
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hamada Masakazu | -- | 3186 | 2022-11-11 09:24:12 | | | |
| 2 | Hamada Masakazu | + 14 word(s) | 3200 | 2022-11-15 13:55:12 | | | | |
| 3 | Hamada Masakazu | Meta information modification | 3200 | 2022-11-15 13:55:13 | | |
Video Upload Options
Immune thrombocytopenic purpura (ITP) is an autoimmune disease characterized by isolated cryptogenic thrombocytopenia due to a transient or persistent reduction in platelet count. However, there have been no studies regarding H. pylori in the oral cavity of patients with ITP. Here, we describe a patient with ITP whose oral samples exhibited H. pylori. A 64-year-old woman with ITP came to our hospital with chief complaints that required oral surgery, including tooth extraction and cystectomy. Bacterial DNA of H. pylori was confirmed on the extracted tooth, but was not detected in saliva taken at the time. Bacterial DNA of H. pylori was detected on the suture around the extraction socket, which was removed at 10 days postoperatively. However, H. pylori DNA was not detected in other oral samples at 10 or 30 days postoperatively. The urea breath test was carried out in the gastrointestinal clinic at 60 days postoperatively, which revealed no presence of H. pylori in the gastrointestinal tract. These results suggest that teeth with severe bacterial infections may be a potential reservoir of H. pylori of patients with ITP.
1. Introduction
Immune thrombocytopenic purpura (also known as idiopathic thrombocytopenic purpura; ITP) is an autoimmune disease characterized by isolated thrombocytopenia with fewer than 100,000/µL platelets due to a transient or persistent reduction in platelet count [1]. Although ITP is presumably caused by autoimmune destruction of platelets in the spleen, the detailed mechanism underlying this phenomenon remains unknown.
Helicobacter pylori is a Gram-negative microaerophilic human gastric pathogen with strong urease activity and a helical structure [2]. H. pylori infection, which causes chronic gastritis, peptic ulcer disease and gastric cancer [2], can be diagnosed via the histological detection of bacteria in gastric biopsies, urea breath test and stool antigen test [3].
Associations between H. pylori and extragastric diseases, such as neurological diseases, respiratory diseases, and hematologic diseases, have been previously reported [4,5]. In 1998, it was reported that the number of platelets increased in patients with ITP after eradication of H. pylori [6]. In addition, studies focusing on the relationship between ITP and H. pylori infection have been reported in recent years [7,8], and eradication of H. pylori is regarded as an effective treatment for ITP [7,8].
There are approximately 700 bacterial species in the oral cavity, and it is not possible to specifically detect H. pylori from the oral cavity by the same methods used to detect H. pylori from the gastric tissue. To detect H. pylori in the oral cavity, molecular microbiological analyses, such as the polymerase chain reaction (PCR), have been widely used [9].
Although molecular biological analysis is used as a tool for diagnosis of H. pylori infection in the oral cavity, there have been no published studies regarding H. pylori detection in oral samples taken from patients with ITP. In the present report, we described the detection of H. pylori in the oral cavity of a patient with ITP who underwent oral surgery comprising tooth extraction and cystectomy.
2. How to detect H. pylori?
2.1. Ethical statement
This study was conducted in full adherence to the Declaration of Helsinki. This study protocol was approved by the Ethics Committee of Osaka University Graduate School of Dentistry (approval: H27-E17-1). Written consent to participate in this study was obtained from the subject patient. The patient first came to our clinic on October 19, 2017, underwent surgery on December 13, 2017, and the consultation at our clinic was completed on January 12, 2018.
2.2. DNA extraction
Bacterial DNA was extracted by previously described methods [10]. Briefly, oral specimens were resuspended in 250 μl of 10 mM Tris-HCl (pH 8.0) containing 100 mM NaCl and 1 mM EDTA. The cells were collected using centrifugation and lysed in 600 µl of Cell Lysis Solution (Qiagen, Düsseldorf, Germany) and incubated at 80 °C for 5 min, followed by the addition of 3 μl of RNase A (10 mg/ml; Qiagen) and incubation at 37 °C for 30 min. Protein Precipitation Solution (Qiagen) was added, vigorously vortexed for 20 s, and then centrifuged at 10,000 × g for 3 min. The supernatant was combined with 600 μl of isopropanol (Wako Pure Chemical Industries, Tokyo, Japan) and centrifuged. The precipitate was then resuspended in 70% ethanol (Wako Pure Chemical Industries), centrifuged, combined with 100 μl of TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]), and used as bacterial DNA for the PCR method.
2.3. PCR detection of bacteria
We previously searched the whole genome sequence of about 50 H. pylori strains in a database to construct a PCR method to detect H. pylori from oral specimens with high sensitivity and specificity [10].
For first-step PCR, 2 μl of bacterial DNA extracted from oral samples was used in a 20 μl reaction. For second-step PCR, 1 μl of the first-step PCR amplification product was used as a template for reaction in a total volume of 20 μl. Both the first and second steps of nested PCR were performed as described previously, using TaKaRa Ex Taq polymerase (Takara Bio. Inc., Otsu, Japan) [11]. During each nested PCR reaction, sterile distilled water was used in place of bacterial DNA as a negative control to ensure that no false positive reactions occurred. PCR amplification was performed with the following cycling parameters: initial denaturation at 95°C for 4 minutes, followed by 30 amplification cycles (95°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds), and a final extension at 72°C for 7 minutes. The amplicon size of the first-step PCR product was 488 bp and the amplicon size of the second-step PCR product was 383 bp [11].
3. Detection of H. pylori from ITP patient
A 64-year-old woman with ITP was presented to the Department of Oral and Maxillofacial Surgery at Osaka University Dental Hospital for tooth extraction and cystectomy due to a radicular cyst. She had been diagnosed with ITP in the Department of Hematology, three years prior. The patient’s platelet count was stable at ap-proximately 30–50×103/μL; thus, the hematologist considered her to be at low risk of hemorrhagic diathesis, and she received a routine dental check-up.
In an intraoral examination, temporary sealing with cement was observed on the right mandibular first molar (Figure 1A). Although intraoral examination showed no obvious gingival swelling around the roots of the right mandibular molar region, panoramic radiography showed a cystic lesion around the root apices of the right mandibular first molar (Figure 2A-C). Therefore, we selected tooth extraction and cystectomy as treatment. Although the patient’s preoperative platelet count was 36×103/μL, which was below the normal range, we performed tooth extraction and cystectomy under intravenous sedation without platelet transfusion based on the hematologist’s opinion that the patient was at low risk of hemorrhagic diathesis. No abnormal bleeding was observed in the socket, and the operative site was packed with Surgicel® (Ethicon Inc., New Brunswick, NJ, USA) and suture; a splint was then placed for hemostasis.

Figure 1. Intraoral photographs taken at the first visit to our hospital. The first molar was temporarily sealed with cement.
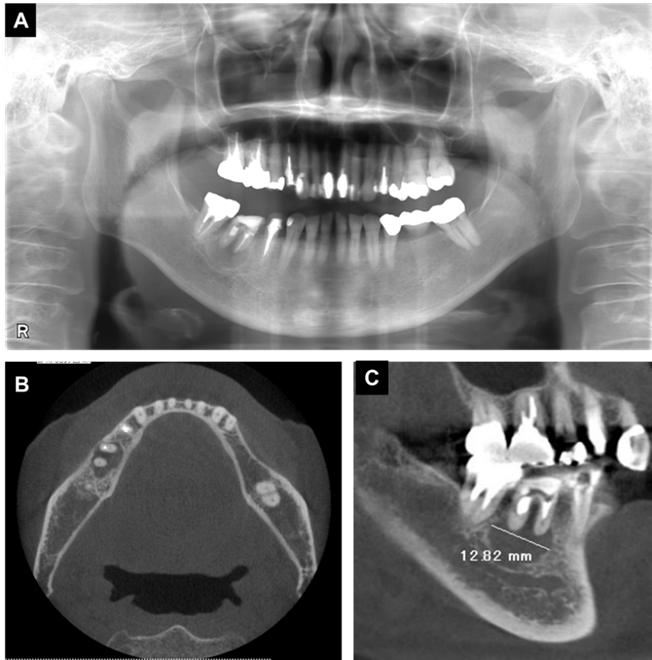
Figure 2. X-ray examination findings at the initial visit. (A) Panoramic radiography, (B, C) Computed tomography images. Radiolucent findings were observed at the root apex of the first molar.
To analyze whether H. pylori was present in the patient’s oral cavity, samples of the extracted tooth were taken intraoperatively with the patient’s consent (Figure 3A). In addition, 1 mL of unstimulated saliva was collected in sterile plastic tubes intraoperatively and postoperatively (after 10 and 30 days). A suture and splint were included in the analysis; these were removed at 10 days postoperatively. Finally, dental plaque samples were taken from four teeth (upper left second premolar, lower left second premolar, lower right second premolar, and lower right second molar) during a follow-up visit at 30 days postoperatively. The patient was not given any restrictions such as brushing the teeth or eating before oral samples collection.
The first-step PCR did not detect H. pylori DNA in oral specimens. Next, second-step PCR detected H. pylori DNA on the extracted teeth, but not detected in saliva taken intraoperatively (Figure 3B). In addition, 2nd step PCR detected H. pylori on the suture used at the extracted tooth site, which was removed from the site at 10 days postoperatively, whereas H. pylori was not detected in saliva or on the splint taken on the same day. Moreover, H. pylori was not detected in dental plaque samples from four teeth or in saliva samples taken at 30 days postoperatively. The bands obtained by the nested PCR method were sequenced using previously described methods [11,12], and confirmed to correspond to the H. pylori genes.
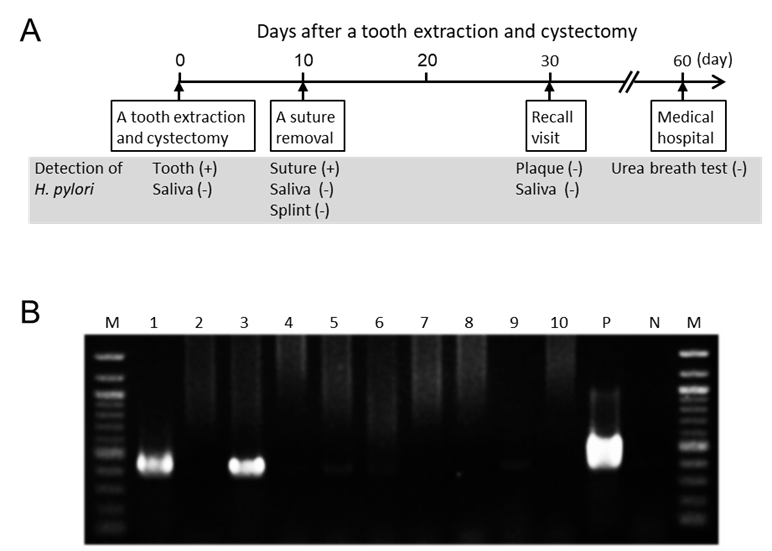
Figure 3. Detection of Helicobacter pylori in oral samples. (A) Time course of the present case after tooth extraction and cystectomy. (B) Polymerase chain reaction results for detection of H. pylori in oral samples. Lanes: 1, Extracted tooth; 2, Saliva taken immediately preoperatively; 3, Suture taken 10 days postoperatively; 4, Saliva taken 10 days postoperatively; 5, Splint taken 10 days postoperatively; 6, Dental plaque (upper left second premolar) taken 30 days postoperatively; 7, Dental plaque (lower left second premolar) taken 30 days postoperatively; 8, Dental plaque (lower right second premolar) taken 30 days postoperatively; 9, Dental plaque (lower right second molar) taken 30 days postoperatively; 10, Saliva taken 30 days postoperatively; P (positive control), H. pylori ATCC 43504; N (negative control), sterile water; M, 100-bp DNA ladder.
Based on these results, we referred the patient to a gastroenterologist to examine the presence of H. pylori in the gastrointestinal tract. On postoperative day 60, the gastroenterologist performed a urea breath test using urea labeled with 13C, which was a negative result for H. pylori.
During the follow-up at our hospital, a one-month postoperative panoramic radiography showed no problems. Then, a family dentist performed subsequent prosthetic treatment and follow-up. More than four years have passed since the tooth extraction, and there have been no visits to our clinic due to postoperative problems.
4. Why was H. pylori detected in ITP patients?
An association between ITP and H. pylori infection was first reported in 1998 [6]. Within the past 20 years, a large number of studies have demonstrated that H. pylori is an etiological factor in the onset of ITP [7,8]. Platelet count was reported to be significantly increased by 3.5–4.2-fold after H. pylori eradication therapy in patients with ITP [7,8]. However, all of the prior studies were focused on the relationship between ITP and H. pylori infection in gastric tissues. Therefore, we examined whether H. pylori was present in the oral cavity of a patient with ITP.
We investigated the distribution of H. pylori in oral samples from the patient, such as saliva and the extracted tooth. Detection of H. pylori in these oral samples was performed by nested PCR using H. pylori detection primer sets targeting the ureA gene, as in our previous study [10]. Nested PCR showed H. pylori DNA on the extracted tooth, but not in saliva taken intraoperatively. In addition, saliva samples taken at 10 and 30 days postoperatively were negative for H. pylori, as were dental plaque samples taken from four different teeth at 30 days postoperatively. However, H. pylori DNA was detected on a suture used in the extraction socket, which was removed at 10 days postoperatively. These results indicated that H. pylori was localized to the extracted tooth, but was not present in saliva or on most other oral surfaces.
Recently, it was reported that a unique oral microbiome forms in subjects under special systemic conditions including disease or congenital anomalies [13]. Furthermore, the oral conditions of individuals in many developing countries in Asia are worse than in Japan [14,15]. The unique oral environment greatly influences H. pylori colonization in the oral cavity. For example, the association between periodontal disease and oral H. pylori infection has attracted attention. H. pylori colonize in periodontal pockets formed by periodontal disease [16], and periodontal treatment reduces H. pylori reinfection into the stomach [17]. H. pylori and Porphyromonas gingivalis, a major periodontopathic bacteria, are often detected together in the oral cavity [37]. H. pylori is also more likely to be detected in the presence of a specific periodontopathic bacterial group called the red complex (Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia) and less likely to be detected in the presence of the orange complex (Prevotella intermedia, Prevotella nigrescens, and Campylobacter rectus) or green complex (Capnocytophaga ochracea, Capnocytophaga sputigena, and Eikenella corrodens) [18]. In the present case report, H. pylori colonized the cystic lesion around the root apices of an ITP patient, and the specific environment or oral bacterial species present in the lesion may be involved in the colonization of H. pylori. These results suggest that the prevention of dental disease is essential in controlling H. pylori colonization.
The oral cavity is a possible reservoir for H. pylori and thus plays a crucial role in both H. pylori transmission and gastric infection [19]. In the present case, the patient completed a urea breath test after tooth extraction, which revealed that no H. pylori was present in the gastrointestinal tract. H. pylori may not have been detected in the gastrointestinal tract because it was present only in a limited portion of the extracted tooth and was not present in the gastrointestinal tract before the tooth extraction. Conversely, H. pylori may have been present in both the extracted tooth and the gastrointestinal tract; thus, H. pylori was eliminated by extraction of the tooth that constituted a reservoir for bacterial colonization. If possible, a urea breath test should have been performed before tooth extraction to demonstrate the presence of H. pylori in the gastric tissue. In future studies, a larger number of patients with ITP should be analyzed, focusing on the presence of H. pylori in both the oral cavity and the gastrointestinal tract, to confirm whether eradication of H. pylori from the oral cavity leads to its eradication from the gastrointestinal tract.
We prescribed 1 g of cefazolin intravenously during tooth extraction and 100 mg of cefditoren pivoxil three times a day after each meal for three days after tooth extraction. The main purpose of antibiotic administration after tooth extraction is to prevent infection. However, β-lactam antibiotics, such as cefazolin and cefditoren pivoxil, are also used to eradicate H. pylori [20], and they may be effective at eradicating H. pylori remaining in the tooth extraction sockets.
This study had some limitations. First, our data have only highlighted a simple casual relationship between oral H. pylori and ITP. To clarify the many unknown factors involved, a longer follow-up of a large number of patients is required to determine the association between H. pylori in the oral cavity and a clinical improvement in ITP. We should monitor changes in patient condition after the removal of H. pylori including improved clinical findings of ITP, and reinfection of H. pylori. In addition, the relationship between H. pylori in the oral cavity and that in the gastric tissues needs to be clarified. Second, since the subject had no subjective symptoms in the digestive system and her platelets were decreased, the subject requested a urea breath test, which is a non-invasive and simple test. However, if possible, a definitive diagnosis should be performed by culture test for H. pylori using tissue samples from gastroscopy. In addition, antibody testing and histopathologic analysis of H. pylori should be performed. Third, since our treatment was completed after tooth extraction, we only had information on platelet count immediately after tooth extraction. Previous reports have shown that platelet count significantly increases in ITP patients 6 months after eradication treatment [7,8]. Therefore, data on platelet count should be collected for at least 6 months even after removal of H. pylori in the oral cavity.
5. Conclusions
H. pylori DNA was detected in the oral cavity, localized around the extracted tooth of a patient with ITP. Although the causal relationship between the oral detection of H. pylori and ITP is still unknown, teeth with severe bacterial infections, such as radicular cysts, are potential reservoirs of H. pylori. Based on these findings, dental treatment or oral care that lead to eradication or reduction of H. pylori from the oral cavity recommend in patients with diseases such as ITP.
References
- Rodeghiero, F.; Stasi, R.; Gernsheimer, T.; Michel, M.; Provan, D.; Arnold, D.M.; Bussel, J.B.; Cines, D.B.; Chong, B.H.; Cooper, N.; et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood 2009, 113, 2386-2393, doi:10.1182/blood-2008-07-162503.
- Fennerty, M.B. Helicobacter pylori. Arch Intern Med 1994, 154, 721-727.
- Wang, Y.K.; Kuo, F.C.; Liu, C.J.; Wu, M.C.; Shih, H.Y.; Wang, S.S.; Wu, J.Y.; Kuo, C.H.; Huang, Y.K.; Wu, D.C. Diagnosis of Helicobacter pylori infection: Current options and developments. World J Gastroenterol 2015, 21, 11221-11235, doi:10.3748/wjg.v21.i40.11221.
- de Korwin, J.D.; Ianiro, G.; Gibiino, G.; Gasbarrini, A. Helicobacter pylori infection and extragastric diseases in 2017. Helicobacter 2017, 22 Suppl 1, doi:10.1111/hel.12411.
- Goni, E.; Franceschi, F. Helicobacter pylori and extragastric diseases. Helicobacter 2016, 21 Suppl 1, 45-48, doi:10.1111/hel.12340.
- Gasbarrini, A.; Franceschi, F.; Tartaglione, R.; Landolfi, R.; Pola, P.; Gasbarrini, G. Regression of autoimmune thrombocytopenia after eradication of Helicobacter pylori. Lancet 1998, 352, 878, doi:10.1016/s0140-6736(05)60004-9.
- Hwang, J.J.; Lee, D.H.; Yoon, H.; Shin, C.M.; Park, Y.S.; Kim, N. The Effects of Helicobacter pylori Eradication Therapy for Chronic IdiopathicThrombocytopenic Purpura. Gut Liver 2016, 10, 356-361, doi:10.5009/gnl14483.
- Amiri, M. Impact of Helicobacter pylori Eradication Therapy on Platelet Counts in Patients With Chronic Idiopathic Thrombocytopenic Purpura. Glob J Health Sci 2015, 8, 35-40, doi:10.5539/gjhs.v8n7p35.
- Westblom, T.U.; Bhatt, B.D. Diagnosis of Helicobacter pylori infection. Curr Top Microbiol Immunol 1999, 241, 215-235, doi:10.1007/978-3-642-60013-5_11.
- Ogaya, Y.; Nomura, R.; Watanabe, Y.; Nakano, K. Detection of Helicobacter pylori DNA in inflamed dental pulp specimens from Japanese children and adolescents. J Med Microbiol 2015, 64, 117-123, doi:10.1099/jmm.0.079491-0.
- Nomura, R.; Ogaya, Y.; Matayoshi, S.; Morita, Y.; Nakano, K. Molecular and clinical analyses of Helicobacter pylori colonization in inflamed dental pulp. BMC Oral Health 2018, 18, 64, doi:10.1186/s12903-018-0526-2.
- Nakano, K.; Inaba, H.; Nomura, R.; Nemoto, H.; Takeda, M.; Yoshioka, H.; Matsue, H.; Takahashi, T.; Taniguchi, K.; Amano, A.; et al. Detection of cariogenic Streptococcus mutans in extirpated heart valve and atheromatous plaque specimens. J Clin Microbiol 2006, 44, 3313-3317, doi:10.1128/jcm.00377-06.
- Mitsuhata, C.; Kado, N.; Hamada, M.; Nomura, R.; Kozai, K. Characterization of the unique oral microbiome of children with Down syndrome. Sci Rep 2022, 12, 14150, doi:10.1038/s41598-022-18409-z.
- Asao, Y.; Iwamoto, Y.; Mitsuhata, C.; Naito, M.; Kozai, K. Three-year survey of oral hygiene conditions of Cambodian public primary school children. J Oral Sci 2022, 64, 208-211, doi:10.2334/josnusd.21-0464.
- Asao, Y.; Asao, Y.; Iwamoto, Y.; Chea, C.; Chher, T.; Mitsuhata, C.; Naito, M.; Kozai K. The effect of improving oral health literacy among teachers on the oral health condition of primary schoolchildren in Cambodia. Eur. J. Paediatr. Dent. in press.
- Lauritano, D.; Cura, F.; Candotto, V.; Gaudio, R.M.; Mucchi, D.; Carinci, F. PERIODONTAL POCKETS AS A RESERVOIR OF HELICOBACTER PYLORI CAUSING RELAPSE OF GASTRIC ULCER: A REVIEW OF THE LITERATURE. J Biol Regul Homeost Agents 2015, 29, 123-126.
- Tongtawee, T.; Wattanawongdon, W.; Simawaranon, T. Effects of periodontal therapy on eradication and recurrence of Helicobacter pylori infection after successful treatment. J Int Med Res 2019, 47, 875-883, doi:10.1177/0300060518816158.
- Kadota, T.; Hamada, M.; Nomura, R.; Ogaya, Y.; Okawa, R.; Uzawa, N.; Nakano, K. Distribution of Helicobacter pylori and Periodontopathic Bacterial Species in the Oral Cavity. Biomedicines 2020, 8, doi:10.3390/biomedicines8060161.
- Payão, S.L.; Rasmussen, L.T. Helicobacter pylori and its reservoirs: A correlation with the gastric infection. World J Gastrointest Pharmacol Ther 2016, 7, 126-132, doi:10.4292/wjgpt.v7.i1.126.
- Kadota, T.; Ogaya, Y.; Hatakeyama, R.; Nomura, R.; Nakano, K. Comparison of oral flora before and after triple therapy for Helicobacter pylori eradication in patient with gastric disease. Odontology 2019, 107, 261-267, doi:10.1007/s10266-018-0393-y.




