Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Qazi Mohammad Sajid Jamal | -- | 3725 | 2022-11-17 07:30:29 | | | |
| 2 | Peter Tang | Meta information modification | 3725 | 2022-11-17 08:54:16 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jamal, Q.M.S. Antiviral Potential of Plants against COVID-19 during Outbreaks. Encyclopedia. Available online: https://encyclopedia.pub/entry/35035 (accessed on 08 February 2026).
Jamal QMS. Antiviral Potential of Plants against COVID-19 during Outbreaks. Encyclopedia. Available at: https://encyclopedia.pub/entry/35035. Accessed February 08, 2026.
Jamal, Qazi Mohammad Sajid. "Antiviral Potential of Plants against COVID-19 during Outbreaks" Encyclopedia, https://encyclopedia.pub/entry/35035 (accessed February 08, 2026).
Jamal, Q.M.S. (2022, November 17). Antiviral Potential of Plants against COVID-19 during Outbreaks. In Encyclopedia. https://encyclopedia.pub/entry/35035
Jamal, Qazi Mohammad Sajid. "Antiviral Potential of Plants against COVID-19 during Outbreaks." Encyclopedia. Web. 17 November, 2022.
Copy Citation
COVID-19 has become a pandemic in most parts of the world. Although vaccines are available to fight the infection, their safety and clinical trial data are still questionable. Social distancing, isolation, the use of sanitizer, and personal productive strategies have been implemented to prevent the spread of the virus. Moreover, the search for a potential therapeutic molecule is ongoing. Based on experiences with outbreaks of SARS and MERS, many research studies reveal the potential of medicinal herbs/plants or chemical compounds extracted from them to counteract the effects of these viral diseases.
antiviral
medicinal plants
COVID-19
SARS
MERS
1. Introduction
Several human diseases are caused by viruses, including cancer, Type I diabetes, Alzheimer’s disease, and hepatocellular carcinoma. In the past, people have greatly suffered from viral diseases such as polio, mumps, measles, dengue fever, SARS, MERS, AIDS, chikungunya fever, encephalitis, and influenza. Human cancer viruses include the hepatitis B virus, the Epstein-Barr virus, the hepatitis C virus, the human T-cell lymphotropic virus type 1, high-risk human papilloma viruses, and Kaposi’s sarcoma-associated herpesvirus [1]. Human enterovirus (HEV) has long been thought to act as an environmental trigger for the onset of Type 1 diabetes (T1D) in people [2]. HHV-6A and HHV-7, two human herpesviruses, may be major causes of AD; however, HSV infection with other viral infections has been documented in some of these cases [3]. The disease 2019-nCoV originated in Wuhan, China, at the end of December 2019, and the WHO declared it an international emergency with public health concerns and issued International Health Regulations [4]. The disease is a pandemic and ongoing; therefore, it is critical to search for new preventive and therapeutic methods as soon as possible. The virus causing 2019-nCov was identified as a β-coronavirus, specifically severe acute respiratory syndrome virus 2 (SARS-CoV-2), belonging to the coronavirus family. COVID-19 is characterized by a series of complex clinical symptoms, including fever, pneumonia, dry cough, and shortness of breath. As of 27 July 2022, the World Health Organization announced 570,005,017 confirmed cases and 6,384,128 deaths worldwide. Few infected people were treated, and few treatments are currently available [5][6].
Recently, an immunization program has led to extensive successful outcomes. However, since its emergence, SARS-CoV-2, the virus causing COVID-19, has challenged public health. Recently, an outbreak of COVID-19 has driven the focus toward traditional herbs that are available in different parts of the world. Many therapeutic and diagnostic strategies are available to manage the disease [7][8]. Moreover, studies have reported that SARS-CoV-2 infection can be spread to reptiles, avian species, and mammals [9]. At least 21 families of viruses can cause disease in humans. Of these, five viral families consist of dsDNA, three families comprise nonenveloped viruses (Adenoviridae, Papillomaviridae, and Polyomaviridae), and two families comprise enveloped viruses (Herpesviridae and Poxviridae). One family consists of partially double-stranded (ds) DNA (Hepadnaviridae and enveloped viruses). The members of seven families are single-stranded RNAs, of which three families are characterized as nonenveloped (Astroviridae, Caliciviridae, and Picornaviridae), and the members of four families are enveloped (Retroviridae, Coronaviridae, and Flaviviridae). All nonenveloped families carry icosahedral nucleocapsids and are in negative ssRNA families (Arenaviridae, Bunyavirales, Filoviridae, Orthomyxoviridae, Paramyxoviridae, and Rhabdoviridae) and are enveloped with helical nucleocapsids. One virus, a dsRNA (Reoviridae, which causes hepatitis D), has not been assigned to a family [8][9]. SARS-CoV-2, the virus causing COVID-19 has been identified as an RNA virus with a special spike protein, nucleocapsid protein, membrane protein, envelope protein, and enzymatic proteins, which have been reported to be therapeutic targets that control disease (Figure 1). Secondary metabolites are synthesized by medicinal plants for plant defense. Many therapeutic molecules, such as antibacterial, antifungal, and antiviral molecules, have been isolated, purified, characterized, and used in the management of various diseases.

Figure 1. Structural features of a coronavirus.
2. Therapeutic Targets for Coronaviruses
Human coronavirus was first detected in the 1960s and was broadly classified into four categories, i.e., alpha, beta, gamma, and delta. There are currently seven known coronaviruses that can harm humans, namely, 229E (alpha coronavirus), NL63 (alpha coronavirus), OC43 (beta coronavirus), HKU1 (beta coronavirus), MERS-CoV (the beta coronavirus that causes Middle East Respiratory Syndrome, or MERS), SARS-CoV (the beta coronavirus that causes severe acute respiratory syndrome, or SARS), and SARS [10].
Molecular level investigation and characterization of the SARS-CoV-2 genome is almost 80% similar to the genetic content of SARS-CoV-2 with characteristics 10b, 13, and 14 different gene regions [11].
SARS-CoV and SARS-CoV-2 viruses use human ACE2 as an entry receptor and human proteases as entry activators, which differentiate from each other [12][13]. Moreover, the most important structural protein, i.e., the spike protein (S), is slightly different in these viruses. SARS-CoV-2 has a furin-like cleavage site that makes the S protein priming easier and may make it more effective at spreading than other beta coronaviruses. Furin inhibitors are thus a possible target for SARS-CoV pharmacological treatments [14][15].
However, both viruses utilize a spike receptor-binding domain for recognition and host cell infection with the support of cellular serine proteases [13].
Currently, no trusted therapeutic intervention is available to target and manage COVID-19. For the development of effective therapy against any disease, the drug-targeting sites or cell-binding proteins must be identified. Recently, the COVID-19 virus genome and potential cell-binding proteins have been discussed, which will help in the design of therapeutics and treatment strategies for this disease (Figure 1).
3. Antiviral Potential of Plant Extracts/Metabolites for Treating SARS-CoV-2 Infection
As COVID-19 rapidly spread, the search for natural antiviral molecules that can fight a SARS-CoV-2 infection was accelerated. Several in silico and in vitro/in vivo studies on plants as well as repurposed drugs have been conducted to identify potential therapeutic molecules. For a significant effect, the binding interactions must be facilitated between ligand and drug molecules. The SARS-CoV-2 infection has also been studied for evaluating drug targets such as spike protein, envelope protein, membrane protein, nucleocapsid protein, and proteases, using various cellular and animal models. Although in vitro studies are helpful for understanding virus biology in highly controlled environments, these models frequently fall short of accurately recapturing the complexity of true bodily systems. However, in vivo research is expensive, requires BSL-3 animal facilities, and raises ethics questions. Two-dimensional (2D) or three-dimensional (3D) cultivation of immortalized cells or primary cells and tissues serves as the foundation for in vitro models [16]. Recently, hydroxychloroquine and chloroquine, and glycyrrhizin, have been evaluated through in vitro studies. turmeric (Curcuma longa L.; Zingiberaceae.) rhizomes, mustard (Brassica nigra W.D.J. Koch; Brassicaceae), and wall rocket (Diplotaxis erucoides subsp. erucoides; Brassicaceae), were reported to considerably suppress 3CLPro activity when administered at 500 g mL−1 [17].
A study proposed that a potential approach involves garlic essential oil. The active molecule in this essential oil simultaneously inhibited ACE-2 protein activity, leading to a loss of this viral receptor in the host cell, and attacked the receptor with PDB ID 6LU7 (the main protease in SARS-CoV-2) (Figure 2). A previous docking study showed that 17 chemical components from 18 chemical constituents of oils inhibited ACE-2 protein–virus binding, and these 17 compounds account for 99.4% of all essential oils [18][19]. Many chemicals extracted from traditional herbal spices, such as allicin, E/Z-ajoene, allin, Diallyl disulfide, Diallyl trisulfide, pyrogallol, protocatechuic acid, quercetin, and gallic acid from the Allium cepa L. bulb (Amaryllidaceae), have been reported to show significant antiviral and antibacterial activities, which could also be active against SARS-CoV-2. Moreover, Singh et al. discussed its antimicrobial potential [20].
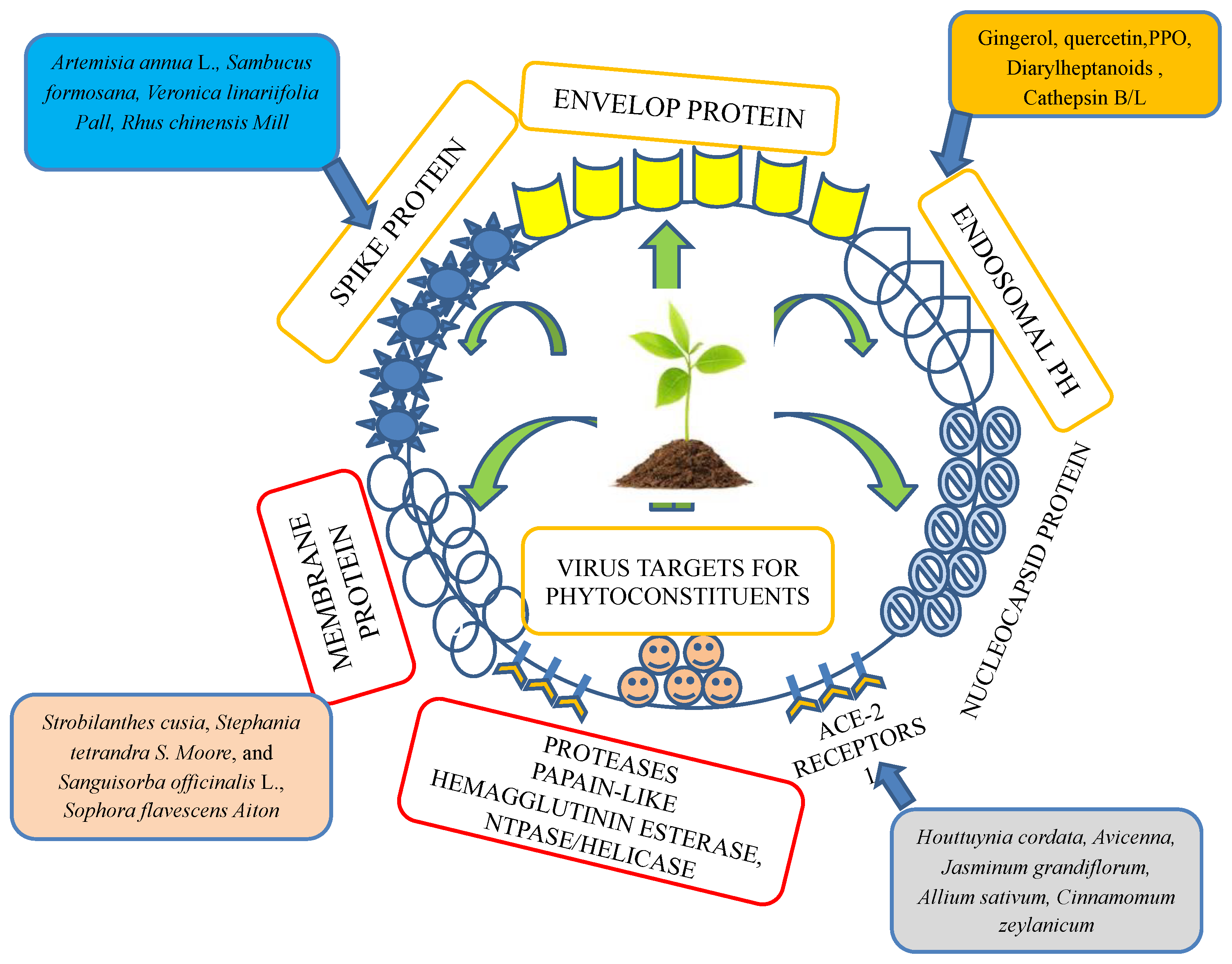
Figure 2. Plant- and herb-targeted envelope protein, spike protein, membrane protein, protease inhibitors, ACE-2 receptors, nuclear capsid protein, and endosomal-like parts of a virus.
Piper longum L. Piperaceae, a fruit commonly known as Indian spice kali mirch, has been reported to show antiviral activity against Coxsackie virus type 3 (CVB3) due to the presence of α-pinene, β-pinene, limonene, myrcene, sabinene, camphene, α-thujone, piperitone, caryophyllene, p-cymene, α-terpinene, and piperamide [21]. A study on curcumin described its inhibitory action on viruses in addition to SARS-CoV-2. Curcuma longa L. (Zingiberaceae) is enriched with curcumenone, bisacumol, bisacurone, curcumenol, curcumadiol, and demethoxycurcumin. Curcumin inhibits SARS-CoV-2 replication in human cells, as previously reported for HIV-AIDS [21][22], chikungunya virus, Zika virus, and herpes simplex virus (HSV).
Moreover, curcumin has been reported to inhibit the penetration of SARS-CoV-2 into host cells to prevent infection. The prevention of chikungunya and Zika virus infectivity by curcumin has been reported, and this feature of turmeric may be beneficial for the therapeutic inactivation of COVID-19 [23].
Syzygium aromaticum (L.) Merr. & L.M. Perry (Myrtaceae) flowering buds have been reported to contain eugenol, acetyl eugenol, β-caryophyllene, vanillin, eugenin, kaempferol, rhamnetin, and eugenitin, and they have antiviral potential against SARS-CoV-2, herpes simplex virus I, herpes simplex virus 2, and the hepatitis C virus.
A docking study reported that eugenol shows low binding energies with viral proteins; for example, its binding energies were −6.1 and −5.4 kcal/mol for the S protein (PDB ID 6VXX) and Mpro (PDB ID 6LU7), respectively [24]. However, this low binding energy showed a less significant effect than the even lower binding energies of nelfinavir with these proteins, which were −8.8 and −8.2 kcal/mol, respectively.
Sage essential oils as phytomedicines have recently been discussed, particularly as antiseptics and sanitizers. The essential oil in Salvia officinalis L. has been reported to exert an effect against SARS-CoV in a patient at the Frankfurt University Hospital, showing an IC50 = 870 mg/mL, which was low [25][26]. The main chemical compounds in the essential oil extracted from Laurus nobilis L. that have been reported to inhibit SARS-CoV and HSV-1 replication were 1,8-cineole, beta-ocimene, beta-pinene and alpha-pinene, each with t an IC(50) value of 120 µg/mL [27]. Thus, it can be concluded from previously conducted research that essential oils have limited antiviral activities against viruses, including SARS-CoV-2 [25]. Thus, symptomatic alleviation after infection may be achieved by using essential oils such as eucalyptus oil, eugenol, cinnamon oils, and neem oil.
Zingiber officinale Roscoe from Zingiberaceae and rhizome have been reported to contain therapeutic chemicals, including 6-gingerol, 6-shogaol, 6-paradol, zingerol, and gingerol, with activity against SARS-CoV-2 and human respiratory syncytial virus (HSRV). The antiviral potential of Zingiber officinale Roscoe against viruses, including SARS-CoV-2, has been described on the basis of computational approaches. The results of docking studies have indicated that 6-Sogaol showed binding energies of −5.5 and −5.8 kcal/mol to the S protein (PDB ID 6VXX) and Mpro (PDB ID 6LU7), which were comparable to the binding energies of nelfinavir for these proteins (−8.8 and −8.2 kcal/mol, respectively). In silico studies indicated that eight chemicals extracted from rhizomes of Alpinia officinarum Hance, Zingiberaceae, and gingerol were potential protease (PLpro) inhibitors of SARS-CoV-2. Thus, the aforementioned studies showed that phytoconstituents or their extracts were SARS-CoV-2 inhibitors, suggesting that these compounds are promising therapeutic molecules against SARS-CoV-2 [28][29]. The role of Schizachyrium urceolatum Stapf Poaceae as an antioxidant suggests that cinnamon shows appreciable immunostimulatory activity by increasing phagocytic activity [30].
The root and flower of clove pink, also known as Dianthus caryophyllus L., has been reported to contain dianthin30, dianthin32, dianthramides, flavonoids against SARS, herpes simplex virus-I (HSV-I), hepatitis-A Virus-27 (HSV-27) [22]. The antiviral activity against herpes simplex virus-I and hepatitis A virus-27 of crude seed extract are found in this plant. At a nontoxic concentration (20 µg/mL), the extract applied to both Vero and HepG-2 cells led to potent antiviral effects on HSV-I and HAV-27, as determined using a plaque infectivity count assay [22]. Dianthus caryophyllus L. as reported to exhibit antiviral activity against HSV-I and HAV-27, so this plant could also be a potential source for antiviral activity against SARS-CoV-2.
The Phytolacca americana L. Phytolaccaceae plant synthesizes ribosome-inactivating protein (RIP). A single-chain ribosome-inactivating protein has been analyzed. Studies provided evidence showing that exogenous application of RIP inhibits zucchini yellow mosaic virus (ZYMV) infection in squash plants in a concentration-dependent manner [31]. The antiviral activity of the Mirabilis jalapa L. plant contains 4-hydroxycoumarin, mirabijalones A-D, and 9-o-methyl-4-hydroxyboeravinone B. The root extract of these compounds inhibits the multiplication of tobacco mosaic virus via the inactivation of ribosomes that had been stimulated by the virus [32]. The antiviral effect of Camellia sinensis (L.) Kuntze extract, also known as green tea, has been reported to inhibit the expression of hepatitis surface antigen (HBsAg) and hepatitis B antigen (HbeAg). Studies reported that the extract exerted an inhibitory effect on intestinal α-glycosidases that are important for processing glycoproteins and glycolipids in viruses [28][33][34]. Kim et al. reported that the methanolic extracts of Acanthopanax (Decne. & Planch.) Miq. Araliaceae and Cimicifuga acemose (L.) Nutt. were administered at doses of 0.9 ± 0.1 μg/mL and 19.4 ± 7.0 μg/mL, inhibiting MHV-A59-type coronavirus, respectively. The IC50 of the ethanolic extract of Artemisia annua L. against SARS-CoV BJ-001 was 34.5 ± 2.6 μg/mL [35][36]. Euphorbia neriifolia L., Euphorbiaceae enriched with 3-β-friedelanol, 3-β-acetoxy friedelane, friedelin, and epitaraxerol-like chemical compounds has also been described to significantly inhibit HCoV [37]. The IC50 of an aqueous extract with 191.6 ± 8.2 μg/mL of Isatis indigotica Fortune ex Lindl; Brassicaceae and other bioactive molecules, e.g., 752 μM Indigo, 217 μM Sinigrin, 1210 μM beta-sitosterol, 366 μM aloe emodin, and 8.3 μM Hesperetin, indicated the antiviral potentiality of this plants against SARS-CoV. Among the compounds from this plant that were tested, low-dose hesperetin showed the greatest antiviral molecule activity. The aquas extract of Polygonum multiflorum Thunb. Polygonaceae (root/vine) has been reported to exhibit significant antiviral activity (IC50 1–10 μg/mL) against SARS-CoV [29]. Chen et al. reported the antiviral effect of a water-based extract of Toona sinensis (Juss.) against SARS-CoV, which showed efficacy at an IC50 of 30–43 μg/mL [38]. When a 6% Epimedium aqueous extract was administered in the test model it led to no diarrhoeal symptoms [39], and intestinal biopsy sample assays revealed complete eradication of the virus from the intestine of the tested animals. The roots of Polypodium glycyrrhiza D.C.Eaton (Licorice) have been used as a traditional medicine for bronchitis, peptic ulcers, allergies, inflammation, and asthma for a long time. Licorice root has been known to be a powerful viral infection inhibitor since ancient times [40][41][42][43]. Glycyrrhizic acid, also referred to as glycyrrhizin, is a key chemical compound in the triterpenoid glycoside class of herbs. SARS-CoV has reportedly been suppressed with glycyrrhizin [44]. Replication of clinical isolates of SARS-CoV have been reportedly inhibited by glycyrrhizin more effectively than a number of synthetic antivirals, including mycophenolic acid, pyrazofurin, 6-azauridine, and ribavirin [45]. Many plants and foods contain high levels of the flavonoid quercetin, which shows a wide range of therapeutic and pharmaceutical effects [46]. Both quercetin 3-galactoside and quercetin abrogate the SARS-CoV 3CLpro protease function in vitro [47].
Wen et al. discovered that specific lignoids and abietane-type diterpenoids show the strongest SARS-CoV-2 inhibiting activities, and they are present in Cryptomeria japonica (Thunb. ex L.f.) D.Don; Cupressaceae Juniperus formosana Hayata Cupressaceae, and Chamaecyparis obtusa Siebold & Zucc. Cupressaceae [48]. Moreover, other bioactive substances related to the aforementioned compound classes are present in Cibotium barometz (L.) J.Sm. Cibotiaceae (dried rhizome), Gentiana scabra Bunge, Gentianaceae (dried rhizome), Dioscorea batatas Decne., Dioscoreaceae (tuber), Taxillus chinensis (DC.) Danser, Loranthaceae (leaf), and Cassia tora L. Fabaceae (dried seed), which explains the anti-SARS-CoV potential confirmed through a cell-based assay with infected Vero E6 cells at concentrations between 25 [48]. Through their potential anti-inflammatory molecules, Boswellia serrata extracts may be promising compounds for the management of inflammatory complications during COVID-19 [49].
The antiviral effect of Bupleurum falcatum subsp. cernuum (Ten.) Arcang; Apiaceae (Radix bupleuri) has been reported to explain the presence of saikosaponins A, B2, C, and D. These active constituents are naturally derived triterpenoid glycosides recovered from the Radix bupleuri plant. By targeting viruses, these compounds effectively prevent the early stage of HCoV-22E9 infection after facilitating the entry of the virus through viral attachment to the host cell [44][50][51].
Clinical studies on Glycyrrhiza glabra L. (Fabaceae) against coronavirus revealed that glycyrrhizin controlled viral SARS-CoV replication. A comparative study was performed using ribavirin, 6-aziridine, pyrazofurin, mycophenolic acid, and glycyrrhizin, to analyze their antiviral activities against coronaviruses (FFM-1 and FFM-2) obtained from clinical patients suffering from SARS. At the end of the study, it was demonstrated that glycyrrhizin at nontoxic concentrations inhibited the replication of SARS-CV; its selectivity index was 67 and its IC50 was 300 mg/mL [45][52]. Glycyrrhizin derivatives, such as glycyrrhetinic acid, derivative GL 1, derivative GL 3, derivative GL 9, derivative GL 10, derivative GL11, derivative GL, and derivative GL 13, from Myosotis radix-palaris A.P.Khokhr; Boraginaceae (Radix scutellariae), are traditional Chinese medicines. The active constituent of this plant is baicalin. In a molecular docking study, baicalin exhibited a binding energy of −8.46 kcal/mol, which was sufficient to bind the receptor ACE-2 in the host cell; thus, baicalin may be a potential candidate for 2019-nCoV treatment. Recently, in vitro antiviral activity against SARS-CoV-2 3CLpro, causing pandemic disease, showed that Scutellaria baicalensis potentially inhibited the virus with an EC50 of 0.74 µg/mL [53][54]. This citrus plant is abundant in China and has been reported to exhibit anti-SARS activity. Another Chinese herb with reported antiviral activity is Houttuynia cordata Thunb.; Saururaceae, which inhibited viral protease 3CL activity and blocked the activity of viral enzyme RNA-dependent RNA polymerase [55][56]. Similarly, Rheum and Polygonum spp. inhibited hepatitis B virus (HBV) in vitro [56]. The papain-like protease (PLpro), which controls the replication of SARS-CoV, has been described as a potential therapeutic target. Diarylheptanoids isolated from Alnus japonica Siebold & Zucc.; Betulaceae showed potent (PLpro) activity, with an inhibitory concentration (IC50) value of 4.1 µM. Oleo europaea has also been shown to be a natural antioxidant [57][58][59].
Thus, the aforementioned compounds clearly indicate the potential of plants/herbs in controlling the disease. Although their compounds are not magic potions to treat disease, they may reduce discomfort and perhaps enhance patients’ overall wellbeing. Only extremely chemically well-characterized and pharmacologically well-researched high-quality preparations are suitable for use as herbal remedies that can be considered medicines. Thorough characterization of formulations in future pharmacological and clinical research is crucial [60].
4. Plants as Biological Factories for the Production of Immunotherapeutics: Applications to SARS-CoV
Various immunomodulators, antibodies, interferons, and other therapeutic proteins have been used in the management of various diseases, including virus-induced diseases. Clinical trials for treatments of MERS and SARS-CoV have revealed the role of interferon in reducing the severity of the diseases caused by these coronaviruses.
Plants have been used as biological factories for the production of immunotherapeutics. Various plant-derived vaccines have been assessed in clinical trials, and a few are now marketed as medications and in conjunction with medical devices for the treatment of infectious and chronic diseases. Plant-based vaccines might enable the rapid production of biological products on an industrial scale, which may help meet needs in urgent situations such as the COVID-19 epidemic. Some vaccinations, including those used for cholera, anthrax, Lyme disease, tetanus, rotavirus, canine parvovirus, and plague, were created through direct particle bombardment or biological means [61]. Vaccines for Ebola, tuberculosis, avian flu, and dengue fever are manufactured indirectly or directly via Agrobacterium-mediated gene transfer [61][62].
One study demonstrated that IFN-α inhibited the replication of human- as well as animal-infecting coronaviruses [63]. Another study reported that interferon-α-2a administered with ribavirin increased survival in patients with MERS-CoV [64]. The very first recombinant medicinal protein made from a plant was human interferon, which was created in turnips. Moreover, tobacco plants and potatoes have been used to manufacture human serum albumin for human use. Similarly, tobacco plants were used to generate the first medication (ZMapp) used experimentally to treat Ebola virus infection.
Intravenous gamma globulin (InIg) has also been studied. It was developed in 1970 but gained popularity during the outbreak of SARS in 2003, when it was used extensively in Singapore. However, severe adverse reactions were noticed during its use [65]. Scientists have developed methods for the production of gamma globulin using plant-based molecular farming.
A peptide hormone, thymosin-α-1, has been isolated from thymic tissues, and its immunomodulatory role has been thoroughly explored [66]. Moreover, a synthetic pentapeptide such as thymopentin interacting with the active site in thymopoietin has been found to boost the production of antibodies for hepatitis B vaccines [67]. Plant bioreactors have been successfully applied to the production of thymosin-α-1 [68][69].
Due to the development and expansion of recombinant techniques, plants are now being assessed as potential alternative platforms for the manufacture of recombinant monoclonal antibodies (mAbs). In a study by Sui et al., r-mAb, a recombinant human monoclonal antibody against the S1 domain of the S protein of SARS-CoV was isolated. Moreover, this mAb had been found to efficiently neutralize SARS-CoV [70]. Recent research demonstrated that plant systems for producing mAbs for immunotherapy have been successfully developed [71].
The immunotherapeutics used as the basis for developing SARS-CoV-2 vaccines are thought to be potentially effective and safe. When considering the potential delivery of SARS-CoV-2 vaccines in the future, current guidelines for immunizing a host must be used [72].
For the first time, the recombinant production of a fully functional human form of the recently identified cytokine IL-37 in plant cells has been reported, with the cytokine functioning as a fundamental suppressor of innate immunity. Interleukin 37 (IL-37), a recently identified member in the interleukin (IL)-1 class, is essential for controlling innate inflammation and inhibiting acquired immunological responses and, therefore, it shows great potential for treating a variety of autoimmune diseases and inflammatory diseases [73]. Recombinant IL-7 is a potential immune therapeutic that acts to promote the proliferation of naive and memory T cells (CD8+ and CD4+ T cells) and may be effective in managing SARS-CoV-2 viral infection. IL-6 is a key mediator of inflammation in COVID-19, and its receptor antagonist (sarilumab/tocilizumab) and IL-6 inhibitors (clazakizumab/siltuximab/sirukumab) have been described as potential immunotherapeutics to manage SARS-CoV-2 viral infections [74].
The IL-1 inhibitors canakinumab (MoAb anti-IL-1beta) and anakinra (recombinant IL-1 receptor) inhibited IL-1β, a proinflammatory cytokine [75][76]. Other significant immunotherapeutic IL-1 inhibitors, such as canakinumab (MoAb anti-IL-1beta) and anakinra (a recombinant IL-1 receptor), as well as IL-18 inhibitors, have been reported to inhibit IL-1β. Suppression of signaling that triggers the cytokine stimulation of cytokines such as IL-7 and alters Type I IFN levels significantly enhanced the risk of thromboembolism after treatment with JAK/STAT inhibitors such as ruxolitinib, baricitinib, and tofacitinib [77]. Anti-VEGF, complement factor C3, C5, and complement system inhibitors are other classes of immune therapeutics that may be effective against COVID-19. Bamlanivimab antibodies have also been described as being effective against the spike protein of SARS-CoV-2. Tomato plants have been examined for use in the development of SARS vaccines; specifically, their ability to express SARS-CoV nucleocapsid proteins, and their immunogenicity for the development of vaccines have been assessed. A COVID-19 vaccine is now being developed by the Kentucky Bio-Processing Company, a British American Tobacco (BAT) subsidiary, using tobacco plants to express the SARS-CoV-2 protein subunit. The receptor binding protein or the sequence of the S1 protein (full polypeptide) may be the intended vaccine target [49]. Hence, these findings indicate that plants have enormous promise for the low-cost and high production of biologically active viral inhibitors.
References
- Sarid, R.; Gao, S.-J. Viruses and human cancer: From detection to causality. Cancer Lett. 2011, 305, 218–227.
- Isaacs, S.R.; Foskett, D.B.; Maxwell, A.J.; Ward, E.J.; Faulkner, C.L.; Luo, J.Y.; Rawlinson, W.D.; Craig, M.E.; Kim, K.W. Viruses and type 1 diabetes: From enteroviruses to the virome. Microorganisms 2021, 9, 1519.
- Readhead, B.; Haure-Mirande, J.-V.; Funk, C.C.; Richards, M.A.; Shannon, P.; Haroutunian, V.; Sano, M.; Liang, W.S.; Beckmann, N.D.; Price, N.D.; et al. Multiscale analysis of independent Alzheimer’s cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron 2018, 99, 64–82.e7.
- Paraskevis, D.; Kostaki, E.G.; Magiorkinis, G.; Panayiotakopoulos, G.; Sourvinos, G.; Tsiodras, S. Full-genome evolutionary analysis of the novel Corona virus (2019-ncov) rejects the hypothesis of emergence as a result of a recent recombination event. Infect. Genet. Evol. 2020, 79, 104212.
- Pal, M.; Berhanu, G.; Desalegn, C.; Kandi, V. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): An Update. Cureus 2020, 12, e7423.
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 21 September 2022).
- Christou, L. The global burden of bacterial and viral zoonotic infections. Clin. Microbiol. Infect. 2011, 17, 326–330.
- Sharma, R. Viral diseases and antiviral activity of some medicinal plants with special reference to ajmer. J. Antivir. Antiretrovir. 2019, 11, 185.
- Guzzi, P.H.; Mercatelli, D.; Ceraolo, C.; Giorgi, F.M. Master regulator analysis of the SARS-CoV-2/human Interactome. J. Clin. Med. 2020, 9, 982.
- Human Coronavirus Types. Available online: https://www.cdc.gov/coronavirus/types.html (accessed on 22 September 2022).
- Chan, J.F.-W.; Kok, K.-H.; Zhu, Z.; Chu, H.; To, K.K.-W.; Yuan, S.; Yuen, K.-Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020, 9, 221–236.
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448.
- Rossi, G.A.; Sacco, O.; Mancino, E.; Cristiani, L.; Midulla, F. Differences and similarities between SARS-COV and SARS-CoV-2: Spike receptor-binding domain recognition and host cell infection with support of cellular serine proteases. Infection 2020, 48, 665–669.
- Coutard, B.; Valle, C.; de Lamballerie, X.; Canard, B.; Seidah, N.G.; Decroly, E. The spike glycoprotein of the new coronavirus 2019-ncov contains a furin-like cleavage site absent in cov of the same clade. Antivir. Res. 2020, 176, 104742.
- Rabaan, A.A.; Al-Ahmed, S.H.; Haque, S.; Sah, R.; Tiwari, R.; Malik, Y.S.; Dhama, K.; Yatoo, M.I.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. SARS-CoV-2, SARS-CoV, and MERS-COV: A comparative overview. Infez. Med. 2020, 28, 174–184.
- Rosa, R.B.; Dantas, W.M.; do Nascimento, J.C.F.; da Silva, M.V.; de Oliveira, R.N.; Pena, L.J. In Vitro and In Vivo Models for Studying SARS-CoV-2, the Etiological Agent Responsible for COVID-19 Pandemic. Viruses 2021, 13, 379.
- Guijarro-Real, C.; Plazas, M.; Rodríguez-Burruezo, A.; Prohens, J.; Fita, A. Potential In Vitro Inhibition of Selected Plant Extracts against SARS-CoV-2 Chymotripsin-Like Protease (3CLPro) Activity. Foods 2021, 10, 1503.
- Yoo, D.Y.; Kim, W.; Nam, S.M.; Yoo, M.; Lee, S.; Yoon, Y.S.; Won, M.-H.; Hwang, I.K.; Choi, J.H. Neuroprotective effects of Z-ajoene, an organosulfur compound derived from oil-macerated garlic, in the gerbil hippocampal CA1 region after transient forebrain ischemia. Food Chem. Toxicol. 2014, 72, 1–7.
- Ankri, S.; Mirelman, D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999, 1, 125–129.
- Singh, P.; Singh, J.; Singh, S.; Singh, B.R. Medicinal values of garlic (Allium sativum L.) in human life: An overview. Greener J. Agric. Sci. 2014, 4, 265–280.
- Mair, C.E.; Liu, R.; Atanasov, A.G.; Schmidtke, M.; Dirsch, V.M.; Rollinger, J.M. Antiviral and anti-proliferative in vitro activities of piperamides from black pepper. Planta Med. 2016, 82, S1–S381.
- Barakat, A.B.; Shoman, S.A.; Dina, N.; Alfarouk, O.R. Antiviral activity and mode of action of Dianthus caryophyllus L. and Lupinus termes L. seed extracts against in vitro herpes simplex and hepatitis A viruses infection. J. Microbiol. Antimicrob. 2010, 2, 23–29.
- Mounce, B.C.; Cesaro, T.; Carrau, L.; Vallet, T.; Vignuzzi, M. Curcumin inhibits zika and chikungunya virus infection by inhibiting cell binding. Antivir. Res. 2017, 142, 148–157.
- Chandra Manivannan, A.; Malaisamy, A.; Eswaran, M.; Meyyazhagan, A.; Arumugam, V.A.; Rengasamy, K.R.; Balasubramanian, B.; Liu, W.-C. Evaluation of clove phytochemicals as potential antiviral drug candidates targeting SARS-CoV-2 main protease: Computational Docking, molecular dynamics simulation, and pharmacokinetic profiling. Front. Mol. Biosci. 2022, 9, 918101.
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential oils as antimicrobial agents—Myth or real alternative? Molecules 2019, 24, 2130.
- Orhan, I.E.; Senol Deniz, F.S. Natural products as potential leads against coronaviruses: Could they be encouraging structural models against SARS-CoV-2? Nat. Prod. Bioprospecting 2020, 10, 171–186.
- Loizzo, M.R.; Saab, A.M.; Tundis, R.; Statti, G.A.; Menichini, F.; Lampronti, I.; Gambari, R.; Cinatl, J.; Doerr, H.W. Phytochemical analysis andin vitro antiviral activities of the essential oils of seven Lebanon species. Chem. Biodivers. 2008, 5, 461–470.
- Khaerunnisa, S.; Kurniawan, H.; Awaluddin, R.; Suhartati, S.; Soetjipto, S. Potential Inhibitor of COVID-19 Main Protease (Mpro) from Several Medicinal Plant Compounds by Molecular Docking Study. Preprints 2020, 2020030226.
- Ho, T.; Wu, S.; Chen, J.; Li, C.; Hsiang, C. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antivir. Res. 2007, 74, 92–101.
- Aggarwal, B.B.; Van Kuiken, M.E.; Iyer, L.H.; Harikumar, K.B.; Sung, B. Molecular targets of nutraceuticals derived from dietary spices: Potential role in suppression of inflammation and tumorigenesis. Exp. Biol. Med. 2009, 234, 825–849.
- Mansouri, S.; Choudhary, G.; Sarzala, P.M.; Ratner, L.; Hudak, K.A. Suppression of human T-cell leukemia virus I gene expression by pokeweed antiviral protein. J. Biol. Chem. 2009, 284, 31453–31462.
- Kaladhar, D.; Nandikolla, S.K. Antimicrobial studies, Biochemical and image analysis in Mirabilis lalapa Linn. Int. J. Farm. Tech. 2010, 2, 683–693.
- Tran, J. Green tea: A potential alternative anti-infectious agent catechins and viral infections. Adv. Anthropol. 2013, 3, 198–202.
- Jin, S. Therapeutic potential of natural catechins in antiviral activity. JSM Biotechnol. Biomed. Eng. 2013, 1, 1002.
- Kim, H.-Y.; Eo, E.-Y.; Park, H.; Kim, Y.-C.; Park, S.; Shin, H.-J.; Kim, K. Medicinal herbal extracts of sophorae radix ACANTHOPANACIS cortex sanguisorbae radix and Torilis fructus inhibit coronavirus replication in vitro. Antivir. Ther. 2010, 15, 697–709.
- Kim, H.-Y.; Shin, H.-S.; Park, H.; Kim, Y.-C.; Yun, Y.G.; Park, S.; Shin, H.-J.; Kim, K. In vitro inhibition of coronavirus replications by the traditionally used medicinal herbal extracts, CIMICIFUGA rhizoma, meliae cortex, coptidis rhizoma, and Phellodendron Cortex. J. Clin. Virol. 2008, 41, 122–128.
- Chang, F.-R.; Yen, C.-T.; EI-Shazly, M.; Lin, W.-H.; Yen, M.-H.; Lin, K.-H.; Wu, Y.-C. Anti-human coronavirus (anti-hcov) triterpenoids from the leaves of Euphorbia neriifolia. Nat. Prod. Commun. 2012, 7, 1415–1417.
- Chen, C.-J.; Michaelis, M.; Hsu, H.-K.; Tsai, C.-C.; Yang, K.D.; Wu, Y.-C.; Cinatl, J.; Doerr, H.W. Toona sinensis roem tender leaf extract inhibits SARS coronavirus replication. J. Ethnopharmacol. 2008, 120, 108–111.
- Cho, J.K.; Curtis-Long, M.J.; Lee, K.H.; Kim, D.W.; Ryu, H.W.; Yuk, H.J.; Park, K.H. Geranylated flavonoids displaying SARS-COV papain-like protease inhibition from the fruits of paulownia tomentosa. Bioorganic Med. Chem. 2013, 21, 3051–3057.
- Fiore, C.; Eisenhut, M.; Krausse, R.; Ragazzi, E.; Pellati, D.; Armanini, D.; Bielenberg, J. Antiviral effects ofglycyrrhiza species. Phytother. Res. 2008, 22, 141–148.
- Asl, M.N.; Hosseinzadeh, H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother. Res. 2008, 22, 709–724.
- Das, S.K.; Das, V.; Gulati, A.K.; Singh, V.P. Deglycyrrhizinated liquorice in aphthous ulcers. J. Assoc. Phys. India 1989, 37, 647.
- Krausse, R. In vitro anti-helicobacter pylori activity of extractum liquiritiae, glycyrrhizin and its metabolites. J. Antimicrob. Chemother. 2004, 54, 243–246.
- Chen, F.; Chan, K.H.; Jiang, Y.; Kao, R.Y.T.; Lu, H.T.; Fan, K.W.; Cheng, V.C.C.; Tsui, W.H.W.; Hung, I.F.N.; Lee, T.S.W. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J. Clin. Virol. 2004, 31, 69–75.
- Cinatl, J.; Morgenstern, B.; Bauer, G.; Chandra, P.; Rabenau, H.; Doerr, H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet 2003, 361, 2045–2046.
- Massi, A.; Bortolini, O.; Ragno, D.; Bernardi, T.; Sacchetti, G.; Tacchini, M.; De Risi, C. Research progress in the modification of quercetin leading to anticancer agents. Molecules 2017, 22, 1270.
- Chen, L.; Li, J.; Luo, C.; Liu, H.; Xu, W.; Chen, G.; Liew, O.W.; Zhu, W.; Puah, C.M.; Shen, X.; et al. Binding interaction of quercetin-3-β-galactoside and its synthetic derivatives with SARS-CoV 3clpro: Structure–activity relationship studies reveal salient pharmacophore features. Bioorganic Med. Chem. 2006, 14, 8295–8306.
- Wen, C.-C.; Shyur, L.-F.; Jan, J.-T.; Liang, P.-H.; Kuo, C.-J.; Arulselvan, P.; Wu, J.-B.; Kuo, S.-C.; Yang, N.-S. Traditional Chinese medicine herbal extracts of Cibotium barometz, Gentiana scabra, Dioscorea batatas, Cassia tora, and Taxillus chinensis inhibit SARS-COV replication. J. Tradit. Complement. Med. 2011, 1, 41–50.
- Brendler, T.; Al-Harrasi, A.; Bauer, R.; Gafner, S.; Hardy, M.L.; Heinrich, M.; Hosseinzadeh, H.; Izzo, A.A.; Michaelis, M.; Nassiri-Asl, M.; et al. Botanical Drugs and supplements affecting the immune response in the time of COVID-19: Implications for research and clinical practice. Phytother. Res. 2020, 35, 3013–3031.
- Cheng, P.-W.; Ng, L.-T.; Chiang, L.-C.; Lin, C.-C. Antiviral effects of SAIKOSAPONINS on human coronavirus 229E in vitro. Clin. Exp. Pharmacol. Physiol. 2006, 33, 612–616.
- Yang, F.; Dong, X.; Yin, X.; Wang, W.; You, L.; Ni, J. Radix bupleuri: A review of traditional uses, botany, phytochemistry, pharmacology, and toxicology. BioMed Res. Int. 2017, 2017, 7597596.
- El-Saber Batiha, G.; Beshbishy, A.M.; El-Mleeh, A.; Abdel-Daim, M.M.; Devkota, H.P. Traditional uses, bioactive chemical constituents, and pharmacological and toxicological activities of Glycyrrhiza glabra L. (fabaceae). Biomolecules 2020, 10, 352.
- Yang, Y.; Islam, M.S.; Wang, J.; Li, Y.; Chen, X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): A review and perspective. Int. J. Biol. Sci. 2020, 16, 1708–1717.
- Liu, H.; Ye, F.; Sun, Q.; Liang, H.; Li, C.; Li, S.; Lu, R.; Huang, B.; Tan, W.; Lai, L. Scutellaria baicalensis extract and Baicalein inhibit replication of SARS-COV-2 and its 3c-like protease in vitro. J. Enzym. Inhib. Med. Chem. 2021, 36, 497–503.
- Lau, K.-M.; Lee, K.-M.; Koon, C.-M.; Cheung, C.S.-F.; Lau, C.-P.; Ho, H.-M.; Lee, M.Y.-H.; Au, S.W.-N.; Cheng, C.H.-K.; Lau, C.B.-S.; et al. Immunomodulatory and anti-SARS activities of Houttuynia Cordata. J. Ethnopharmacol. 2008, 118, 79–85.
- Dang, S.-S.; Jia, X.-L.; Song, P.; Cheng, Y.-A.; Zhang, X.; Sun, M.-Z.; Liu, E.-Q. Inhibitory effect of emodin and astragalus polysaccharideon the replication of HBV. World J. Gastroenterol. 2009, 15, 5669.
- Sethu, S.; Shetty, R.; Ghosh, A.; Honavar, S.G.; Khamar, P. Therapeutic opportunities to manage COVID-19/SARS-CoV-2 infection: Present and future. Indian J. Ophthalmol. 2020, 68, 693.
- Park, J.-Y.; Jae Jeong, H.; Hoon Kim, J.; Min Kim, Y.; Park, S.-J.; Kim, D.; Hun Park, K.; Song Lee, W.; Bae Ryu, Y. Diarylheptanoids from alnus japonica inhibit papain-like protease of severe acute respiratory syndrome coronavirus. Biol. Pharm. Bull. 2012, 35, 2036–2042.
- Capell, T.; Twyman, R.M.; Armario-Najera, V.; Ma, J.K.-C.; Schillberg, S.; Christou, P. Potential applications of plant biotechnology against SARS-CoV-2. Trends Plant Sci. 2020, 25, 635–643.
- Heinrich, M.; Appendino, G.; Efferth, T.; Fürst, R.; Izzo, A.A.; Kayser, O.; Pezzuto, J.M.; Viljoen, A. Best practice in research—Overcoming common challenges in phytopharmacological research. J. Ethnopharmacol. 2020, 246, 112230.
- Dhama, K.; Natesan, S.; Iqbal Yatoo, M.; Patel, S.K.; Tiwari, R.; Saxena, S.K.; Harapan, H. Plant-based vaccines and antibodies to combat COVID-19: Current status and prospects. Hum. Vaccines Immunother. 2020, 16, 2913–2920.
- Kurup, V.M.; Thomas, J. Edible vaccines: Promises and challenges. Mol. Biotechnol. 2019, 62, 79–90.
- Turner, R.B.; Felton, A.; Kosak, K.; Kelsey, D.K.; Meschievitz, C.K. Prevention of experimental coronavirus colds with intranasal -2b interferon. J. Infect. Dis. 1986, 154, 443–447.
- Mustafa, S.; Balkhy, H.; Gabere, M.N. Current treatment options and the role of peptides as potential therapeutic components for Middle East respiratory syndrome (MERS): A Review. J. Infect. Public Health 2018, 11, 9–17.
- Lew, T.W. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. JAMA 2003, 290, 374.
- Costantini, C.; Bellet, M.M.; Pariano, M.; Renga, G.; Stincardini, C.; Goldstein, A.L.; Garaci, E.; Romani, L. A reappraisal of Thymosin Alpha1 in cancer therapy. Front. Oncol. 2019, 9, 873.
- Záruba, K.; Rastorfer, M.; Grob, P.J.; Joller-Jemelka, H.; Bolla, K. Thymopentin as adjuvant in non-responders or hyporesponders to hepatitis B vaccination. Lancet 1983, 322, 1245.
- Wei, L. Study on Expression of Thymosin α1 with Plant Bioreactor. J. Sichuan Norm. Univ. 2009, 1, 112–117.
- Cui, L.; Chen, Y.; Shen, G.; Zhao, L.; Tang, K. Expression of bioactive thymosin alpha 1 (TA1) in marker-free transgenic lettuce (Lactuca sativa). Plant Mol. Biol. Report. 2010, 29, 466–472.
- Sui, J.; Li, W.; Murakami, A.; Tamin, A.; Matthews, L.J.; Wong, S.K.; Moore, M.J.; Tallarico, A.S.; Olurinde, M.; Choe, H.; et al. Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human MAB to S1 protein that blocks receptor association. Proc. Natl. Acad. Sci. USA 2004, 101, 2536–2541.
- Moussavou, G.; Ko, K.; Lee, J.-H.; Choo, Y.-K. Production of monoclonal antibodies in plants for cancer immunotherapy. BioMed Res. Int. 2015, 2015, 306164.
- Boechat, J.L.; Chora, I.; Morais, A.; Delgado, L. The immune response to SARS-CoV-2 and COVID-19 immunopathology—Current perspectives. Pulmonology 2021, 27, 423–437.
- Alqazlan, N.; Diao, H.; Jevnikar, A.M.; Ma, S. Production of functional human interleukin 37 using plants. Plant Cell Rep. 2019, 38, 391–401.
- Michot, J.-M.; Albiges, L.; Chaput, N.; Saada, V.; Pommeret, F.; Griscelli, F.; Balleyguier, C.; Besse, B.; Marabelle, A.; Netzer, F.; et al. Tocilizumab, an anti-IL-6 receptor antibody, to treat COVID-19-related respiratory failure: A case report. Ann. Oncol. 2020, 31, 961–964.
- Wampler Muskardin, T.L. Intravenous anakinra for macrophage activation syndrome may hold lessons for treatment of cytokine storm in the setting of coronavirus disease 2019. ACR Open Rheumatol. 2020, 2, 283–285.
- Cai, C.; Koch, B.; Morikawa, K.; Suda, G.; Sakamoto, N.; Rueschenbaum, S.; Akhras, S.; Dietz, J.; Hildt, E.; Zeuzem, S.; et al. Macrophage-derived extracellular vesicles induce long-lasting immunity against hepatitis C virus which is blunted by polyunsaturated fatty acids. Front. Immunol. 2018, 9, 723.
- Favalli, E.G.; Ingegnoli, F.; De Lucia, O.; Cincinelli, G.; Cimaz, R.; Caporali, R. COVID-19 infection and rheumatoid arthritis: Faraway, so close! Autoimmun. Rev. 2020, 19, 102523.
More
Information
Subjects:
Others
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
17 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




