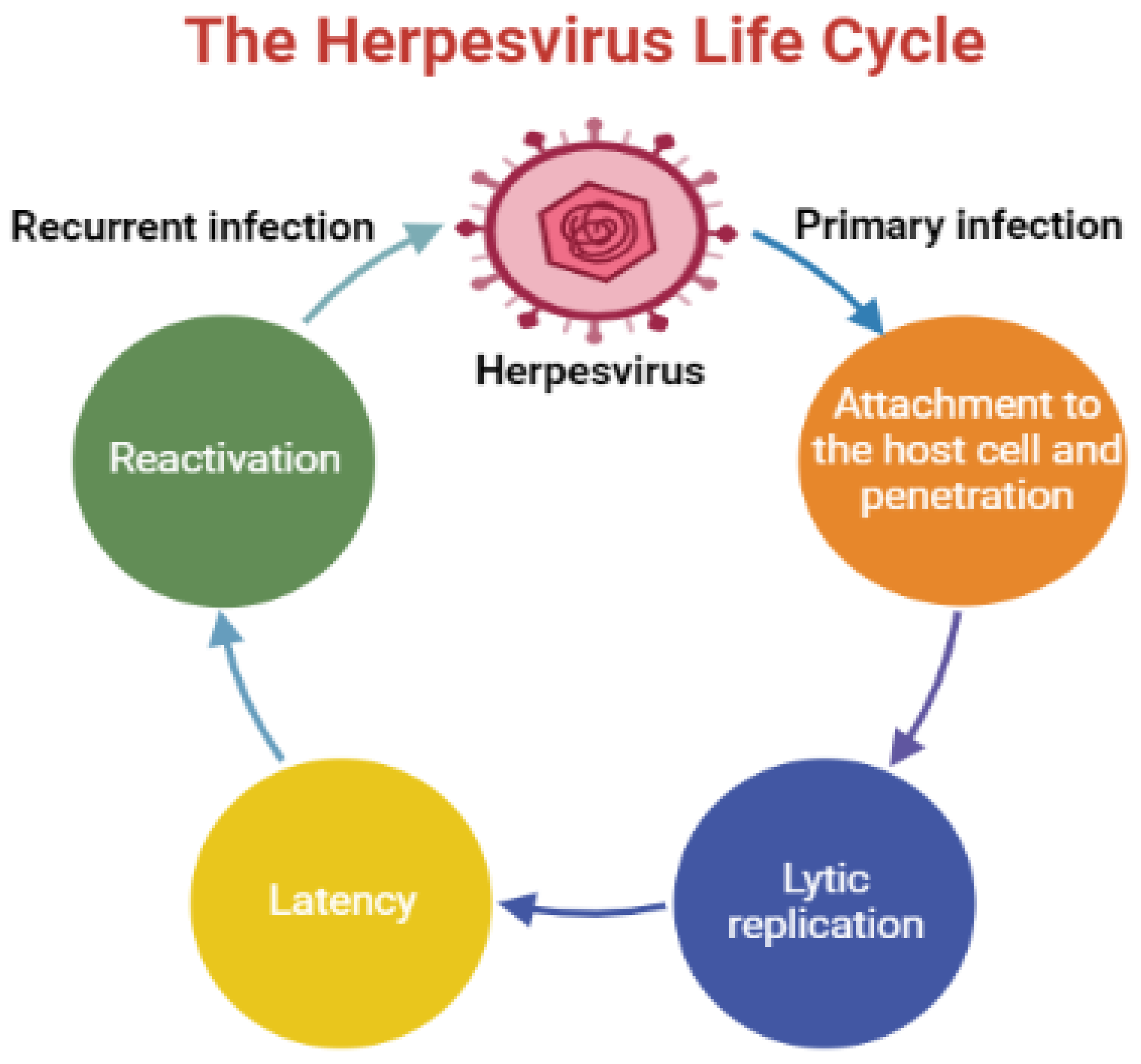
Figure 1. An illustration displays an overview of the herpesvirus life cycle.
The current effective medical treatment of HHVs is based on acyclovir and other related antiviral medications that target viral DNA polymerases. The overuse of these drugs has led to the developing of the problem of drug resistance, leading to unsuccessful treatment efficacy [8][9]. Moreover, acyclovir and related nucleoside or nucleotide analogs do not cure herpes infections but reduce the severity and frequency of symptoms [10]. Therefore, the search for new sources such as natural products that provide effective drugs with the ability to inhibit the herpesvirus at diverse stages of the life cycle with no toxicity and resistance is urgently needed [11][12].
2. An Overview of Polyphenols and Their Health Benefits as Antivirals
Polyphenols are a superfamily of a large group of phytochemicals that naturally occur in plants with different structures and properties. Their primary function is to protect plants from pathogenic infections, UV radiation damage, herbivores, and promote the development process [13][14]. Protection against other abiotic stresses, including salinity, drought, toxic metals, and extremely low or high temperatures, has been reported as a significant role of polyphenols in plants [15][16]. Polyphenols are present in many commonly consumed vegetables, fruits, herbs, and grains and their multiple therapeutic activities make them important factors contributing to maintaining the health of the organism [17][18]. The amount of polyphenols in plants varies and depends on several factors, including the plant maturation state, growing, and storage conditions, and the extraction process [19][20]. Information regarding polyphenols classification and their chemical structures has been well-documented in a recently published review article by Durazzo et al. [15]. Readers who are interested in this information can refer to the recommended reference.
In various preclinical and clinical studies, polyphenols have proven therapeutic efficacy for many diseases including viral infections [18]. It has been shown that polyphenols, including their subclass flavonoids, inhibit the replication of diverse human DNA and RNA viruses by various mechanisms of action at different molecular levels [21][22].
3. Antiviral Properties of Non-Flavonoid Polyphenols against Alpha-Herpesviruses
Human alpha-herpesviruses are a group of infectious DNA viruses that includes important human pathogens such as HSV-1, HSV-2, and VZV. HSV-1 is the causative agent of herpes labialis (the symptoms are recognized as cold sores) with the capacity to generate genital herpes (a sexually transmitted infection), while HSV-2 induces primarily genital herpes characterized by the presence of ulcerative or vesicular lesions [23][24]. VZV causes both varicella (chickenpox) by initial infection and herpes zoster (shingles) by reactivation from latency [25][26]. Human alpha-herpesviruses are transmitted in different ways and direct contact with an infected individual is the most common mode. Their infections are usually asymptomatic, and slight symptoms might appear that go unnoticed in some cases [27][28].
Researchers critically discuss the recent experiments that feature the anti-herpesvirus properties of polyphenols (excluding flavonoids) against HSV-1, HSV-2, and VZV. The molecular mechanisms and effective concentrations or doses are also highlighted.
3.1. Phenolic Acids
In an in vitro assay, ginkgolic acid, a phenolic compound detected in the leaves and fruits of Ginkgo biloba, was observed to inhibit the replication of HSV-1 in infected HEp-2 and 293T cells at various concentrations ranging from 2.5 to 50 µM. The results showed that ginkgolic acid has blocked HSV-1 infection by inhibiting viral protein synthesis such as immediate early (ICP27), early (ICP8), and late (US11) proteins. Moreover, ginkgolic acid has effectively repressed viral progeny production [29]. In another work, an extract of Ginkgo biloba containing ginkgolic acid (<5 ppm) possessed anti-HSV-1 action before viral adsorption to the cell surface, interrupted the viral structure, and impeded HSV-1 virion entry. The anti-HSV-1 action was proposed to be linked to the presence of ginkgolic acid [30]. An additional study has also explored the antiviral activity of ginkgolic acid against HSV-1 skin infection in BALB/cJ mice. At an effective dose of 10 mM prepared in 2.5% hydroxyethyl cellulose gel (administered twice daily for 14 days), ginkgolic acid was found to significantly decrease mortality, infection score, and durations of HSV-1 cutaneous infection in a zosteriform model in BALB/cJ mice. It also lessened the replication of an acyclovir-resistant strain of HSV-1 in Vero cells at a concentration of 10 µM [31].
Protocatechuic acid from Hibiscus sabdariffa L. was identified to deactivate the replication of HSV-2 DNA in Vero cells with a 50% effective concentration (EC50) value of 0.92 µg/mL. Inhibition of the replication of HSV-2 DNA was mentioned as a mechanism of action [12].
In a molecular docking study, Todorova and colleagues [32] showed that trans-ferulic acid, gentisic acid, vanillic acid, syringic acid, and gallic acid extracted from the phenolic fraction of Graptopetalum paraguayense E. Walther might inhibit HSV-1 DNA polymerase, a critical enzyme required for HSV replication. The test compounds showed the capacity to effectively bind to the active site of the enzyme, leading to possible inhibition of HSV-1 activity.
AbouAitah et al. [33] have recently designed a nano-formulation composed of ellagic acid and functionalized zinc oxide nanoparticles (ZnO NPs) with enhanced anti-HSV-2 activity. The hybrid nano-formulation, ZnO NPs, and ellagic acid selectively inactivated HSV-2 DNA replication with 50% inhibitory concentration (IC50) values of 3.6, 5.4, and 4.0 µg/mL, respectively, and selectivity index (SI) 57.5, 41.2, and 28.1, respectively.
Treatment of HSV-1-infected Vero cells with a polyphenol-rich extract from Solanum melongena L. for 24 h after viral adsorption hindered the viral replication with an IC50 value of 83.4 µg/mL by targeting the viral gB expression. The presence of chlorogenic acid, caffeic acid, and vanillic acid, along with other polyphenolic compounds identified in the extract, was proposed to be correlated to the induced anti-HSV-1 properties [34]. In another work, Langland et al. [35] evaluated the anti-HSV effects of phenolic acids when paired with metal and inorganic ions. Metal chelates of caffeic acid (addition of cations such as Fe3+ and anionic molecules such as molybdate and phosphate) increased antiviral activity upwards of 100-fold. Specifically, caffeic acid chelates exerted the best effects against HSV-1 and HSV-2. Besides, due to their action on extra-cellular processes such as inhibition of virion binding to the cell, the authors concluded that the potential of adding caffeic acid chelates to existing medications such as acyclovir could provide more efficient management of HSV infections.
3.2. Tannins and Their Derivatives
In an ethnopharmacological study, chebulagic and chebulinic acids were isolated from the fruits of Terminalia chebula Retz. and were further examined for their anti-HSV-2 properties. Both compounds inhibited the viral activity with IC50 values of 1.41 and 0.06 µg/mL, respectively. Moreover, in the post-infection plaque reduction test, both molecules suppressed the replication of HSV-2 with IC50 values of 31.84 and 8.69 µg/mL, respectively. The study outcome suggests that both compounds might prevent the attachment and penetration of the virus to Vero cells [36].
The antiherpetic action of geraniin (extracted from the leaves of Spondias mombin L.) against HSV-1 was studied in a combined in vitro and in silico experiment. The in vitro results demonstrated that geraniin at a concentration of 20.40 µg/mL exhibited virucidal action via blocking viral attachment. The mechanism of action was predicted using a molecular docking analysis via targeting the glycoprotein gB of the HSV-1 surface [37].
Tannic acid (TA), a plant-derived hydrolyzable tannin with five digalloyl units connected with one glucose molecule, has recently been designed by Szymańska et al. [38] in mucoadhesive gelling delivery systems using silver nanoparticles modified with tannic acid (TA-AgNPs) for possible enhanced HSV therapy. The authors have demonstrated in vitro the ability of TA-AgNPs-based hydrogels (at concentrations of 25 or 50 ppm) to potently inhibit HSV-1 and HSV-2 replications. The mechanism of HSV-1 inhibition was revealed by blocking the viral attachment to the immortal human keratinocyte cell line by interfering with gC and gB glycoproteins, while the anti-HSV-2 activity was mediated by hindering viral attachment and penetration. Additionally, treatment of a murine model of HSV-2 genital infection with TA-AgNPs-based hydrogels (at a concentration of 25 ppm) has successfully prevented vaginal HSV-2 transmission.
In an animal study, another research team [39] provided interesting results in which treatment of an HSV-2-infected mouse model (with primary and recurrent vaginal infections) with TA-AgNPs (size 33 nm) improved the anti-HSV-2 immune response by boosting a virus-specific cellular and humoral response with activation of B cells.
Selected groups of natural tannins such as ellagitannins and gallotannin-type compounds have been assayed for their antiviral effects on HSV-1 replication performed on monolayer cultures of Madin-Darbey bovine kidney (MDBK) cells. Based on the calculation of selectivity index (SI; CC50/IC50), ellagitannins such as epiacutissimin B (SI > 60.6), epiacutissimin A (SI > 55.5), acutissimin A (SI > 34.8), and mongolicain (SI > 32.5) demonstrated greater inhibition of HSV-1 replication than gallotannin-type compounds such as 1,2,3,4,5-penta-O-digalloyl-β-D-glucopyranose (SI > 35.7), 1,2,3,4,5-penta-O-digalloyl-α-D-glucopyranose (SI > 28.5), tannic acid (SI > 25), and α/β-3-O-digalloyl-D-glucopyranose (1:1 mixture; SI = 15.6). The non-nucleoside structure of the test compounds was suggested to be accountable for the anti-HSV-1 properties. The mechanisms of action have been proposed to be related to the ability to target HSV-1 glycoproteins. [40]. In another experiment, the combination of C-glucosidic ellagitannins castalagin and vescalagin (isolated from Quercus robur) with acyclovir applied against acyclovir-resistant HSV-1 possessed a much stronger synergistic effect compared with the effect detected against acyclovir-resistant HSV-2 with IC50 values ranging from 0.04 to 0.46 µM [41].
Punicalagin, an ellagitannin isolated from Punica granatum (pomegranate), demonstrated 100% anti-HSV-2 activity determined at 31.25 µg/mL and showed an inhibitory effect equivalent to the standard drug acyclovir. The mechanism of action was predicted via targeting HSV-2 protease using molecular docking analysis [42]. Likewise, Houston et al. [43] revealed that the virucidal activity of pomegranate extract and punicalagin against HSV-1 can be potentiated when co-administered with zinc ions.
Pentagalloylglucose (1,2,3,4,6-penta-O-galloyl-β-D-glucose; PGG), a bioactive gallotannin extracted from Elaeocarpus sylvestris, has significantly suppressed VZV replication with an IC50 value of 14.67 µg/mL. The anti-VZV mechanism of PGG was disclosed via inhibiting VZV-induced c-Jun N-terminal kinase (JNK) activation and expression of VZV-IE62 protein [44].
3.3. Xanthones
Mangiferin, a bioactive compound of Mangifera indica with C-glycosylxanthone structure, has been examined in vitro and in vivo for its anti-herpesvirus properties against HSV-1. Mangiferin in vitro suppressed the replication of two HSV-1 strains (ACV-resistant HSV-1 (AR-29) and sensitive (KOS)) with IC50 values of 2.9 and 3.5 µg/mL, respectively, with a suggested mechanism that affects viral particles. Furthermore, treatment of Balb/c mice with mangiferin topical formulation (0.7%) improved the healing course by reducing the lesions [45]. Another in vitro research has evaluated the anti-HSV-1 properties of a mixture containing mangiferin combined with a polysaccharide galactomannan derived from the tree Dimorphandra gardneriana (DgGmM). DgGmM repressed the replication of HSV-1 with an IC50 value of 287.5 µg/mL, while at post-infection treatment (1 h; 500 µg/mL), maximum inhibition was observed [46].
3.4. Stilbenes and Their Derivatives
Resveratrol, a stilbene compound, has been detected in several plants, including grape vines, berries, pomegranates, pines, soybeans, legumes, and peanuts with promising antiviral properties against various human and animal viruses [47][48]. Previous preclinical studies have asserted the capacity of resveratrol to inhibit the replication of HSV-1 and HSV-2 in a dose-dependent and reversible manners via targeting viral immediate-early (IE) genes [49][50]. However, Ding et al. [51] reported in their recent investigation that resveratrol (in a dose-dependent manner) can promote HSV-2 replication and hence HSV-2 infection by boosting histone acetylation and regulating the activation of nuclear factor-κB (NF-κB). Their results also revealed that suppression of HSV-2 replication by resveratrol at a concentration of 30 µM might be achieved via repressing the activity of cyclin-dependent kinase 9 (CDK9), an enzyme necessary for HSV-2 replication. Based on the reviewed data, researchers can assume that resveratrol induces anti-HSV activities in a dose-dependent manner; however, the validation of its activity and mechanisms should be in-depth evaluated in further in vivo experiments. Unlike the vital role of thymidine kinase (TK; an HSV-encoded gene product and a mediator enzyme that plays a critical role in HSV replication) in HSV pathogenesis, resveratrol at concentrations of 10 and 20 µM was found to boost the bystander action induced by the HSV-TK/ganciclovir gene therapy for hepatocellular carcinoma associated with herpesvirus infections by improving connexin-mediated gap junctional communication [52].
Piceatannol, a resveratrol metabolite extracted from Cassia abbreviata, was determined to be active against HSV-1 and HSV-2 with IC50 values of 47.5 and 45.0 µM, respectively, by affecting viral particles. However, further studies are needed to elucidate the exact mechanism of action beyond the inhibition of viral DNA replication [53].
Tarbeeva and co-workers [54] isolated a stilbenoid with a 1,2-diketone fragment named bicoloketone from the stem bark of Lespedeza bicolor with potential antiherpetic activity. Treatment of HSV-1-infected Vero cells with bicoloketone has led to notable inhibition of HSV-1 replication with an IC50 value of 44.2 µM.
Recently, a study performed in Italy analyzed the efficacy of grape cane extract (named ‘’Greco’’ from Vitis vinifera L.) against HSV-1 infection. The results of high-performance liquid chromatography combined with multistage ion trap mass spectrometry (HPLC/ITMSn) analysis indicated that Greco extract at pH = 13 is rich in stilbenoids such as resveratrol C-glucoside, resveratrol, and epsilon-viniferin. Furthermore, the extract (pH = 13; 10 µg/mL) was identified to act directly with HSV-1 particles by blocking HSV-1 replication with an IC50 value of 0.9 µg/mL. The study outcome suggests that the anti-HSV-1 activity of the test extract is related to the presence of the detected stilbenoids [55].
3.5. Lignans and Neolignans
Honokiol is a bioactive component of the genus Magnolia with multiple pharmacological actions. Its antiviral effect on HSV-1 infection has been disclosed by Liu and colleagues [56] with an IC50 value of 10.51 µg/mL. Its mechanism of action against HSV-1 is related to inhibiting the virus replication, ICP27 and VP16 gene expressions, and viral progeny production. Moreover, honokiol (5 µg/mL) showed a synergy effect with acyclovir (1 µM), leading to significant inhibition of HSV-1 infection via blocking ICP27, VP16, and gD expressions.
In a combined phytochemical profiling and antiviral study, Dias et al. [57] tested the anti-HSV-1 properties of Arctium lappa L. crude extract, which is rich in dibenzylbutyrolactone-type lignans arctiin and arctigenin. The crude extract at a concentration of 400 µg/mL exhibited a remarkable reduction in viral load and hence viral DNA replication. The authors suggest that the anti-HSV-1 action of the crude extract is related to the presence of arctiin and arctigenin.
Deightonin, a neolignan, has recently been obtained from the aerial parts of Euphorbia deightonii Croizat. with anti-herpesvirus activity. This compound has successfully inactivated the replication of HSV-2 in infected Vero cells with an IC50 value of 11.73 µM and SI = 3.39 [58].
3.6. Anthraquinones and Their Derivatives
Emodin is an anthraquinone derivative distributed in various plant species, including Aloe vera, Rheum palmatum, Polygonum multiflorum, and Cassia occidentalis with proven in vitro and in vivo efficacy against HSV-1 and HSV-2 at an effective concentration of 50 µg/mL (in vitro) and doses of 3.3, 6.7, and 11.3 g/kg/day, respectively (in vivo; 7 days), according to study published in 2011 [59]. Later, in an animal experiment published in 2021 [60], several mechanisms were disclosed in which emodin was found to reduce the TLR3 pathway and its downstream molecules, TRIF, TRADD, TRAF6, traf3, Nemo, IRF3, and p38, by 20–60% as well as the expressions of IL-6 (interleukin-6), TNF-α (tumor necrosis factor-α), and IFN-β (tumor necrosis factor-β) by 30-50% in HSV-1-infected brain tissues of mice.
Mugas and colleagues [61] isolated from the aerial parts of Heterophyllaea pustulata seven anthraquinone-type compounds with ani-HSV-1 properties. The investigated compounds 5,5′-Bisoranjidiol, rubiadin 1-methyl ether, soranjidiol 1-methyl ether, damnacanthol, soranjidiol, rubiadin, and heterophylline suppressed the replication of HSV-1 with IC50 values of 15.6, 69.4, 57.1, 61.6, 27.2, 20.5, and 72.2 µM, respectively. Moreover, the test molecules possessed notable photo-inactivation (>80%) of HSV-1 particles, suggesting their potential use in treating localized herpetic lesions.
Treatment of HSV-1-infected Vero cells with a plant-derived 1,4-Anthraquinone (6.25 µg/mL) notably impeded HSV-1 activity with a reduced value of viral titer (Rf; 1 × 102) [62].
3.7. Curcuminoids
Curcumin is a bioactive molecule that belongs to the class of curcuminoids and has broadly been detected in diverse Curcuma spp. with various health benefits [63][64]. This compound at a concentration of 30 µM hindered the replication of HSV-1 and HSV-2 by blocking the adsorption of both viruses [65]. Previous work has also uncovered additional mechanisms against HSV-1 via inhibiting IE gene expression by repressing histone acetyltransferase activity of the transcriptional coactivator proteins p300 and CBP [66]. In another experiment, nanoparticle-containing curcumin (0.5 mg) decreased tissue inflammation and the severity of HSV-2 infection in animal models (female C57BL/6 mice). The mechanism of action was claimed to be linked with the anti-inflammatory properties of curcumin [67].
HSV encodes several enzymes that are necessary for viral replication, representing valuable drug targets useful for the therapy of HSV infections [23][68]. Accordingly, in an in silico assay, curcumin was studied for its capability to bind to the active site of HSV-1 TK. The results depicted that curcumin has successfully bound to HSV-1 TK active site by establishing critical molecular interactions (hydrogen bonding and hydrophobic interactions) with the amino acid residues of the enzyme active site. Hydroxyl and carbonyl groups and phenyl rings of curcumin were proposed as functional groups responsible for the anti-HSV-1 TK action [69].
Badria et al. [70], in their remarkable work, enhanced the anti-HSV-1 properties of curcumin by fabricating and optimizing a formulation of curcumin-loaded proniosomes delivery system (F5). The optimized F5 (30 µM) has notably reduced the viral plaques by 90% and hence HSV-1 replication with an acceptable level of cytotoxicity (CC50 = 200 µM). Also, the research team has positively determined the binding mode and molecular interaction of curcumin with HSV-1 DNA polymerase, a critical enzyme required for HSV-1 replication, by molecular docking analysis.
3.8. Coumarins
Saidu and colleagues [58] have recently isolated scoparon, a coumarin type molecule, from the aerial parts of Euphorbia deightonii Croizat. with promising antiviral properties. Scoparon showed strong inhibitory action against HSV-2 DNA replication with an IC50 value of 0.032 µM and SI = 10.93.
Coumarin-type molecules such as imperatorin and phellopterin, acquired from the fruits of Angelica archangelica L., were noticed to be active against HSV-1 replication. Imperatorin at concentrations of 15.62 and 31.25 µg/mL reduced the titer of HSV-1 by 3.48 log and 4.7 log, respectively. Phellopterin at the concentration of 7.81 µg/mL decreased the virus titer by 3.01 log, while the mixture of imperatorin and phellopterin diminished the virus titer by 3.73 log at a concentration of 31.25 µg/mL. Direct inactivation of HSV-1 DNA replication was recorded as a mechanism of action [71].
3.9. Other Polyphenols: Phloroglucinol
Okba and her research team [72] evaluated the efficacy of phloroglucinol-rich extract (PGRE) from the plant Eucalyptus sideroxylon A.Cunn. ex Woolls against HSV-2 infection. PGRE suppressed HSV-2 replication and attachment to Vero cells with an IC50 value of 189.36 µg/mL and an inhibition percentage of 87.65%. The mechanism was claimed to be attributed to impeding viral protein synthesis.
References
- Šudomová, M.; Berchová-Bímová, K.; Mazurakova, A.; Šamec, D.; Kubatka, P.; Hassan, S.T.S. Flavonoids Target Human Herpesviruses That Infect the Nervous System: Mechanisms of Action and Therapeutic Insights. Viruses 2022, 14, 592.
- Šudomová, M.; Berchová-Bímová, K.; Marzocco, S.; Liskova, A.; Kubatka, P.; Hassan, S.T.S. Berberine in Human Oncogenic Herpesvirus Infections and Their Linked Cancers. Viruses 2021, 13, 1014.
- Cohen, J.I. Herpesvirus Latency. J. Clin. Investig. 2020, 130, 3361–3369.
- Wu, Y.; Yang, Q.; Wang, M.; Chen, S.; Jia, R.; Yang, Q.; Zhu, D.; Liu, M.; Zhao, X.; Zhang, S.; et al. Multifaceted Roles of ICP22/ORF63 Proteins in the Life Cycle of Human Herpesviruses. Front. Microbiol. 2021, 12, 668461.
- Frappier, L. Regulation of Herpesvirus Reactivation by Host MicroRNAs. J. Virol. 2015, 89, 2456–2458.
- Dochnal, S.A.; Francois, A.K.; Cliffe, A.R. De Novo Polycomb Recruitment: Lessons from Latent Herpesviruses. Viruses 2021, 13, 1470.
- Jarosinski, K.W. Interindividual Spread of Herpesviruses. Adv. Anat. Embryol. Cell Biol. 2017, 223, 195–224.
- Poole, C.L.; James, S.H. Antiviral Therapies for Herpesviruses: Current Agents and New Directions. Clin. Ther. 2018, 40, 1282–1298.
- Majewska, A.; Mlynarczyk-Bonikowska, B. 40 Years after the Registration of Acyclovir: Do We Need New Anti-Herpetic Drugs? Int. J. Mol. Sci. 2022, 23, 3431.
- Kłysik, K.; Pietraszek, A.; Karewicz, A.; Nowakowska, M. Acyclovir in the Treatment of Herpes Viruses—A Review. Curr. Med. Chem. 2020, 27, 4118–4137.
- Hassan, S.T.S.; Masarčíková, R.; Berchová, K. Bioactive Natural Products with Anti-Herpes Simplex Virus Properties. J. Pharm. Pharmacol. 2015, 67, 1325–1336.
- Hassan, S.T.S.; Švajdlenka, E.; Berchová-Bímová, K. Hibiscus sabdariffa L. and Its Bioactive Constituents Exhibit Antiviral Activity against HSV-2 and Anti-Enzymatic Properties against Urease by an ESI-MS Based Assay. Molecules 2017, 22, 722.
- Lattanzio, V. Phenolic Compounds: Introduction. In Natural Products; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1543–1580. ISBN 978-3-642-22143-9.
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212.
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A Concise Overview on the Chemistry, Occurrence, and Human Health. Phytother. Res. 2019, 33, 2221–2243.
- Tuladhar, P.; Sasidharan, S.; Saudagar, P. Role of Phenols and Polyphenols in Plant Defense Response to Biotic and Abiotic Stresses. In Biocontrol Agents and Secondary Metabolites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 419–441. ISBN 978-0-12-822919-4.
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87.
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273.
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of Dietary Polyphenols: The Role of Metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659.
- Zhang, L.; Han, Z.; Granato, D. Polyphenols in Foods: Classification, Methods of Identification, and Nutritional Aspects in Human Health. Adv. Food Nutr. Res. 2021, 98, 1–33.
- Chojnacka, K.; Skrzypczak, D.; Izydorczyk, G.; Mikula, K.; Szopa, D.; Witek-Krowiak, A. Antiviral Properties of Polyphenols from Plants. Foods 2021, 10, 2277.
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Reig, M.; Valderrama, C.; Granados, M.; Cortina, J.L.; Saurina, J. Polyphenols and Their Potential Role to Fight Viral Diseases: An Overview. Sci. Total Environ. 2021, 801, 149719.
- Hassan, S.T.S.; Šudomová, M.; Berchová-Bímová, K.; Šmejkal, K.; Echeverría, J. Psoromic Acid, a Lichen-Derived Molecule, Inhibits the Replication of HSV-1 and HSV-2, and Inactivates HSV-1 DNA Polymerase: Shedding Light on Antiherpetic Properties. Molecules 2019, 24, 2912.
- Zhu, S.; Viejo-Borbolla, A. Pathogenesis and Virulence of Herpes Simplex Virus. Virulence 2021, 12, 2670–2702.
- Gershon, A.A.; Breuer, J.; Cohen, J.I.; Cohrs, R.J.; Gershon, M.D.; Gilden, D.; Grose, C.; Hambleton, S.; Kennedy, P.G.E.; Oxman, M.N.; et al. Varicella Zoster Virus Infection. Nat. Rev. Dis. Prim. 2015, 1, 15016.
- Kennedy, P.G.E.; Gershon, A.A. Clinical Features of Varicella-Zoster Virus Infection. Viruses 2018, 10, E609.
- Azab, W.; Dayaram, A.; Greenwood, A.D.; Osterrieder, N. How Host Specific Are Herpesviruses? Lessons from Herpesviruses Infecting Wild and Endangered Mammals. Annu. Rev. Virol 2018, 5, 53–68.
- Lum, K.K.; Cristea, I.M. Host Innate Immune Response and Viral Immune Evasion During Alphaherpesvirus Infection. Curr. Issues Mol. Biol. 2021, 42, 635–686.
- Borenstein, R.; Hanson, B.A.; Markosyan, R.M.; Gallo, E.S.; Narasipura, S.D.; Bhutta, M.; Shechter, O.; Lurain, N.S.; Cohen, F.S.; Al-Harthi, L.; et al. Ginkgolic Acid Inhibits Fusion of Enveloped Viruses. Sci. Rep. 2020, 10, 4746.
- Sochocka, M.; Sobczyński, M.; Ochnik, M.; Zwolińska, K.; Leszek, J. Hampering Herpesviruses HHV-1 and HHV-2 Infection by Extract of Ginkgo Biloba (EGb) and Its Phytochemical Constituents. Front. Microbiol. 2019, 10, 2367.
- Bhutta, M.S.; Shechter, O.; Gallo, E.S.; Martin, S.D.; Jones, E.; Doncel, G.F.; Borenstein, R. Ginkgolic Acid Inhibits Herpes Simplex Virus Type 1 Skin Infection and Prevents Zosteriform Spread in Mice. Viruses 2021, 13, 86.
- Todorova, N.; Rangelov, M.; Dincheva, I.; Badjakov, I.; Enchev, V.; Markova, N. Potential of Hydroxybenzoic Acids from Graptopetalum Paraguayense for Inhibiting of Herpes Simplex Virus DNA Polymerase–Metabolome Profiling, Molecular Docking and Quantum-Chemical Analysis. Pharmacia 2022, 69, 113–123.
- AbouAitah, K.; Allayh, A.K.; Wojnarowicz, J.; Shaker, Y.M.; Swiderska-Sroda, A.; Lojkowski, W. Nanoformulation Composed of Ellagic Acid and Functionalized Zinc Oxide Nanoparticles Inactivates DNA and RNA Viruses. Pharmaceutics 2021, 13, 2174.
- Di Sotto, A.; Di Giacomo, S.; Amatore, D.; Locatelli, M.; Vitalone, A.; Toniolo, C.; Rotino, G.L.; Lo Scalzo, R.; Palamara, A.T.; Marcocci, M.E.; et al. A Polyphenol Rich Extract from Solanum Melongena L. DR2 Peel Exhibits Antioxidant Properties and Anti-Herpes Simplex Virus Type 1 Activity In Vitro. Molecules 2018, 23, E2066.
- Langland, J.; Jacobs, B.; Wagner, C.E.; Ruiz, G.; Cahill, T.M. Antiviral Activity of Metal Chelates of Caffeic Acid and Similar Compounds towards Herpes Simplex, VSV-Ebola Pseudotyped and Vaccinia Viruses. Antivir. Res. 2018, 160, 143–150.
- Kesharwani, A.; Polachira, S.K.; Nair, R.; Agarwal, A.; Mishra, N.N.; Gupta, S.K. Anti-HSV-2 Activity of Terminalia Chebula Retz Extract and Its Constituents, Chebulagic and Chebulinic Acids. BMC Complement. Altern. Med. 2017, 17, 110.
- Siqueira, E.M.D.S.; Lima, T.L.; Boff, L.; Lima, S.G.; Lourenço, E.M.; Ferreira, É.G.; Barbosa, E.G.; Machado, P.R.; Farias, K.J.; Ferreira, L.D.S.; et al. Antiviral Potential of Spondias Mombin L. Leaves Extract Against Herpes Simplex Virus Type-1 Replication Using In Vitro and In Silico Approaches. Planta Med. 2020, 86, 505–515.
- Szymańska, E.; Orłowski, P.; Winnicka, K.; Tomaszewska, E.; Bąska, P.; Celichowski, G.; Grobelny, J.; Basa, A.; Krzyżowska, M. Multifunctional Tannic Acid/Silver Nanoparticle-Based Mucoadhesive Hydrogel for Improved Local Treatment of HSV Infection: In Vitro and In Vivo Studies. IJMS 2018, 19, 387.
- Orłowski, P.; Kowalczyk, A.; Tomaszewska, E.; Ranoszek-Soliwoda, K.; Węgrzyn, A.; Grzesiak, J.; Celichowski, G.; Grobelny, J.; Eriksson, K.; Krzyzowska, M. Antiviral Activity of Tannic Acid Modified Silver Nanoparticles: Potential to Activate Immune Response in Herpes Genitalis. Viruses 2018, 10, 524.
- Vilhelmova-Ilieva, N.; Jacquet, R.; Deffieux, D.; Pouységu, L.; Sylla, T.; Chassaing, S.; Nikolova, I.; Quideau, S.; Galabov, A.S. Anti-Herpes Simplex Virus Type 1 Activity of Specially Selected Groups of Tannins. Drug Res. 2019, 69, 373–374.
- Vilhelmova-Ilieva, N.; Jacquet, R.; Quideau, S.; Galabov, A.S. Ellagitannins as Synergists of ACV on the Replication of ACV-Resistant Strains of HSV 1 and 2. Antivir. Res. 2014, 110, 104–114.
- Arunkumar, J.; Rajarajan, S. Study on Antiviral Activities, Drug-Likeness and Molecular Docking of Bioactive Compounds of Punica Granatum L. to Herpes Simplex Virus-2 (HSV-2). Microb. Pathog. 2018, 118, 301–309.
- Houston, D.M.J.; Bugert, J.J.; Denyer, S.P.; Heard, C.M. Potentiated Virucidal Activity of Pomegranate Rind Extract (PRE) and Punicalagin against Herpes Simplex Virus (HSV) When Co-Administered with Zinc (II) Ions, and Antiviral Activity of PRE against HSV and Aciclovir-Resistant HSV. PLoS ONE 2017, 12, e0179291.
- Bae, S.; Kim, S.Y.; Do, M.H.; Lee, C.H.; Song, Y.-J. 1,2,3,4,6-Penta-O-Galloyl-ß-D-Glucose, a Bioactive Compound in Elaeocarpus Sylvestris Extract, Inhibits Varicella-Zoster Virus Replication. Antivir. Res. 2017, 144, 266–272.
- Rechenchoski, D.Z.; Agostinho, K.F.; Faccin-Galhardi, L.C.; Lonni, A.A.S.G.; da Silva, J.V.H.; de Andrade, F.G.; Cunha, A.P.; Ricardo, N.M.P.S.; Nozawa, C.; Linhares, R.E.C. Mangiferin: A Promising Natural Xanthone from Mangifera Indica for the Control of Acyclovir - Resistant Herpes Simplex Virus 1 Infection. Bioorg. Med. Chem. 2020, 28, 115304.
- Rechenchoski, D.Z.; Samensari, N.L.; Faccin-Galhardi, L.C.; de Almeida, R.R.; Cunha, A.P.; Ricardo, N.M.P.S.; Nozawa, C.; Linhares, R.E.C. The Combination of Dimorphandra Gardneriana Galactomannan and Mangiferin Inhibits Herpes Simplex and Poliovirus. Curr. Pharm. Biotechnol. 2019, 20, 215–221.
- Abba, Y.; Hassim, H.; Hamzah, H.; Noordin, M.M. Antiviral Activity of Resveratrol against Human and Animal Viruses. Adv. Virol. 2015, 2015, 184241.
- Chen, X.; Song, X.; Zhao, X.; Zhang, Y.; Wang, Y.; Jia, R.; Zou, Y.; Li, L.; Yin, Z. Insights into the Anti-Inflammatory and Antiviral Mechanisms of Resveratrol. Mediat. Inflamm. 2022, 2022, 7138756.
- Docherty, J.J.; Fu, M.M.; Stiffler, B.S.; Limperos, R.J.; Pokabla, C.M.; DeLucia, A.L. Resveratrol Inhibition of Herpes Simplex Virus Replication. Antivir. Res. 1999, 43, 145–155.
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Tenore, G.C.; Novellino, E. Resveratrol as a Novel Anti-Herpes Simplex Virus Nutraceutical Agent: An Overview. Viruses 2018, 10, 473.
- Ding, L.; Jiang, P.; Xu, X.; Lu, W.; Yang, C.; Zhou, P.; Liu, S. Resveratrol Promotes HSV-2 Replication by Increasing Histone Acetylation and Activating NF-ΚB. Biochem. Pharmacol. 2020, 171, 113691.
- Xiao, J.; Wang, X.; Wu, Y.; Zhao, Q.; Liu, X.; Zhang, G.; Zhao, Z.; Ning, Y.; Wang, K.; Tan, Y.; et al. Synergistic Effect of Resveratrol and HSV-TK/GCV Therapy on Murine Hepatoma Cells. Cancer Biol. Ther. 2019, 20, 183–191.
- Zheng, Y.; Yang, X.-W.; Schols, D.; Mori, M.; Botta, B.; Chevigné, A.; Mulinge, M.; Steinmetz, A.; Schmit, J.-C.; Seguin-Devaux, C. Active Components from Cassia Abbreviata Prevent HIV-1 Entry by Distinct Mechanisms of Action. Int. J. Mol. Sci. 2021, 22, 5052.
- Tarbeeva, D.V.; Krylova, N.V.; Iunikhina, O.V.; Likhatskaya, G.N.; Kalinovskiy, A.I.; Grigorchuk, V.P.; Shchelkanov, M.Y.; Fedoreyev, S.A. Biologically Active Polyphenolic Compounds from Lespedeza Bicolor. Fitoterapia 2022, 157, 105121.
- Squillaci, G.; Zannella, C.; Carbone, V.; Minasi, P.; Folliero, V.; Stelitano, D.; Cara, F.L.; Galdiero, M.; Franci, G.; Morana, A. Grape Canes from Typical Cultivars of Campania (Southern Italy) as a Source of High-Value Bioactive Compounds: Phenolic Profile, Antioxidant and Antimicrobial Activities. Molecules 2021, 26, 2746.
- Liu, S.; Li, L.; Tan, L.; Liang, X. Inhibition of Herpes Simplex Virus-1 Replication by Natural Compound Honokiol. Virol. Sin. 2019, 34, 315–323.
- Dias, M.M.; Zuza, O.; Riani, L.R.; de Faria Pinto, P.; Pinto, P.L.S.; Silva, M.P.; de Moraes, J.; Ataíde, A.C.Z.; de Oliveira Silva, F.; Cecílio, A.B.; et al. In Vitro Schistosomicidal and Antiviral Activities of Arctium Lappa L. (Asteraceae) against Schistosoma Mansoni and Herpes Simplex Virus-1. Biomed. Pharmacother. 2017, 94, 489–498.
- Saidu, M.B.; Kúsz, N.; Tsai, Y.-C.; Vágvölgyi, M.; Berkecz, R.; Kókai, D.; Burián, K.; Hohmann, J.; Rédei, D. Triterpenes and Phenolic Compounds from Euphorbia Deightonii with Antiviral Activity against Herpes Simplex Virus Type-2. Plants 2022, 11, 764.
- Xiong, H.-R.; Luo, J.; Hou, W.; Xiao, H.; Yang, Z.-Q. The Effect of Emodin, an Anthraquinone Derivative Extracted from the Roots of Rheum Tanguticum, against Herpes Simplex Virus in Vitro and in Vivo. J. Ethnopharmacol. 2011, 133, 718–723.
- Huang, Y.; Li, X.; Pan, C.; Cheng, W.; Wang, X.; Yang, Z.; Zheng, L. The Intervention Mechanism of Emodin on TLR3 Pathway in the Process of Central Nervous System Injury Caused by Herpes Virus Infection. Neurol. Res. 2021, 43, 307–313.
- Mugas, M.L.; Marioni, J.; Martinez, F.; Aguilar, J.J.; Cabrera, J.L.; Contigiani, M.S.; Konigheim, B.S.; Núñez-Montoya, S.C. Inactivation of Herpes Simplex Virus by Photosensitizing Anthraquinones Isolated from Heterophyllaea Pustulata. Planta Med. 2021, 87, 716–723.
- Roa-Linares, V.C.; Miranda-Brand, Y.; Tangarife-Castaño, V.; Ochoa, R.; García, P.A.; Castro, M.Á.; Betancur-Galvis, L.; San Feliciano, A. Anti-Herpetic, Anti-Dengue and Antineoplastic Activities of Simple and Heterocycle-Fused Derivatives of Terpenyl-1,4-Naphthoquinone and 1,4-Anthraquinone. Molecules 2019, 24, 1279.
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma Longa) and Its Major Constituent (Curcumin) as Nontoxic and Safe Substances: Review. Phytother. Res. 2018, 32, 985–995.
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930.
- Flores, D.J.; Lee, L.H.; Adams, S.D. Inhibition of Curcumin-Treated Herpes Simplex Virus 1 and 2 in Vero Cells. AiM 2016, 6, 276–287.
- Kutluay, S.B.; Doroghazi, J.; Roemer, M.E.; Triezenberg, S.J. Curcumin Inhibits Herpes Simplex Virus Immediate-Early Gene Expression by a Mechanism Independent of P300/CBP Histone Acetyltransferase Activity. Virology 2008, 373, 239–247.
- Vitali, D.; Bagri, P.; Wessels, J.M.; Arora, M.; Ganugula, R.; Parikh, A.; Mandur, T.; Felker, A.; Garg, S.; Kumar, M.N.V.R.; et al. Curcumin Can Decrease Tissue Inflammation and the Severity of HSV-2 Infection in the Female Reproductive Mucosa. IJMS 2020, 21, 337.
- Xie, Y.; Wu, L.; Wang, M.; Cheng, A.; Yang, Q.; Wu, Y.; Jia, R.; Zhu, D.; Zhao, X.; Chen, S.; et al. Alpha-Herpesvirus Thymidine Kinase Genes Mediate Viral Virulence and Are Potential Therapeutic Targets. Front. Microbiol. 2019, 10, 941.
- El-Halim, S.M.A.; Mamdouh, M.A.; El-Haddad, A.E.; Soliman, S.M. Fabrication of Anti-HSV-1 Curcumin Stabilized Nanostructured Proniosomal Gel: Molecular Docking Studies on Thymidine Kinase Proteins. Sci. Pharm. 2020, 88, 9.
- Badria, F.A.; Abdelaziz, A.E.; Hassan, A.H.; Elgazar, A.A.; Mazyed, E.A. Development of Provesicular Nanodelivery System of Curcumin as a Safe and Effective Antiviral Agent: Statistical Optimization, In Vitro Characterization, and Antiviral Effectiveness. Molecules 2020, 25, 5668.
- Rajtar, B.; Skalicka-Woźniak, K.; Świątek, Ł.; Stec, A.; Boguszewska, A.; Polz-Dacewicz, M. Antiviral Effect of Compounds Derived from Angelica Archangelica L. on Herpes Simplex Virus-1 and Coxsackievirus B3 Infections. Food Chem. Toxicol. 2017, 109, 1026–1031.
- Okba, M.M.; El Gedaily, R.A.; Ashour, R.M. UPLC-PDA-ESI-QTOF-MS Profiling and Potent Anti-HSV-II Activity of Eucalyptus Sideroxylon Leaves. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1068–1069, 335–342.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.8K
Revisions:
2 times
(View History)
Update Date:
21 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




