
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Beatrix Zheng | -- | 3928 | 2022-11-17 01:43:44 |
Video Upload Options
Medication-related osteonecrosis of the jaw (MON, MRONJ) is progressive death of the jawbone in a person exposed to a medications known to increase the risk of disease, in the absence of a previous radiation treatment. It may lead to surgical complication in the form of impaired wound healing following oral and maxillofacial surgery, periodontal surgery, or endodontic therapy. Particular medications can result in MRONJ, a serious but uncommon side effect in certain individuals. Such medications are frequently used to treat diseases that cause bone resorption such as osteoporosis, or to treat cancer. The main groups of drugs involved are anti-resorptive drugs, and anti-angiogenic drugs. This condition was previously known as bisphosphonate-related osteonecrosis of the jaw (BON or BRONJ) because osteonecrosis of the jaws correlating with bisphosphate treatment was frequently encountered, with its first incident occurring in 2003. Osteonecrotic complications associated with denosumab, another antiresorptive drug from a different drug category, were soon determined to be related to this condition. Newer medications such as anti-angiogenic drugs have been potentially implicated causing a very similar condition and consensus shifted to refer to the related conditions as MRONJ; however, this has not been definitively demonstrated. There is no known prevention for bisphosphonate-associated osteonecrosis of the jaw. Avoiding the use of bisphosphonates is not a viable preventive strategy on a general-population basis because the medications are beneficial in the treatment and prevention of osteoporosis (including prevention of bony fractures) and treatment of bone cancers. Current recommendations are for a 2 month drug holiday prior to dental surgery for those who are at risk (intravenous drug therapy, greater than 4 years of by-mouth drug therapy, other factors that increase risk such as steroid therapy). It usually develops after dental treatments involving exposure of bone or trauma, or may arise spontaneously. Patients who develop MRONJ may experience prolonged healing, pain, swelling, infection, exposed bone, after dental procedures, though some patients may have no signs/symptoms.
1. Definition
This condition is defined as;
- 1. exposed bone, or bone that can be probed through an in the mouth or through a fistula in the skin of the face, and
- 2. has persisted for more than eight weeks, and
- 3. in patients with a history of treatment with anti-resorptive or anti-angiogenic drugs, and
- 4. without a history of radiation therapy to the jaw or no obvious metastatic disease to the jaws.[1]
Osteonecrosis, or localized death of bone tissue, of the jaws is a rare potential complication in cancer patients receiving treatments including radiation, chemotherapy, or in patients with tumors or infectious embolic events. In 2003,[2][3] reports surfaced of the increased risk of osteonecrosis in patients receiving these therapies concomitant with intravenous bisphosphonate.[4] Matrix metalloproteinase 2 may be a candidate gene for bisphosphonate-associated osteonecrosis of the jaws, since it is the only gene known to be associated with both bone abnormalities and atrial fibrillation, another side effect of bisphosphonates.[5]
In response to the growing base of literature on this association, the United States Food and Drug Administration issued a broad drug class warning of this complication for all bisphosphonates in 2005.[6]
2. Signs and Symptoms

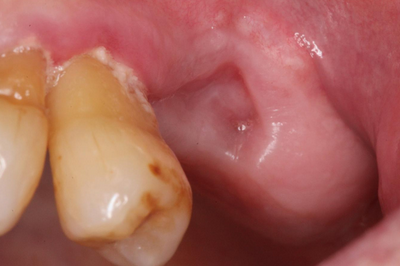
Classically, MRONJ will cause an ulcer or areas of necrotic bone for weeks, months, or even years following a tooth extraction.[7] While the exposed, dead bone does not cause symptoms these areas often have mild pain from the inflammation of the surrounding tissues.[8] Clinical signs and symptoms associated with, but not limited to MRONJ, include:
- Jaw pain and neuropathy[9]
- Loose teeth[10]
- Mucosal swelling[10]
- Erythema
- Suppuration[10]
- Soft tissue ulceration[10] persisting for more than 8 weeks[11]
- Trismus[10]
- Non-healing extraction sockets[10]
- Paraesthesia or numbness in the jaw[12]
- Bad breath
- Exposed necrotic jaw bone[8]
3. Pathogenesis
Although the methods of action are not yet completely understood, it is hypothesized that medication-associated osteonecrosis of the jaw is related to a defect in jaw bone healing and remodelling. The condition is predominantly confined within the maxillofacial region.
The inhibition of osteoclast differentiation and function, precipitated by drug therapy, leads to decreased bone resorption and remodelling.[13][14] Evidence also suggests bisphosphonates induce apoptosis of osteoclasts, resulting in resorption of bones.[15] Another suggested factor is the inhibition of angiogenesis due to bisphosphonates but its effect remains uncertain.[16][17][18] Several studies have proposed that bisphosphonates cause excessive reduction of bone turnover, resulting in a higher risk of bone necrosis when repair is needed.[19][20][21]
It is also thought that bisphosphonates bind to osteoclasts and interfere with the remodeling mechanism for bones. To be more specific, the drug interferes with the cholesterol biosynthesis pathway through the inhibition of farnesyl diphosphate synthase. Over time, the cytoskeleton of the osteoclasts loses its function and the essential border needed for bone resorption does not form.[1] Like aminobisphosphonates, bisphosphonates have shown to have antiangiogenic properties. Therefore, effects include an overall decrease in bone recycling/turnover as well as an increased inhibition of the absorptive bone abilities.
As for the isolation to the facial skeleton, one theory is that because bisphosphonates are preferentially deposited in bone with high turnover rates, it is possible that the levels of bisphosphonate within the jaw are selectively elevated. To date, there have been no reported cases of bisphosphonate-associated complications within bones outside the craniofacial skeleton.[6]
4. Diagnosis
A diagnosis of bisphosphonate-associated osteonecrosis of the jaw relies on three criteria:[22]
- the patient possesses an area of exposed bone in the jaw persisting for more than 8 weeks,
- the patient must present with no history of radiation therapy to the head and neck
- the patient must be taking or have taken bisphosphonate medication.
According to the updated 2009 BRONJ Position Paper published by the American Association of Oral and Maxillofacial Surgeons, both the potency of and the length of exposure to bisphosphonates are linked to the risk of developing bisphosphonate-associated osteonecrosis of the jaw.[23]
In the 2014 AAOMS update[1] on MRONJ, a staging and treatment strategies table was created:
| MRONJ Staging | Criteria* (>8 weeks) | Treatment Strategies** | Picture |
|---|---|---|---|
| At risk | No apparent necrotic bone in patients who have been treated with either oral or IV bisphosphonates | No treatment indicated, Patient education | N/A |
| Stage 0 | No clinical evidence of necrotic bone, but non-specific clinical findings, radiographic changes and symptoms | Systemic management, including the use of pain medication and antibiotics | N/A |
| Stage 1 | Exposed and necrotic bone, or fistulae that probes to bone, in patients who are asymptomatic and have no evidence of infection | Antibacterial mouth rinse, Clinical follow-up on a quarterly basis, Patient education and review of indications for continued bisphosphonate therapy |  |
| Stage 2 | Exposed and necrotic bone, or fistulae that probes to bone, associated with infection as evidenced by pain and erythema in the region of the exposed bone with or without purulent drainage | Symptomatic treatment with oral antibiotics, Oral antibacterial mouth rinse, Pain control, Debridement to relieve soft tissue irritation and infection control |  |
| Stage 3 | Exposed and necrotic bone or a fistula that probes to bone in patients with pain, infection, and one or more of the following: exposed and necrotic bone extending beyond the region of alveolar bone,(i.e., inferior border and ramus in the mandible, maxillary sinus and zygoma in the maxilla) resulting in pathologic fracture, extra-oral fistula, oral antral/oral nasal communication, or osteolysis extending to the inferior border of the mandible of sinus floor | Antibacterial mouth rinse, Antibiotic therapy and pain control, Surgical debridement/resection for longer term palliation of infection and pain |  |
5. Cause
Cases of MRONJ have also been associated with the use of the following two intravenous and three oral bisphosphonates, respectively: Zometa (zoledronic acid) and Aredia (pamidronate) and Fosamax (alendronate), Actonel (risedronate), and Boniva (ibandronate).[25][26] Despite the fact that it remains vague as to what the actual cause is, scientists and doctors believe that there is a correlation between the necrosis of the jaw and time of exposure to bisphosphonates.[27] Causes are also thought to be related to bone injury in patients using bisphosphonates as stated by Remy H Blanchaert in an article about the matter.
5.1. Risk Factors
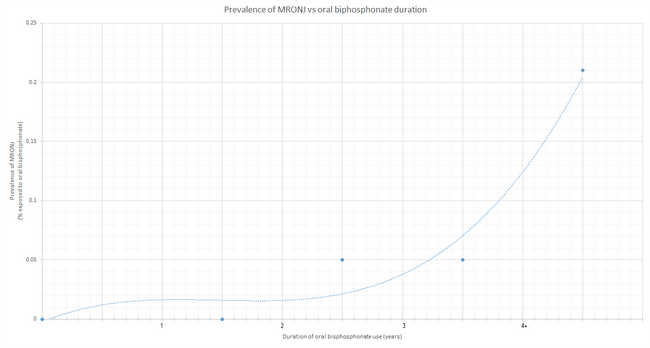
The overwhelming majority of MRONJ diagnoses, however, were associated with intravenous administration of bisphosphonates (94%). Only the remaining 6% of cases arose in patients taking bisphosphonates orally.[22]
Although the total United States prescriptions for oral bisphosphonates exceeded 30 million in 2006, less than 10% of MRONJ cases were associated with patients taking oral bisphosphonate drugs.[28] Studies have estimated that BRONJ occurs in roughly 20% of patients taking intravenous zoledronic acid for cancer therapy and in between 0–0.04% of patients taking orally administered bisphosphonates.[29]
Owing to prolonged embedding of bisphosphonate drugs in the bone tissues, the risk for MRONJ is high even after stopping the administration of the medication for several years.[30]
Patients who stopped taking anti-angiogenic drugs are exposed to the same risk as patients who have never taken the drugs because anti-angiogenic drugs are not normally supposed to remain in the body for long period of time.[31]
Risk factors include:[31]
- Dental treatment (e.g. dentoalveolar surgery/procedure that impacts bone) – it is possible for MRONJ to occur spontaneously without any recent invasive dental treatment
- Duration of bisphosphonate drug therapy – increased risk with increased cumulative dose of drug
- Other concurrent medication – use of chronic systemic glucocorticoid increases risk when they are taken in combination with anti-resorptive drugs
- Dental implants
- Drug holidays – no evidence to support a reduction in MRONJ risk if patients stop taking bisphosphonates temporarily/permanently, as drugs can persist in skeletal tissues for many years
- Treatment in the past with anti-resorptive/anti-angiogenic drugs
- Patient being treated for cancer – higher risk
- Patients being treated for osteoporosis/non-malignant bone diseases (e.g. Paget's disease) – lower risk
5.2. Research Findings
‘The risk of MRONJ after dental extraction was significantly higher in patients treated with ARD (antiresorptive drugs) for oncological reasons (3.2%) than in those treated with ARD for OP (osteoporosis) (0.15%) (p < 0.0001). Dental extraction performed with adjusted extraction protocols decreased significantly MRONJ development. Potential risk indicators such as concomitant medications and pre-existing osteomyelitis were identified.’[32]
Research found that patients on BsPs alone for stage II oncological disease – mainly females- were most frequently diagnosed with MRONJ mainly after dental treatment and bone surgery. ‘ A new population affected by MRONJ is emerging. Men affected by kidney cancer treated with new antiresorptive-antiangiogenic drugs will represent a growing portion of the pool of patients at risk. '
[33]
5.3. Patient Risk Categories
Low:[31]
- Treatment of osteoporosis or non-malignant bone disease with oral bisphosphonates for <5 years (not taking systemic glucocorticoids)
- Treatment of osteoporosis or non-malignant bone disease with quarterly/yearly infusions of intravenous bisphosphonates for <5 years (not taking systemic glucocorticoids)
- Treatment of osteoporosis or non-malignant bone disease with denosumab (not taking systemic glucocorticoids)
High:
- Patients being treated for osteoporosis or non-malignant bone disease with oral bisphosphonates/quarterly or yearly infusions of intravenous bisphosphonates for >5 years
- Patients being treated for osteoporosis or non-malignant bone disease with bisphosphonates/denosumab for any length of time as well as being treated with systemic glucocorticoids
- Patients being treated with anti-resorptive/anti-angiogenic drugs/both as part of cancer management
- Previous MRONJ diagnosis
"N.B. Patients who have taken bisphosphonate drugs at any time in the past and those who have taken denosumab in the last nine months are allocated to a risk group as if they are still taking the drug."[31]
5.4. Anti-Resorptive Drugs
Anti-resorptive drugs inhibit osteoclast differentiation and function, slowing down the breakdown of bone.[34] It is usually prescribed for patients with osteoporosis or other metastatic bone diseases, such as Paget's disease, osteogenesis imperfecta and fibrous dysplasia.[35][36]
The two main types of anti-resorptive drugs are bisphosphonate and denosumab. These drugs help to decrease the risk of bone fracture and bone pain.
Because the mandible has a faster remodeling rate compared to other bones in the body, it is more affected by the effects of these drugs.[37]
- Bisphosphonate
- Bisphosphonate could be administrated orally or intravenously. It reduces bone resorption.[38]
- Mechanism of action: Bisphosphonate binds to the mineral component of the bone and inhibits enzymes (i.e. farnesyl-pyrophosphate synthase) that is responsible for bone formation, recruitment and osteoclast function.[36][38]
- This type of drug has a high affinity to hydroxyapatite[35] and stays in bone tissue for a long period of time,[36] with alendronate, it has a half-life of approximately ten years.[37]
- The risk of patient having MRONJ after stop taking this medication is unknown.[37]
- There are suggestions that bisphosphonate may inhibit the proliferation of soft tissue cells and increases apoptosis. This may result in delayed soft tissue healing.[37]
- Examples of bisphosphonate: : Zoledronic acid (Reclast, Zometa), Risedronate (Actonel), Alendronate (Fosamax), Etidronate (Didronel), Ibandronate (Boniva), Pamidronate (Aredia), Tiludronate (Skelid).[39]
- Denosumab
- Denosumab is a monoclonal antibody[40][41] which is administrated subcutaneously. It inhibits osteoclast differentiation and activation, reduces bone resorption, improves bone density and lessens skeletal-related events associated with metastasis.[38]
- Mechanism of action: The drug binds to receptor activator nuclear factor κB ligand (RANKL), preventing the interaction with RANK.[38][40][41]
- It does not bind to bone and its effect on bone diminishes in 9 months.[37]
5.5. Anti-Angiogenic Drugs
Osteonecrosis of the jaw has been identified as one of the possible complications after taking anti-angiogenic drugs, the association the disease and medication is known as MRONJ. This have been stated in the Drug Safety Updates by MHRA.[31]
Anti-angiogenic drugs, which is as known as angiogenesis inhibitors obstruct the blood vessels formation by interfering the angiogenesis signalling cascade. They are used primarily to treat cancer. It is different from other conventional cancer drugs as these cancer-fighting agents tend to hinder the growth of blood vessels that supply the tumour rather than killing tumour cells directly.[42] It does not necessary eliminate tumours but it has indirect on treating cancer by preventing the tumour from growing. For example, bevacizumab/ aflibercept is a monoclonal antibody that specifically bind to the vascular endothelial growth factor (VEGF), so the VEGF is no longer available to bind to the receptors on the surface of normal endothelial cells.[43] Sunitinib is a different example of anti-angiogenic drugs as it inhibits cellular signalling by targeting multiple receptor tyrosine kinases. It reduces blood/ nutrients supply to tumour by inhibiting new blood vessels formation from the cancer cells.[44] Hence, the tumour stops growing or even shrinks.[45]
6. Prevention
Tooth extraction is the major risk factor for development of MRONJ. Prevention including the maintenance of good oral hygiene, comprehensive dental examination and dental treatment including extraction of teeth of poor prognosis and dentoalveolar surgery should completed prior to commencing any medication which is likely to cause osteonecrosis (ONJ). Patients with removable prostheses should be examined for areas of mucosal irritation. Procedures which are likely to cause direct osseous trauma, e.g. tooth extraction, dental implants, complex restoration, deep root planning, should be avoided in preference of other dental treatments. There are limited data to support or refute the benefits of a drug holiday for osteoporotic patients receiving antiresorptive therapy. However, a theoretical benefit may still apply for those patients with extended exposure histories (>4 yr), and current recommendations are for a 2 month holiday for those at risk.[1]
7. Management
MRONJ is an adverse reaction which can occur as a result of medicines used to treat cancer and osteoporosis.[46] Some medications which induce these effects are Bisphosphonates, Denosumab and Antiangiogenic agents. They involve the destruction of bone in a progressive manner, particularly associated with the mandible or maxilla. The overall effects depend on which drug is being used, the dose and the duration of taking this drug. MRONJ is associated with significant severe disease, negative affects on the quality of life and remains to be increasingly challenging to treat.[47] It is of high debate whether the various management techniques used for MRONJ are effective or not but due to the severity of the disease it is continually understood action must be taken.[48] The management of patients taking the drugs of concern undergo initial management and continuing management. Before either of these are considered the patient must be as dentally fit as possible.
7.1. Initial Management
This involves patients who are about to start, or very recently have started, taking the drugs of concern. There is a small portion of observational studies which promote the idea of preventative dental treatment to decrease oral complications in patients taking these drugs. These preventative measures may require a change in the patients’ oral hygiene technique and lifestyle factors such as smoking and alcohol consumption. There is also a benefit in prescribing high fluoridated toothpaste if the patient is of high caries risk. Before prescribing of any kind or when noticing a patient is on the treatment already, it is encouraged to tell the patient of the risk of developing MRONJ, although this risk is small. This is followed by personalised advice given to the patient, involving: a healthy diet, excellent oral hygiene, stop smoking, limited alcohol consumption and regular dental appointments. If a patient has a complex medical history and is of particular high risk it is advised before any treatment to commence, communication with a specialist with regards to the clinical assessment and treatment plan. It is also advised for individuals who take bisphosphonates to never allow the tablet to dissolve in the mouth as this causes damage to the oral mucosa. The patient must follow the instructions given with the tablets.
7.2. Continuing Management
This involves patients who have a regime which actively incorporates the drugs of concern and also for the patients whom undergone initial management. In terms of dental treatment all must be done as normal, accompanied by personalised advice to the patient. If there is a need for an extraction or any procedure which implicates bone a discussion with the patient about the risks and benefits must occur. Due to bacterial resistance and possible side effects of antibiotic therapy, they are only prescribed if there is a necessity for them. There is minimal evidence to say the use of prophylactic antibiotics will reduce MRONJ.[31]
For some patients, it is possible to have a drug holiday during which bisphosphonates are discontinued if the benefit of discontinuing the drug outweighs the risks. If it is possible to have a drug holiday, it is recommended that treatment be carried out during that period. Some patients however have been taking the drug for a prolonged period of time and so the bisphosphonate levels have accumulated in the body. In this case, a drug holiday would be of no benefit.[49]
Medical management of MRONJ is most commonly performed for patients who have less severe cases or those whom have contraindicating health conditions. The antimicrobials therapies commonly used are topical, oral and intravenous.
7.3. Topical Antimicrobials
A commonly used medicament, chlorhexidine gluconate 0.12% is bacteriostatic and bacteriocidal making an effective agent against MRONJ. Advantages of this topical gel is the low cost, ease of use, availability and patient acceptance. The disadvantages of this are the low compliance, patient acceptance, dental staining and risk of opportunistic bacterial resistance. For some patients, it is possible to have a drug holiday during which bisphosphonates are discontinued if the benefit of discontinuing the drug outweighs the risks. If it is possible to have a drug holiday, it is recommended that treatment be carried out during that period. Some patients however have been taking the drug for a prolonged period of time and so the bisphosphonate levels have accumulated in the body. In this case, a drug holiday would be of no benefit. For some patients, it is possible to have a drug holiday during which bisphosphonates are discontinued if the benefit of discontinuing the drug outweighs the risks. If it is possible to have a drug holiday, it is recommended that treatment be carried out during that period. Some patients however have been taking the drug for a prolonged period of time and so the bisphosphonate levels have accumulated in the body. In this case, a drug holiday would be of no benefit.
7.4. Oral Antimicrobials
The use of these are based on the clinical evaluation of the condition and if pathogenic bacteria presence is indicated. This is generally a 2-week course for a patient with a persistent presentation of the disease or a 4-6 week course for more severe cases. Penicillin is the first line of choice, although if this is contraindicated commonly used antimicrobials are: clindamycin, fluoroquinolones and/or metronidazole.
7.5. Intravenous Antimicrobials
This means of therapy may be of benefit with patients who possess specific pathogenic organisms which resist oral therapies. Although this method has perceived greater penetration of tissue there is little evidence of being a substantial greater efficacy when compared to other methods of management.[50]
8. Treatment
Treatment usually involves antimicrobial mouth washes and oral antibiotics to help the immune system fight the attendant infection, and it also often involves local resection of the necrotic bone lesion. Many patients with MRONJ have successful outcomes after treatment, meaning that the local osteonecrosis is stopped, the infection is cleared, and the mucosa heals and once again covers the bone.
The treatment the person receives depends on the severity of osteonecrosis of the jaw.
8.1. Conservative
Indicated in patients who have evidence of exposed bone but no evidence of infection. It may not necessarily eliminate all the lesions, but it may provide patients with a long term relief. This approach involves a combination of antiseptic mouthwashes and analgesics and the use of teriparatide.[51] However, note that the teriparatide treatment should not be used in cancer patients, or patients with a history of skeletal radiation or active bone metastases. Splints may be used to protect sites of exposed necrotic bone.
8.2. Non-Surgical
Indicated for patients with exposed bone with symptoms of infection. This treatment modality may also be utilised for patients with other co-morbidities which precludes invasive surgical methods. This approach requires antimicrobial mouthwashes, systemic antibiotics and antifungal medication and analgesics.[52]
8.3. Surgery
Surgical intervention is indicated in patients with symptomatic exposed bone with fistula formation and one or more of the following: exposed and necrotic bone extending beyond the alveolar bone resulting in pathological fracture; extra-oral fistula; oral antral communication or osteolysis extending from the inferior border of the mandible or the sinus floor. Surgical management involves necrotic bone resection, removal of loose sequestra of necrotic bone and reconstructive surgery. The objective of surgical management is to eliminate areas of exposed bone to prevent the risk of further inflammation and infection. The amount of surgical debridement required remains controversial.
8.4. Other
9. Epidemiology
The likelihood of this condition developing varies widely from less than 1/10,000 to 1/100, as many other factors need to be considered, such as the type, dose and frequency of intake of drug, how long it has been taken for, and why it has been taken.[55]
In patients taking drugs for cancer, the likelihood of MRONJ development varies from 0 - 12%. This again, varies with the type of cancer, although prostate cancer and multiple myeloma are reported to be at a higher risk.[31]
In patients taking oral drugs for osteoporosis, the likelihood of MRONJ development varies from 0 - 0.2%.[1]
References
- "American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw - 2014 update.". Journal of Oral and Maxillofacial Surgery 72 (10): 1938–1956. 2014. doi:10.1016/j.joms.2014.04.031. PMID 25234529. https://dx.doi.org/10.1016%2Fj.joms.2014.04.031
- Marx RE (September 2003). "Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic". J. Oral Maxillofac. Surg. 61 (9): 1115–7. doi:10.1016/S0278-2391(03)00720-1. PMID 12966493. https://dx.doi.org/10.1016%2FS0278-2391%2803%2900720-1
- Migliorati CA (November 2003). "Bisphosphanates and oral cavity avascular bone necrosis". J. Clin. Oncol. 21 (22): 4253–4. doi:10.1200/JCO.2003.99.132. PMID 14615459. http://www.jco.org/cgi/pmidlookup?view=long&pmid=14615459.
- Appendix 11: Expert Panel Recommendation for the Prevention, Diagnosis and Treatment of Osteonecrosis of the Jaw https://www.fda.gov/ohrms/dockets/ac/05/briefing/2005-4095B2_02_12-Novartis-Zometa-App-11.htm
- "Bisphosphonate-induced osteonecrosis of the jaws, bone markers, and a hypothesized candidate gene". J. Oral Maxillofac. Surg. 67 (1): 159–61. January 2009. doi:10.1016/j.joms.2008.09.015. PMID 19070762. https://dx.doi.org/10.1016%2Fj.joms.2008.09.015
- Ruggiero SL (March 2008). "Bisphosphonate-related Osteonecrosis of the Jaws". Compendium of Continuing Education in Dentistry 29 (2): 97–105.
- Allen, Matthew R.; Ruggiero, Salvatore L. (2009-07-01). "Higher bone matrix density exists in only a subset of patients with bisphosphonate-related osteonecrosis of the jaw". Journal of Oral and Maxillofacial Surgery 67 (7): 1373–1377. doi:10.1016/j.joms.2009.03.048. ISSN 1531-5053. PMID 19531405. https://dx.doi.org/10.1016%2Fj.joms.2009.03.048
- Khan, Aliya A.; Morrison, Archie; Hanley, David A.; Felsenberg, Dieter; McCauley, Laurie K.; O'Ryan, Felice; Reid, Ian R.; Ruggiero, Salvatore L. et al. (2015-01-01). "Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus". Journal of Bone and Mineral Research 30 (1): 3–23. doi:10.1002/jbmr.2405. ISSN 1523-4681. PMID 25414052. https://dx.doi.org/10.1002%2Fjbmr.2405
- "Painful trigeminal neuropathy induced by oral bisphosphonate-related osteonecrosis of the jaw: a new etiology for the numb-chin syndrome". Quintessence Int. 43 (2): 97–104. February 2012. PMID 22257870. http://www.quintpub.com/journals/qi/abstract.php?iss2_id=1012&article_id=11722&article=3&title=Painful+trigeminal+neuropathy+induced+by+oral+bisphosphonate%EF%BF%BDrelated+osteonecrosis+of+the+jaw%3A+A+new+etiology+for+the+numb-chin+syndrome#.UGfs5ZjA_IZ.
- Sharma, Dileep; Ivanovski, Saso; Slevin, Mark; Hamlet, Stephen; Pop, Tudor S.; Brinzaniuc, Klara; Petcu, Eugen B.; Miroiu, Rodica I. (2013-01-01). "Bisphosphonate-related osteonecrosis of jaw (BRONJ): diagnostic criteria and possible pathogenic mechanisms of an unexpected anti-angiogenic side effect". Vascular Cell 5 (1): 1. doi:10.1186/2045-824X-5-1. ISSN 2045-824X. PMID 23316704. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3606312
- Goodell, Dr. Gary G. (Fall 2012). "Endodontics: Colleagues for Excellence". 211 E. Chicago Ave., Suite 1100 Chicago, IL 60611-2691: American Association of Endodontists. https://www.aae.org/uploadedfiles/publications_and_research/endodontics_colleagues_for_excellence_newsletter/fall2012ecfe.pdf.
- Otto, Sven; Hafner, Sigurd; Grötz, Knut A. (2009-03-01). "The role of inferior alveolar nerve involvement in bisphosphonate-related osteonecrosis of the jaw". Journal of Oral and Maxillofacial Surgery 67 (3): 589–592. doi:10.1016/j.joms.2008.09.028. ISSN 1531-5053. PMID 19231785. https://dx.doi.org/10.1016%2Fj.joms.2008.09.028
- Baron, Roland; Ferrari, Serge; Russell, R. Graham G. (2011-04-01). "Denosumab and bisphosphonates: different mechanisms of action and effects". Bone 48 (4): 677–692. doi:10.1016/j.bone.2010.11.020. ISSN 1873-2763. PMID 21145999. https://dx.doi.org/10.1016%2Fj.bone.2010.11.020
- Russell, R. G. G.; Watts, N. B.; Ebetino, F. H.; Rogers, M. J. (2008-06-01). "Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy". Osteoporosis International 19 (6): 733–759. doi:10.1007/s00198-007-0540-8. ISSN 0937-941X. PMID 18214569. https://dx.doi.org/10.1007%2Fs00198-007-0540-8
- Lindsay R, Cosman F. Osteoporosis. In: Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, eds. Harrison’s principles of internal medicine. New York:McGraw-Hill, 2001:2226-37.
- Wood, J; Bonjean, K; Ruetz, S; Bellahcene, A; Devy, L; Foidart, JM; Castronovo, V; Green, JR (2002). "Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid". J Pharmacol Exp Ther 302 (3): 1055–61. doi:10.1124/jpet.102.035295. PMID 12183663. https://dx.doi.org/10.1124%2Fjpet.102.035295
- Vincenzi, B; Santini, D; Dicuonzo, G; Battistoni, F; Gavasci, M; La Cesa, A; Grilli, C; Virzi, V et al. (2005). "Zoledronic acid-related angiogenesis modifications and survival in advanced breast cancer patients". J Interferon Cytokine Res 25 (3): 144–51. doi:10.1089/jir.2005.25.144. PMID 15767788. https://dx.doi.org/10.1089%2Fjir.2005.25.144
- Santini, D; Vincenzi, B; Dicuonzo, G; Avvisati, G; Massacesi, C; Battistoni, F; Gavasci, M; Rocci, L et al. (2003). "Zoledronic acid induces significant and long-lasting modifications of circulating angiogenic factors in cancer patients". Clin Cancer Res 9 (8): 2893–7. PMID 12912933. http://www.ncbi.nlm.nih.gov/pubmed/12912933
- Chapurlat, Roland D; Arlot, Monique; Burt-Pichat, Brigitte; Chavassieux, Pascale; Roux, Jean Paul; Portero-Muzy, Nathalie; Delmas, Pierre D (2007-10-01). "Microcrack Frequency and Bone Remodeling in Postmenopausal Osteoporotic Women on Long-Term Bisphosphonates: A Bone Biopsy Study" (in en). Journal of Bone and Mineral Research 22 (10): 1502–1509. doi:10.1359/jbmr.070609. ISSN 1523-4681. PMID 17824840. https://dx.doi.org/10.1359%2Fjbmr.070609
- Stepan, Jan J.; Burr, David B.; Pavo, Imre; Sipos, Adrien; Michalska, Dana; Li, Jiliang; Fahrleitner-Pammer, Astrid; Petto, Helmut et al. (2007-09-01). "Low bone mineral density is associated with bone microdamage accumulation in postmenopausal women with osteoporosis". Bone 41 (3): 378–385. doi:10.1016/j.bone.2007.04.198. ISSN 8756-3282. PMID 17597017. https://dx.doi.org/10.1016%2Fj.bone.2007.04.198
- Woo, Sook-Bin; Hellstein, John W.; Kalmar, John R. (2006-05-16). "Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws". Annals of Internal Medicine 144 (10): 753–761. doi:10.7326/0003-4819-144-10-200605160-00009. ISSN 1539-3704. PMID 16702591. https://dx.doi.org/10.7326%2F0003-4819-144-10-200605160-00009
- Osteoporosis medications and your dental health pamphlet #W418, American Dental Association/National Osteoporosis Foundation, 2008
- Medical News Today AAOMS Updates BRONJ Position Paper, January 23, 2009 http://www.medicalnewstoday.com/articles/136380.php
- "Table 6. ONJ Staging System" (in en). PDQ Supportive and Palliative Care Editorial Board, National Center for Biotechnology Information, U.S. National Library of Medicine. 16 December 2016. https://www.ncbi.nlm.nih.gov/books/NBK65881/table/CDR0000062870__878/. This article incorporates text from this source, which is in the public domain.
- American Dental Association Osteonecrosis of the Jaw http://www.ada.org/prof/resources/topics/osteonecrosis.asp
- "Osteonecrosis of the jaw (ONJ) and drug treatments for osteoporosis". The National Osteoporosis Society. https://nos.org.uk/media/1593/k-drug-treatments-for-osteoporosis-osteonecrosis-of-the-jaw-onj-_.pdf.
- Bamias, Aristotle; Kastritis, Efstathios; Bamia, Christina; Moulopoulos, Lia A.; Melakopoulos, Ioannis; Bozas, George; Koutsoukou, Vassiliki; Gika, Dimitra et al. (2005-12-01). "Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors". Journal of Clinical Oncology 23 (34): 8580–8587. doi:10.1200/JCO.2005.02.8670. ISSN 0732-183X. PMID 16314620. https://dx.doi.org/10.1200%2FJCO.2005.02.8670
- "Incidence of osteonecrosis of the jaw in women with postmenopausal osteoporosis in the health outcomes and reduced incidence with zoledronic acid once yearly pivotal fracture trial". J Am Dent Assoc 139 (1): 32–40. January 2008. doi:10.14219/jada.archive.2008.0017. PMID 18167382. http://jada.ada.org/cgi/pmidlookup?view=long&pmid=18167382.
- "Bisphosphonate use and the risk of adverse jaw outcomes: a medical claims study of 714,217 people". J Am Dent Assoc 139 (1): 23–30. January 2008. doi:10.14219/jada.archive.2008.0016. PMID 18167381. http://jada.ada.org/cgi/pmidlookup?view=long&pmid=18167381.
- "The importance of a thorough medical and pharmacological history before dental implant placement.". Aust Dent J 57 (3): 388–392. September 2012. doi:10.1111/j.1834-7819.2012.01717.x. PMID 22924366. https://dx.doi.org/10.1111%2Fj.1834-7819.2012.01717.x
- "Oral Health Management of Patients at Risk of Medication-related Osteonecrosis of the Jaw". Scottish Dental Clinical Effectiveness Programme. March 2017. http://www.sdcep.org.uk/wp-content/uploads/2017/04/SDCEP-Oral-Health-Management-of-Patients-at-Risk-of-MRONJ-Guidance-full.pdf.
- Gaudin, Seidel, Bacevic, Rompen, Lambert, Elise, Laurence , Miljana , Eric, Lambert (October 2015). Occurrence and risk indicators of medication-related osteonecrosis of the jaw after dental extraction: a systematic review and meta-analysis.
- G.Ghidini, M.Manfredi, I.Giovannacci, G.Mergoni, A.Sarraj, M.Mureddu, G.Giunta, M.Bonanini , M.Meleti, P. Vescovi (August 2017). Medication-related osteonecrosis of the jaw: risk factors in patients under biphosphonate versus patients under antiresorptive-antiangiogenic drugs..
- "Anti-resorptive Medical Definition". https://www.merriam-webster.com/medical/antiresorptive. Retrieved 19 February 2018.
- Drake, Matthew T.; Clarke, Bart L.; Khosla, Sundeep (September 2008). "Bisphosphonates: Mechanism of Action and Role in Clinical Practice". Mayo Clinic Proceedings 83 (9): 1032–1045. doi:10.4065/83.9.1032. ISSN 0025-6196. PMID 18775204. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2667901
- Martin, T John (2000-06-01). "Experimental and Clinical Pharmacology: Bisphosphonates - mechanisms of action" (in en). Australian Prescriber 23 (6): 130–132. doi:10.18773/austprescr.2000.144. https://dx.doi.org/10.18773%2Faustprescr.2000.144
- Michaelina Macluskey, Stephanie Sammut (March 2017). Oral Health Management of Patients at Risk of Medication-related Osteonecrosis of the Jaw Dental Clinical Guidance. SDCEP. pp. 4, 5.
- Baron, Roland; Ferrari, Serge; Russell, R. Graham G. (2011). "Denosumab and bisphosphonates: Different mechanisms of action and effects". Bone 48 (4): 677–692. doi:10.1016/j.bone.2010.11.020. PMID 21145999. https://dx.doi.org/10.1016%2Fj.bone.2010.11.020
- Samuel Durham, Rebecca Miller, Caryn Davis, Brian M (May 20, 2010). "Bisphosphonate Nephrotoxicity Risks and Use in CKD Patients". U.S. Pharmacist.
- Dahiya, Navdeep; Khadka, Anjan; Sharma, A.K.; Gupta, A.K.; Singh, Nishith; Brashier, D.B.S. (January 2015). "Denosumab: A bone antiresorptive drug". Medical Journal, Armed Forces India 71 (1): 71–75. doi:10.1016/j.mjafi.2014.02.001. ISSN 0377-1237. PMID 25609868. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=4297848
- McClung, Michael R. (2017). "Denosumab for the treatment of osteoporosis". Osteoporosis and Sarcopenia 3 (1): 8–17. doi:10.1016/j.afos.2017.01.002. PMID 30775498. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=6372782
- "Angiogenesis Inhibitors" (in en). May 2018. https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/angiogenesis-inhibitors-fact-sheet#q3. Retrieved 2018-02-19.
- Rosella, Daniele; Papi, Piero; Giardino, Rita; Cicalini, Emauele; Piccoli, Luca; Pompa, Giorgio (2016). "Medication-related osteonecrosis of the jaw: Clinical and practical guidelines". Journal of International Society of Preventive & Community Dentistry 6 (2): 97–104. doi:10.4103/2231-0762.178742. ISSN 2231-0762. PMID 27114946. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=4820581
- Al-Husein, Belal; Abdalla, Maha; Trepte, Morgan; Deremer, David L.; Somanath, Payaningal R. (December 2012). "Antiangiogenic therapy for cancer: an update". Pharmacotherapy 32 (12): 1095–1111. doi:10.1002/phar.1147. ISSN 1875-9114. PMID 23208836. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3555403
- Hao, Zhonglin; Sadek, Ibrahim (2016-09-08). "Sunitinib: the antiangiogenic effects and beyond". OncoTargets and Therapy 9: 5495–5505. doi:10.2147/OTT.S112242. ISSN 1178-6930. PMID 27660467. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=5021055
- Beth-Tasdogan, N. H., Mayer, B., Hussein, H., & Zolk, O. (2017). "Interventions for managing medication-related osteonecrosis of the jaw". Cochrane Database of Systematic Reviews. doi:10.1002/14651858. http://espace.library.uq.edu.au/view/UQ:263806/UQ263806_OA.pdf.
- Institute of Pharmacology of Natural Products & Clinical Pharmacology, Ulm University, Helmholtzstr. 20, Ulm, Germany, 89081.
- Effectiveness of treatments for medication-related osteonecrosis of the jaw: A systematic review and meta-analysis. El-Rabbany M, Sgro A, Lam DK, Shah PS, Azarpazhooh A. J Am Dent Assoc. 2017 Aug
- Lam, David K.; Sándor, George K. B.; Holmes, Howard I.; Evans, A. Wayne; Clokie, Cameron M. L. (June 2007). "A review of bisphosphonate-associated osteonecrosis of the jaws and its management". Journal of the Canadian Dental Association 73 (5): 417–422. ISSN 1488-2159. PMID 17555652. http://www.worldcat.org/issn/1488-2159
- Management of Medication-related Osteonecrosis of the Jaw, An Issue of Oral and Maxillofacial Clinics of North America 27-4. 7 Jan 2016. Salvatore L. Ruggiero
- Aliya A Khan; Archie Morrison; David A Hanley; Dieter Felsenberg; Laurie K McCauley; Felice O’Ryan; Ian R Reid; Salvatore L Ruggiero et al. (April 2014). "Diagnosis and Management of Osteonecrosis of the Jaw: A Systematic Review and International Consensus". JBMR 30 (1): 3–23. doi:10.1002/jbmr.2405. PMID 25414052. https://dx.doi.org/10.1002%2Fjbmr.2405
- Svejda, B.; Muschitz, Ch; Gruber, R.; Brandtner, Ch; Svejda, Ch; Gasser, R. W.; Santler, G.; Dimai, H. P. (1946). "[Position paper on medication-related osteonecrosis of the jaw (MRONJ)]". Wiener Medizinische Wochenschrift 166 (1–2): 68–74. doi:10.1007/s10354-016-0437-2. ISSN 1563-258X. PMID 26847441. https://dx.doi.org/10.1007%2Fs10354-016-0437-2
- R. Fliefel; M. Tro¨ltzsch; J. Kühnisch; M. Ehrenfeld; S. Otto (May 2015). "Treatment strategies and outcomes of bisphosphonaterelated osteonecrosis of the jaw (BRONJ) with characterization of patients: a systematic review". International Journal of Oral and Maxillofacial Surgery 44 (5): 568–85. doi:10.1016/j.ijom.2015.01.026. PMID 25726090. https://dx.doi.org/10.1016%2Fj.ijom.2015.01.026
- Blus, Cornelio (23 August 2013). "Use of Ultrasonic Bone Surgery (Piezosurgery) to Surgically Treat Bisphosphonate-Related Osteonecrosis of the Jaws (BRONJ). A Case Series Report with at Least 1 Year of Follow-Up". The Open Dentistry Journal 7 (1): 94–101. doi:10.2174/1874210601307010094. PMID 24044030. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3772575
- Dodson, TB (2015). "The frequency of medication-related ssteonecrosis of the jaw and its associated risk factors". Oral and Maxillofacial Surgery Clinics of North America 27 (4): 509–16. doi:10.1016/j.coms.2015.06.003. PMID 26362367. https://dx.doi.org/10.1016%2Fj.coms.2015.06.003




