
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Manik Prabhu Narsing Rao | -- | 2052 | 2022-11-14 17:09:21 | | | |
| 2 | Conner Chen | -61 word(s) | 1991 | 2022-11-18 01:45:51 | | |
Video Upload Options
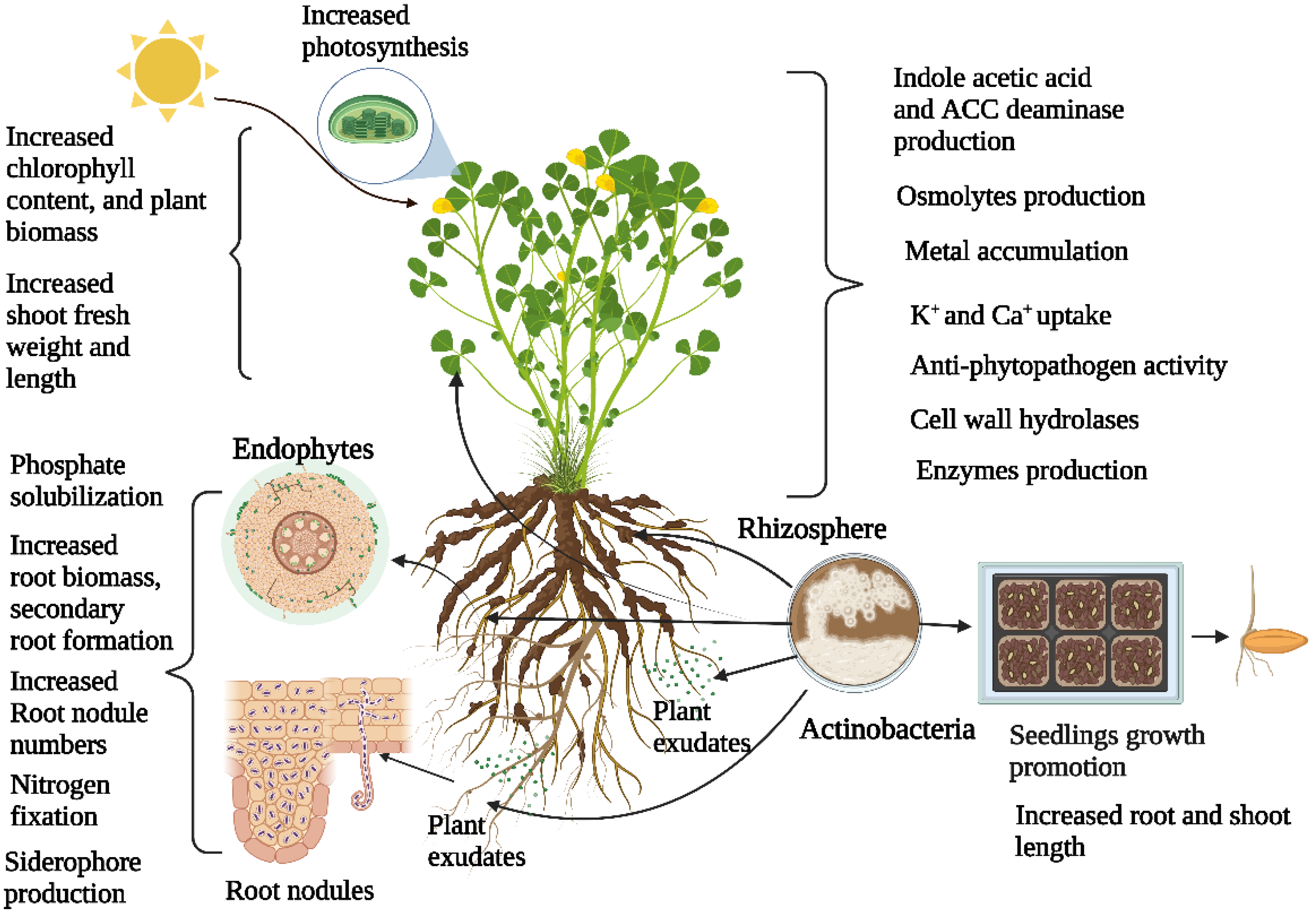
Abiotic stressors, such as drought, flooding, extreme temperature, soil salinity, and metal toxicity, are the most important factors limiting crop productivity. Plants use their innate biological systems to overcome these abiotic stresses caused by environmental and edaphic conditions. Microorganisms that live in and around plant systems have incredible metabolic abilities in mitigating abiotic stress. Recent advances in multi-omics methods, such as metagenomics, genomics, transcriptomics, and proteomics, have helped to understand how plants interact with microbes and their environment. These methods aid in the construction of various metabolic models of microbes and plants, resulting in a better knowledge of all metabolic exchanges engaged during interactions. Actinobacteria are ubiquitous and are excellent candidates for plant growth promotion because of their prevalence in soil, the rhizosphere, their capacity to colonize plant roots and surfaces, and their ability to produce various secondary metabolites. Mechanisms by which actinobacteria overcome abiotic stress include the production of osmolytes, plant hormones, and enzymes, maintaining osmotic balance, and enhancing nutrient availability. With these characteristics, actinobacteria members are the most promising candidates as microbial inoculants.
1. Introduction
2. Actinobacteria Diversity Associated with Plants and Plant Growth Promotion
References
- Imran, Q.M.; Falak, N.; Hussain, A.; Mun, B.-G.; Yun, B.-W. Abiotic stress in plants; Stress perception to molecular response and role of biotechnological tools in stress resistance. Agronomy 2021, 11, 1579.
- Zhang, H.; Zhao, Y.; Zhu, J.-K. Thriving under stress: How plants balance growth and the stress response. Dev. Cell 2020, 55, 529–543.
- Boyer, J.S. Plant productivity and environment. Science 1982, 218, 443–448.
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria. Environ. Dev. Sustain. 2021, 5, 100032.
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163.
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav. 2011, 6, 1720–1731.
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86.
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and drought stresses in crop plants: Implications, cross talk, and potential management opportunities. Front. Plant Sci. 2018, 9, 393.
- McDowell, N.G.; Beerling, D.J.; Breshears, D.D.; Fisher, R.A.; Raffa, K.F.; Stitt, M. The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends Ecol. Evol. 2011, 26, 523–532.
- Osakabe, K.; Osakabe, Y. Plant light stress. In Encyclopaedia of Life Sciences; Robinson, S., Ed.; Nature Publishing Group: London, UK, 2012.
- Yu, J.; Su, D.; Yang, D.; Dong, T.; Tang, Z.; Li, H.; Han, Y.; Li, Z.; Zhang, B. Chilling and heat stress-Induced physiological changes and microRNA-Related mechanism in sweetpotato (Ipomoea batatas L.). Front. Plant Sci. 2020, 11, 687.
- Hazel, J.R. Thermal adaptation in biological membranes: Is homeoviscous adaptation the explanation? Annu. Rev. Physiol. 1995, 57, 19–42.
- He, M.; He, C.Q.; Ding, N.Z. Abiotic stresses: General defenses of land plants and chances for engineering multistress tolerance. Front. Plant Sci. 2018, 9, 1771.
- Narsing Rao, M.P.; Dong, Z.-Y.; Xiao, M.; Li, W.-J. Effect of salt stress on plants and role of microbes in promoting plant growth under salt stress. In Microorganisms in Saline Environments: Strategies and Functions; Giri, B., Varma, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 423–435.
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596.
- Ghori, N.H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828.
- Liu, J.J.; Wei, Z.; Li, J.H. Effects of copper on leaf membrane structure and root activity of maize seedling. Bot. Stud. 2014, 55, 47.
- Rizvi, A.; Zaidi, A.; Ameen, F.; Ahmed, B.; AlKahtani, M.D.F.; Khan, M.S. Heavy metal induced stress on wheat: Phytotoxicity and microbiological management. RSC Adv. 2020, 10, 38379–38403.
- Inbaraj, M.P. Plant-Microbe interactions in alleviating abiotic stress-A mini review. Front. Agron. 2021, 3, 667903.
- Ram, K.; Devi, S.; Singh, A.; Kaur, V.; Kumar, J.; Arya, S.S. Microorganisms: The viable approach for mitigation of abiotic stress. In Plant Stress Mitigators: Action and Application; Vaishnav, A., Arya, S.S., Choudhary, D.K., Eds.; Springer Nature Singapore: Singapore, 2022; pp. 323–339.
- Yandigeri, M.S.; Meena, K.K.; Singh, D.; Malviya, N.; Singh, D.P.; Solanki, M.K.; Yadav, A.K.; Arora, D.K. Drought-Tolerant endophytic actinobacteria promote growth of wheat (Triticum aestivum) under water stress conditions. Plant Growth Regul. 2012, 68, 411–420.
- Berg, G.; Köberl, M.; Rybakova, D.; Müller, H.; Grosch, R.; Smalla, K. Plant microbial diversity is suggested as the key to future biocontrol and health trends. FEMS Microbiol. Ecol. 2017, 93, fix050.
- Jaemsaeng, R.; Jantasuriyarat, C.; Thamchaipenet, A. Molecular interaction of 1-aminocyclopropane-1-carboxylate deaminase (ACCD)-Producing endophytic Streptomyces sp. GMKU 336 towards salt-stress resistance of Oryza sativa L. cv. KDML105. Sci. Rep. 2018, 8, 1950.
- Dastogeer, K.M.G.; Tumpa, F.H.; Sultana, A.; Akter, M.A.; Chakraborty, A. Plant microbiome—An account of the factors that shape community composition and diversity. Curr. Plant Biol. 2020, 23, 100161.
- Pritchard, L.; Birch, P. A systems biology perspective on plant-microbe interactions: Biochemical and structural targets of pathogen effectors. Plant Sci. 2011, 180, 584–603.
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37.
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631.
- Zhou, J.; Deng, Y.; Shen, L.; Wen, C.; Yan, Q.; Ning, D.; Qin, Y.; Xue, K.; Wu, L.; He, Z.; et al. Temperature mediates continental-scale diversity of microbes in forest soils. Nat. Commun. 2016, 7, 12083.
- Jia, T.; Yao, Y.; Wang, R.; Wu, T.; Chai, B. Dynamics eelationship of phyllosphere and rhizosphere bacterial communities during the development of Bothriochloa ischaemum in copper tailings. Front. Microbiol. 2020, 11, 869.
- Liu, L.; Zhu, K.; Wurzburger, N.; Zhang, J. Relationships between plant diversity and soil microbial diversity vary across taxonomic groups and spatial scales. Ecosphere 2020, 11, e02999.
- Dong, C.J.; Wang, L.L.; Li, Q.; Shang, Q.M. Bacterial communities in the rhizosphere, phyllosphere and endosphere of tomato plants. PLoS ONE 2019, 14, e0223847.
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590.
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Meier-Kolthoff, J.P.; Klenk, H.P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, physiology, and natural products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43.
- Amin, D.H.; Abdallah, N.A.; Abolmaaty, A.; Tolba, S.; Wellington, E.M. Microbiological and molecular insights on rare Actinobacteria harboring bioactive prospective. Bull. Natl. Res. Cent. 2020, 44, 5.
- Stackebrandt, E.; Rainey, F.A.; Ward-Rainey, N.L. Proposal for a new hierarchic classification system, Actinobacteria classis nov. Int. J. Syst. Evol. Microbiol. 1997, 47, 479–491.
- Salam, N.; Jiao, J.-Y.; Zhang, X.-T.; Li, W.-J. Update on the classification of higher ranks in the phylum Actinobacteria. Int. J. Syst. Evol. Microbiol. 2020, 70, 1331–1355.
- Li, Q.; Chen, X.; Jiang, Y.; Jiang, C. Morphological identification of actinobacteria. In Actinobacteria; Dhanasekaran, D., Jiang, Y., Eds.; IntechOpen: London, UK, 2016; pp. 59–86.
- Gonzalez, D.; Huber, K.J.; Tindall, B.; Hedrich, S.; Rojas-Villalobos, C.; Quatrini, R.; Dinamarca, M.A.; Ibacache-Quiroga, C.; Schwarz, A.; Canales, C.; et al. Acidiferrimicrobium australe gen. nov., sp. nov., an acidophilic and obligately heterotrophic, member of the Actinobacteria that catalyses dissimilatory oxido-reduction of iron isolated from metal-rich acidic water in Chile. Int. J. Syst. Evol. Microbiol. 2020, 70, 3348–3354.
- Liu, X.Y.; Wang, B.J.; Jiang, C.Y.; Liu, S.J. Micrococcus flavus sp. nov., isolated from activated sludge in a bioreactor. Int. J. Syst. Evol. Microbiol. 2007, 57, 66–69.
- Busse, H.J. Review of the taxonomy of the genus Arthrobacter, emendation of the genus Arthrobacter sensu lato, proposal to reclassify selected species of the genus Arthrobacter in the novel genera Glutamicibacter gen. nov., Paeniglutamicibacter gen. nov., Pseudoglutamicibacter gen. nov., Paenarthrobacter gen. nov. and Pseudarthrobacter gen. nov., and emended description of Arthrobacter roseus. Int. J. Syst. Evol. Microbiol. 2016, 66, 9–37.
- Prabhu, D.M.; Quadri, S.R.; Cheng, J.; Liu, L.; Chen, W.; Yang, Y.; Hozzein, W.N.; Lingappa, K.; Li, W.J. Sinomonas mesophila sp. nov., isolated from ancient fort soil. J. Antibiot. 2015, 68, 318–321.
- Locci, R.; Schaal, K.P. Apical growth in facultative Anaerobic actinomycetes as determined by immunofluorescent labeling. Zentralbl. Bakteriol. A 1980, 246, 112–118.
- Takeuchi, M.; Sakane, T.; Nihira, T.; Yamada, Y.; Imai, K. Corynebacterium terpenotabidum sp. nov., a bacterium capable of degrading squalene. Int. J. Syst. Bacteriol. 1999, 49 Pt 1, 223–229.
- Lechevalier, M.P. Description of a new species, Oerskovia xanthineolytica, and emendation of Oerskovia. Int. J. Syst. Evol. Microbiol. 1972, 22, 260–264.
- Trujillo, M.E.; Riesco, R.; Benito, P.; Carro, L. Endophytic actinobacteria and the interaction of Micromonospora and nitrogen fixing plants. Front. Microbiol. 2015, 6, 1341.
- Narsing Rao, M.P.; Li, W.-J. Diversity of actinobacteria in various habitats. In Actinobacteria: Microbiology to Synthetic Biology; Karthik, L., Ed.; Springer Nature Singapore: Singapore, 2022; pp. 37–58.
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.-S.; Patra, J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140.
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266.
- Yadav, A.N.; Verma, P.; Kumar, S.; Kumar, V.; Kumar, M.; Kumari Sugitha, T.C.; Singh, B.P.; Saxena, A.K.; Dhaliwal, H.S. Chapter 2-Actinobacteria from rhizosphere: Molecular diversity, distributions, and potential biotechnological applications. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, B.P., Gupta, V.K., Passari, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 13–41.
- Jog, R.; Pandya, M.; Nareshkumar, G.; Rajkumar, S. Mechanism of phosphate solubilization and antifungal activity of Streptomyces spp. isolated from wheat roots and rhizosphere and their application in improving plant growth. Microbiology 2014, 160, 778–788.
- Thilagam, R.; Hemalatha, N. Plant growth promotion and chilli anthracnose disease suppression ability of rhizosphere soil actinobacteria. J. Appl. Microbiol. 2019, 126, 1835–1849.
- Tokala, R.K.; Strap, J.L.; Jung, C.M.; Crawford, D.L.; Salove, M.H.; Deobald, L.A.; Bailey, J.F.; Morra, M.J. Novel plant-microbe rhizosphere interaction involving Streptomyces lydicus WYEC108 and the pea plant (Pisum sativum). Appl. Environ. Microbiol. 2002, 68, 2161–2171.
- AbdElgawad, H.; Abuelsoud, W.; Madany, M.M.Y.; Selim, S.; Zinta, G.; Mousa, A.S.M.; Hozzein, W.N. Actinomycetes enrich soil rhizosphere and improve seed quality as well as productivity of legumes by boosting nitrogen availability and metabolism. Biomolecules 2020, 10, 1675.
- Arunachalam Palaniyandi, S.; Yang, S.H.; Damodharan, K.; Suh, J.W. Genetic and functional characterization of culturable plant-beneficial actinobacteria associated with yam rhizosphere. J. Basic Microbiol. 2013, 53, 985–995.
- Zhang, H.; Han, L.; Jiang, B.; Long, C. Identification of a phosphorus-solubilizing Tsukamurella tyrosinosolvens strain and its effect on the bacterial diversity of the rhizosphere soil of peanuts growth-promoting. World J. Microbiol. Biotechnol. 2021, 37, 109.
- Tamreihao, K.; Ningthoujam, D.S.; Nimaichand, S.; Singh, E.S.; Reena, P.; Singh, S.H.; Nongthomba, U. Biocontrol and plant growth promoting activities of a Streptomyces corchorusii strain UCR3–16 and preparation of powder formulation for application as biofertilizer agents for rice plant. Microbiol. Res. 2016, 192, 260–270.
- Alekhya, G.; Gopalakrishnan, S. Biological control and plant growth-promotion traits of Streptomyces species under greenhouse and field conditions in chickpea. Agric. Res. 2017, 6, 410–420.
- Álvarez-Pérez, J.M.; González-García, S.; Cobos, R.; Olego, M.; Ibañez, A.; Díez-Galán, A.; Garzón-Jimeno, E.; Coque, J.J.R. Use of endophytic and rhizosphere Actinobacteria from grapevine plants to reduce nursery fungal graft infections that lead to young grapevine decline. Appl. Environ. Microbiol. 2017, 83, e01564-17.
- Liu, H.; Carvalhais, L.C.; Crawford, M.; Singh, E.; Dennis, P.G.; Pieterse, C.M.J.; Schenk, P.M. Inner plant values: Diversity, colonization and benefits from endophytic bacteria. Front. Microbiol. 2017, 8, 2552.
- Golinska, P.; Wypij, M.; Agarkar, G.; Rathod, D.; Dahm, H.; Rai, M. Endophytic actinobacteria of medicinal plants: Diversity and bioactivity. Antonie Van Leeuwenhoek 2015, 108, 267–289.
- Madhurama, G.; Sonam, D.; Urmil, P.G.; Ravindra, N.K. Diversity and biopotential of endophytic actinomycetes from three medicinal plants in India. Afr. J. Microbiol. Res. 2014, 8, 184–191.
- van der Meij, A.; Willemse, J.; Schneijderberg, M.A.; Geurts, R.; Raaijmakers, J.M.; van Wezel, G.P. Inter- and intracellular colonization of Arabidopsis roots by endophytic actinobacteria and the impact of plant hormones on their antimicrobial activity. Antonie Van Leeuwenhoek 2018, 111, 679–690.
- Callaham, D.; Deltredici, P.; Torrey, J.G. Isolation and cultivation in vitro of the Actinomycete causing root nodulation in Comptonia. Science 1978, 199, 899–902.
- Marappa, N.; Ramachandran, L.; Dharumadurai, D.; Nooruddin, T. Plant growth-promoting active metabolites from Frankia spp. of Actinorhizal Casuarina spp. Appl. Biochem. Biotechnol. 2020, 191, 74–91.
- Verma, V.C.; Gond, S.K.; Kumar, A.; Mishra, A.; Kharwar, R.N.; Gange, A.C. Endophytic actinomycetes from Azadirachta indica A. Juss.: Isolation, diversity, and anti-microbial activity. Microb. Ecol. 2009, 57, 749–756.
- Coombs, J.T.; Franco, C.M. Isolation and identification of actinobacteria from surface-sterilized wheat roots. Appl. Environ. Microbiol. 2003, 69, 5603–5608.
- Sessitsch, A.; Reiter, B.; Berg, G. Endophytic bacterial communities of field-grown potato plants and their plant-growth-promoting and antagonistic abilities. Can. J. Microbiol. 2004, 50, 239–249.
- Xu, T.; Cui, K.; Chen, J.; Wang, R.; Wang, X.; Chen, L.; Zhang, Z.; He, Z.; Liu, C.; Tang, W.; et al. Biodiversity of culturable endophytic Actinobacteria isolated from high yield Camellia oleifera and their plant growth promotion potential. Agriculture 2021, 11, 1150.
- Shan, W.; Zhou, Y.; Liu, H.; Yu, X. Endophytic actinomycetes from tea plants (Camellia sinensis): Isolation, abundance, antimicrobial, and plant-growth-promoting activities. Biomed. Res. Int. 2018, 2018, 1470305.
- Xu, T.; Vo, Q.A.T.; Barnett, S.J.; Ballard, R.A.; Zhu, Y.; Franco, C.M.M. Revealing the underlying mechanisms mediated by endophytic actinobacteria to enhance the rhizobia-chickpea (Cicer arietinum L.) symbiosis. Plant Soil. 2022, 474, 299–318.
- Kruasuwan, W.; Thamchaipenet, A. Diversity of culturable plant growth-promoting bacterial endophytes associated with sugarcane roots and their effect of growth by co-inoculation of diazotrophs and actinomycetes. J. Plant Growth Regul. 2016, 35, 1074–1087.
- Baoune, H.; Ould El Hadj-Khelil, A.; Pucci, G.; Sineli, P.; Loucif, L.; Polti, M.A. Petroleum degradation by endophytic Streptomyces spp. isolated from plants grown in contaminated soil of southern Algeria. Ecotoxicol. Environ. Saf. 2018, 147, 602–609.




