
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tuba Esatbeyoglu | + 1990 word(s) | 1990 | 2020-12-08 09:26:55 | | | |
| 2 | Catherine Yang | Meta information modification | 1990 | 2020-12-15 07:24:39 | | |
Video Upload Options
Phenolic compounds (quercetin, rutin, cyanidin, tangeretin, hesperetin, curcumin, resveratrol, etc.) are known to have health‐promoting effects and they are accepted as one of the main proposed nutraceutical group. However, their application is limited owing to the problems
related with their stability and water solubility as well as their low bioaccessibility and bioavailability. These limitations can be overcome by encapsulating phenolic compounds by physical, physicochemical and chemical encapsulation techniques.
1. Introduction
The dynamic market of so called “superfoods” grows steadily worldwide and offers new health-improving products regularly, although some of these foods have evolved into established products or food additives, e.g., Goji berries and Chia seeds [1]. While the demand for superfoods and healthier foods rose over the last years, the positive relationship between nutrition and health became more and more pronounced and forced the development of these kinds of products [2]. Moreover, the individual-related and specific nutrient supply, especially for the elderly, comes into focus. In 2050, nearly 16% of the world population will be aged over 65, whereby the demand for personalized functional foods will be increased in parallel as the population age [3].
Despite market growth in functional and healthier foods, their beneficial effects are controversially discussed, e.g., as reviewed by Marian (2017) [4]. In this entry, nutrition studies with healthy humans consuming dietary supplements were summarized. Conclusively, most of the studies showed health-improving effects induced by the supplements, but at rather high doses which are unusual for the dietary intake [4]. For example, one of these food supplements is resveratrol, a naturally occurring phytoalexin that is synthesized in plants, e.g. grapes, as a response to injuries [5]. Resveratrol, as a food additive, possesses various health promoting effects including high antioxidant and anti-inflammatory potential, anticarcinogenicity in breast and liver tissue, prevention of osteoporosis, improving ischemic diseases and muscle regeneration, etc. [6][7] Unfortunately, these health-improving effects have been mainly analyzed in cell culture studies or preclinical models, which makes the application of effective concentrations and substances more difficult in humans [6]. For example, the functionality of resveratrol is limited owing to its low bioavailability [5]. While the solubility of resveratrol in aqueous solutions is 3 mg/L, the solubility is enhanced to 50 g/L in ethanol, which results in a higher uptake and plasma concentration of resveratrol with a lipophilic-based food matrix. Besides, the bioavailability is too low to reach effective doses up to 1 g/day only by consumption of resveratrol-containing food. Theoretically, the consumption of about 3500 L of rose wine, 2600 kg of white grapes, up to 35,000 kg of peanuts or 2500 kg of apples per day were found to be necessary to reach these daily intake doses [6].
These results illustrate the need for developing new delivery systems for bioactive compounds, which show low bioavailability values [8], by altering the molecular structure or the physiochemical characteristics of bioactive compounds [7]. The pharmaceutical industry has developed technologies to improve drug delivery systems, which could be transferred to the food industry and may be also helpful for nanoscale delivery systems for food products [3]. The encapsulation of these compounds using nanoparticles, nanodelivery carriers or various emulsions could protect them against enzymatic degradation during digestion and increase the intestinal uptake, resulting in a higher gut concentration as well as increased plasma levels of encapsulated food additives [9].
2. Overview of Phenolic Compounds Bioaccessibility/Bioavailability
Phenolic substances are secondary metabolites which are present in a wide variety of foods such as fruits, vegetables, cereals, horticultural crops, legumes, chocolate, etc. and in beverages, i.e., tea and coffee [10]. Polyphenols with at least one aromatic ring and one or more hydroxyl groups can be categorized primarily as flavonoids and nonflavonoids. The basic structure of the common classes of flavonoids and nonflavonoids are shown in Figure 1. Flavonoids, as the most widespread and diverse group of polyphenols, can be further subdivided into flavonols (myricetin, quercetin, rutin, kaempferol etc.), flavones (aspigenin, luteolin, tangeretin etc.), flavanones (hesperetin, hesperidin, naringenin etc.), isoflavones (genistein, daidzein etc.) and anthocyanidins (cyanidin, delphinidin, malvidin, pelargonidin etc.) depending on the degree of hydroxylation, methoxylation, prenylation and glycosylation [11]. Nonflavonoids include diverse classes of polyphenols, such as stilbenes (resveratrol), lignans, hydrolyzable tannins and phenolic acids (hydroxybenzoic acids and hydroxycinnamic acids) [12].
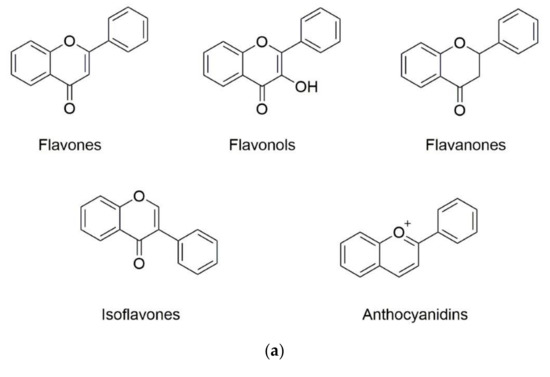
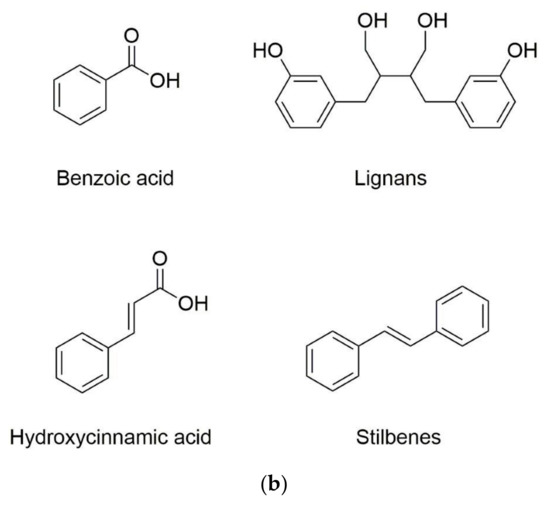
Figure 1. Basic structure of (a) common classes of flavonoids and (b) nonflavonoid-type phenolic compounds.
Phenolic compounds have been used for the production of functional foods due to their many benefits to human health through antioxidant, anti-inflammatory, anticancer, antiobesity, antiviral, antibacterial, antiaging and/or antiallergenic activities [13]. In vitro studies reported that flavonoids showed a high anticancer potential by inhibition of the proliferation, metastasis and angiogenesis of tumor cell lines, while the process of apoptotic cell death was activated. Such beneficial effects were also detected in mice fed with citrus peel extract, rich in phenolic compounds. The skin and colon carcinogenesis as well as the tumor size and volume of mice suffering from prostate cancer was significantly reduced in treated animals. In addition to the health-improving effects of a phenolic-enriched extract, the specific effects of each individual phenolic compound can also be allocated; such as an anti-inflammatory potential of tangeretin and sinensetin or the suitability of hesperidin as an antioxidant [14].
Bioaccessibility and bioavailability of phenolic compounds are the main factors which effect the biofunctional properties and possible beneficial effects. Bioaccessibility as a clue for the release and solubility of bioactive compounds during gastrointestinal digestion for further uptake, is a considerable factor for bioavailability [15]. Furthermore, various external and internal factors are also determinants of the bioavailability of phenolic compounds. The external factors comprise the nature of the bioactive agent, including solubility, crystallinity, etc., as well as the composition and structure of the food matrix, while the internal factors include gender, age, health, nutrient status, and life phase [16].
The bioavailability of macronutrients such as carbohydrates, proteins, and fats are mostly higher than 90%. However, most of the phenolic substances, especially lipophilic ones, possess low levels of solubility, stability, bioavailability and target tissue specificity in the body [17] depending on their molecular and physicochemical characteristics [3]. Besides, each class of phenolic substances has different chemical structures, solubility (hydrophilic or lipophilic) and sensitivity to oxidation [13]. For example, the bioavailability of lipophilic bioactives such as curcumin, quercetin, rutin or polymethoxylated flavonoids (PMFs) is limited due to their poor solubility, high melting point and chemical instability [18][19][20][21]. Overall, it is essential to have high bioavailability leading to a sufficient substance concentration in the blood stream and finally enabling the production of effective functional foods with beneficial health effects [3].
Several approaches have been used to enhance the bioaccessibility and bioavailability of bioactive ingredients, including chemical modifications of the molecules, dosing formulations, combination with other dietary components as well as incorporating them within micro-/nanoparticle delivery systems [22]. The rapid dissolution of bioactive compounds within the gastrointestinal tract could be achieved by the relatively high surface area of these systems [23]. Consequently, there is a great attempt to develop phenolic compound loaded micro/nanoscale delivery systems by pharmaceutical and food industries.
3. Intestinal Transport Mechanisms and Effective Factors on Phenolic Compound Bioavailability
An increase in bioaccessibility by encapsulation is the initial step for higher exploitation of phenolic compounds. Nevertheless, the bioavailability is equally essential and represents the second step, which can be positively affected by encapsulation. With increasing intestinal absorption of phenolic compounds, their biological activities will be increased. The intestinal epithelial transport mechanisms can be divided into four different routes: the paracellular route, the transcellular route, the carrier-mediated transport and transcytosis (Figure 2) [24][25]. While on the transcellular route substances diffuse through the membranes and the intracellular space of the epithelial cells, on the paracellular route ions and small molecules can passively diffuse through the tight junctions. More complex and hydrophilic molecules use vesicles along transcytosis or they bind to specific transporters, which are integrated into the membrane of the intestine, in the case of carrier-mediated transport [25]. Phenolic compounds are mainly absorbed by passive diffusion, where the lipophilicity and molecular weight of each molecule are crucial [12]. While such substance-specific features represent the first group of effective factors on polyphenolic bioavailability, the second group consists of all possibly consumed compounds of the dietary matrix, which may influence the digestion processes and the composition of the person-related microbiome.
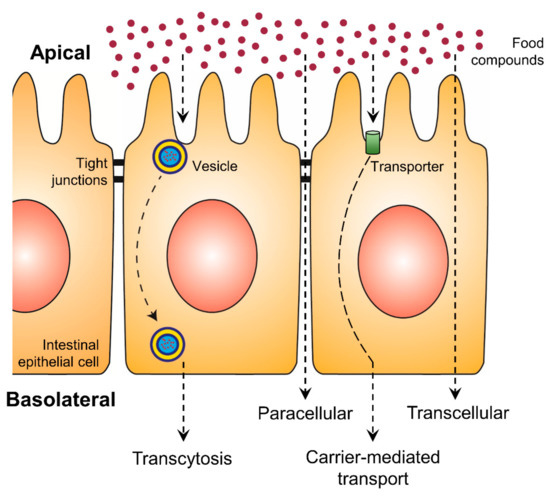
Figure 2. Uptake of food compounds by intestinal epithelial transport mechanisms from the gut lumen (apical side) to the blood vessel (basolateral side).
One of the most important factors for high bioavailability is the degree of polymerization as well as the methylation of the phenolic compound [26][27][28][29]. (−)-Epicatechin, a flavan-3-ol, possesses moderate bioavailability in in vivo studies with an average absorption of 23% after 90 min [26] or 46% after 2.5 h [30]. While 95.8% of transferred flavanol-related compounds were identified as (−)-epicatechin, the epicatechin dimers B2 and B5 showed a significantly lower content of <1% of the total transferred value [26]. Similar results were detected for further flavan-3-ols, whose monomers can directly be absorbed in the small intestine. More complex substances, e.g., polymeric forms will be transferred to the colon, where gut bacteria metabolize the compounds by glucuronidation or sulfation prior to absorption [29]. Unfortunately, the health-improving potential of these microbial-derived metabolites are largely unknown. Whether a flavone will be directly absorbed or possibly metabolized, depends on the methylation state likewise. Wen and Walle [27] and Wen and Walle [31] analyzed the stability of methylated and nonmethylated flavones in addition to liver S9 fraction or in the presence of human hepatocytes. The methylated compounds showed a high resistance against metabolization in all assays compared to the nonmethylated forms, suggesting that the methylation of flavonoids eventually protects them from metabolization and excretion [31] . In further in vitro transport experiments, up to 8 times higher absorption rates were documented for methylated compounds, while the rate of the nonmethylated forms was lower and correlated with their high potential of metabolic transformation [27]. Therefore, the replacement of hydroxyl groups by methylated groups may be another suitable method for increasing phenolic compound bioavailability.
The metabolization of phenolic compounds by the microbiome and/or intestine epithelial cells plays an important role in bioavailability. Nevertheless, elements of the dietary matrix can influence the bacterial growth and the composition of the microbiome, resulting in different digestion and metabolization pathways. Roowi et al. [32] detected a high content of phenolic acids (3‑hydroxyphenylacetic acid, 3-hydroxyphenylhydracrylic acid, dihydroferulic acid, 3-methoxy-4-hydroxyphenylhydracrylic acid and 3-hydroxyhippuric acid) in the urine of participants after consumption of orange juice, which corresponded to 37% of total ingested flavanones. The excretion of these acids was significantly reduced by parallel consumption of orange juice with yoghurt, suggesting an increased metabolization by gut bacteria [32]. Similar to the effects of yoghurt, the naturally occurring dietary fiber pectin influenced the metabolic activity and/or composition of the intestinal flora and induced a higher quercetin plasma concentration after rutin digestion [33]. Moreover, glucose and insulin are effective factors on bioavailability. While the total anthocyanin content in red wine and red grape juice was comparable, the uptake of anthocyanins of red grape juice was significantly higher than that of red wine, which might be due to the lower glucose content in red wine [34]. Such synergistic effects of glucose and the phenolic compound absorption may be based on the stimulation of bacterial growth, whereby the bacteria use glucose as an energy source [33] or alternatively the high glucose content induces the release of insulin, which is able to influence the microbiome and the phenolic bioavailability [35]. Further, bacteria-independent effects may be induced by protein complexes and fat-enriched diets. Proteins, e.g. the salivary protein histatine 5 can bind phenolic compounds and form insoluble complexes, which are related to reduced absorption [36]. Otherwise, experiments with milk protein had no effect on the uptake of cocoa polyphenols [37]. However, a high dietary fat content is associated with greater absorption in a dose-dependent manner [38]. Lesser et al. [39] analyzed the bioavailability of quercetin in pigs, whereby the dietary fat content was increased from 3 to 17%, resulting in an enhanced absorption of 50%. It is assumed that quercetin was incorporated in micelles, derived from the dietary fat, followed by absorption in the small intestine due to a higher solubility [39]. This principle of using a lipid carrier is already used as an effective encapsulation method for higher phenolic absorption.
References
- Wetters, S.; Thomas, H.; Peter, N. Goji Who? Morphological and DNA Based Authentication of a “Superfood”. Front. Plant Sci. 2018, 9, 1859.
- Tichy, H.V.; Bruhs, A.; Palisch, A. Development of Real-Time Polymerase Chain Reaction Systems for the Detection of So-Called “Superfoods” Chia and Quinoa in Commercial Food Products. J. Agric. Food Chem. 2020.
- Jafari, S.M.; McClements, D.J. Nanotechnology Approaches for Increasing Nutrient Bioavailability, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 81.
- Marian, M.J. Dietary Supplements Commonly Used by Cancer Survivors: Are There Any Benefits? Nutr. Clin. Pract. 2017, 32, 607–627.
- Pannu Naveet, A.B. Resveratrol: From Enhanced Biosynthesis and Bioavailability to Multitargeting Chronic Diseases. Biomed. Pharmacother. 2019, 109, 2237–2251.
- Weiskirchen Sabine, R.W. Resveratrol: How Much Wine Do You Have to Drink to Stay Healthy? Adv. Nutr. 2016, 7, 706–718.
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 71.
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901.
- Kumari, A.; Yadav, S.K.; Pakade, Y.B.; Singh, B.; Yadav, S.C. Development of Biodegradable Nanoparticles for Delivery of Quercetin. Colloids Surf. B Biointerfaces 2010, 2, 184–192.
- Shahidi, F.; Ambigaipalan, P. Phenolics and Polyphenolics in Foods, Beverages and Spices: Antioxidant Activity and Health Effects—A Review. J. Funct. Foods 2015, 18, 820–897.
- Gonzales, G.B.; Raes, K.; Coelus, S.; Struijs, K.; Smagghe, G.; Van Camp, J. Ultra(High)-Pressure Liquid Chromatography-Electrospray Ionization-Time-of-Flight-Ion Mobility-High Definition Mass Spectrometry for the Rapid Identification and Structural Characterization of Flavonoid Glycosides from Cauliflower Waste. J. Chromatogr. A 2014, 1323, 39–48.
- Faridi Esfanjani, A.; Assadpour, E.; Jafari, S.M. Improving the Bioavailability of Phenolic Compounds by Loading Them within Lipid-Based Nanocarriers. Trends Food Sci. Technol. 2018, 76, 56–66.
- Bao, C.; Jiang, P.; Chai, J.; Jiang, Y.; Li, D.; Bao, W.; Liu, B.; Liu, B.; Norde, W.; Li, Y. The Delivery of Sensitive Food Bioactive Ingredients: Absorption Mechanisms, Influencing Factors, Encapsulation Techniques and Evaluation Models. Food Res. Int. 2019, 120, 130–140.
- Koolaji, N.; Shammugasamy, B.; Schindeler, A.; Dong, Q.; Dehghani, F.; Valtchev, P. Citrus Peel Flavonoids as Potential Cancer Prevention Agents. Curr. Dev. Nutr. 2020, 4, 1–20.
- Parada, J.; Aguilera, J.M. Food Microstructure Affects the Bioavailability of Several Nutrients. J. Food Sci. 2007, 72, 21–32.
- Heaney, P.R. Factors Influencing the Measurement of Bioavailability, Taking Calcium as a Model. J. Nutr. 2001, 131, 1344S–1348S.
- Wang, S.; Su, R.; Nie, S.; Sun, M.; Zhang, J.; Wu, D.; Moustaid-Moussa, N. Application of Nanotechnology in Improving Bioavailability and Bioactivity of Diet-Derived Phytochemicals. J. Nutr. Biochem. 2014, 25, 363–376.
- Ozkan, G.; Franco, P.; Capanoglu, E.; De Marco, I. PVP/Flavonoid Coprecipitation by Supercritical Antisolvent Process. Chem. Eng. Process. Process Intensif. 2019, 146.
- Edelman, R.; Engelberg, S.; Fahoum, L.; Meyron-Holtz, E.G.; Livney, Y.D. Potato Protein- Based Carriers for Enhancing Bioavailability of Astaxanthin. Food Hydrocoll. 2019, 96, 72–80.
- Ahmed, K.; Li, Y.; McClements, D.J.; Xiao, H. Nanoemulsion- and Emulsion-Based Delivery Systems for Curcumin: Encapsulation and Release Properties. Food Chem. 2012, 132, 799–807.
- Manthey, J.A.; Cesar, T.B.; Jackson, E.; Mertens-Talcott, S. Pharmacokinetic Study of Nobiletin and Tangeretin in Rat Serum by High-Performance Liquid Chromatography-Electrospray Ionization-Mass Spectrometry. J. Agric. Food Chem. 2011, 59, 145–151.
- Yang, C.S.; Sang, S.; Lambert, J.D.; Lee, M.J. Bioavailability Issues in Studying the Health Effects of Plant Polyphenolic Compounds. Mol. Nutr. Food Res. 2008, 52 (Suppl. 1), 139–151.
- Erfanian, A.; Mirhosseini, H.; Manap, M.Y.A.; Rasti, B.; Bejo, M.H. Influence of Nano-Size Reduction on Absorption and Bioavailability of Calcium from Fortified Milk Powder in Rats. Food Res. Int. 2014, 66, 1–11.
- Artursson, P.; Palm, K.; Luthman, K. Caco-2 Monolayers in Experimental and Theoretical Predictions of Drug Transport. Adv. Drug Deliv. Rev. 2012, 64, 280–289.
- Laksitorini, M.; Prasasty, V.D.; Kiptoo, P.K.; Teruna, J.S. Pathways and Progress in Improving Drug Delivery through the Intestinal Mucosa and Blood–Brain Barriers. Ther. Deliv. 2014, 5, 1143–1163.
- Spencer, J.P.E.; Schroeter, H.; Shenoy, B.S.; Srai, S.K.; Debnam, E.S.; Rice-Evans, C. Epicatechin Is the Primary Bioavailable Form of the Procyanidin Dimers B2 and B5 after Transfer across the Small Intestine. Biochem. Biophys. Res. Commun. 2001, 285, 588–593.
- Wen, X.; Walle, T. Methylated Flavonoids Have Greatly Improved Intestinal Absorption and Metabolic Stability. Drug Metab. Dispos. 2006, 34, 1786–1792.
- Appeldoorn, M.M.; Vincken, J.P.; Gruppen, H.; Hollman, P.C.H. Procyanidin Dimers A1, A2, and B2 Are Absorbed without Conjugation or Methylation from the Small Intestine of Rats. J. Nutr. 2009, 139, 1469–1473.
- Monagas, M.; Urpi-Sarda, M.; Sánchez-Patán, F.; Llorach, R.; Garrido, I.; Gómez-Cordovés, C.; Andres-Lacueva, C.; Bartolomé, B. Insights into the Metabolism and Microbial Biotransformation of Dietary Flavan-3-Ols and the Bioactivity of Their Metabolites. Food Funct. 2010, 1, 233–253.
- Actis-Goretta, L.; Lévèques, A.; Rein, M.; Teml, A.; Schäfer, C.; Hofmann, U.; Li, H.; Schwab, M.; Eichelbaum, M.; Williamson, G. Intestinal Absorption, Metabolism, and Excretion of (-)-Epicatechin in Healthy Humans Assessed by Using an Intestinal Perfusion Technique. Am. J. Clin. Nutr. 2013, 98, 924–933.
- Wen, X.; Walle, T. Methylation Protects Dietary Flavonoids from Rapid Hepatic Metabolism. Xenobiotica 2006, 36, 387–397.
- Roowi, S.; Mullen, W.; Edwards, C.A.; Crozier, A. Yoghurt Impacts on the Excretion of Phenolic Acids Derived from Colonic Breakdown of Orange Juice Flavanones in Humans. Mol. Nutr. Food Res. 2009, 53 (Suppl. 1), 68–75.
- Tamura, M.; Nakagawa, H.; Tsushida, T.; Hirayama, K.; Itoh, K. Effect of Pectin Enhancement on Plasma Quercetin and Fecal Flora in Rutin-Supplemented Mice. J. Food Sci. 2007, 72, 648–651.
- Bitsch, R.; Netzel, M.; Frank, T.; Strass, G.; Bitsch, I. Bioavailability and Biokinetics of Anthocyanins From Red Grape Juice and Red Wine. J. Biomed. Biotechnol. 2004, 5, 293–298.
- Piazza, C.; Privitera, M.G.; Melilli, B.; Incognito, T.; Marano, M.R.; Leggio, G.M.; Roxas, M.A.; Drago, F. Influence of Inulin on Plasma Isoflavone Concentrations in Healthy Postmenopausal Women. Am. J. Clin. Nutr. 2007, 86, 775–780.
- Cai, K.; Bennick, A. Effect of Salivary Proteins on the Transport of Tannin and Quercetin across Intestinal Epithelial Cells in Culture. Biochem. Pharmacol. 2006, 72, 974–980.
- Keogh, J.B.; McInerney, J.; Clifton, P.M. The Effect of Milk Protein on the Bioavailability of Cocoa Polyphenols. J. Food Sci. 2007, 72.
- Chen, D.; Xi, H.; Guo, X.; Qin, Z.; Pang, X.; Hu, X.; Liao, X.; Wu, J. Comparative Study of Quality of Cloudy Pomegranate Juice Treated by High Hydrostatic Pressure and High Temperature Short Time. Innov. Food Sci. Emerg. Technol. 2013, 19, 85–94.
- Content, F.; Lesser, S.; Cermak, R.; Wolffram, S. Nutrient Metabolism—Research Communication Bioavailability of Quercetin in Pigs. J. Nutr. 2004, 134, 1508–1511.





