
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maurizio Muraca | + 1045 word(s) | 1045 | 2020-05-27 05:55:48 | | | |
| 2 | Nicole Yin | + 1 word(s) | 1046 | 2020-12-08 05:05:58 | | |
Video Upload Options
Bronchopulmonary Dysplasia is a chronic respiratory disease that affects a significant fraction of former extremely premature infants, this disease is a heterogeneous condition that develops on an extremely preterm lung exposed to different pathogenetic noxae.
1. Introduction
The most often-mentioned consensus on how to diagnose BPD comes from the NICHD 2001[1]. This definition considers two criteria: weeks of gestation (WG); and the need for supplemental oxygen beyond 28 days of life and/or oxygen dependence at 36 weeks post-menstrual age (PMA). To improve on this diagnosis, a physiological measure, like the “room challenge test”, was suggested[2].
In 2018, the NICHD proposed a revision[3] of their definition, considering newer methods of noninvasive ventilation, a reclassification based on grades, and radiographic evidence of pulmonary parenchymal disease.
Though useful to neonatologists, these definitions do not seem to predict long-term outcomes. Those caring for patients with BPD are more interested in establishing new diagnostic criteria that can shed light on a patient’s likely outcome. They also seek a more comprehensive definition of the disease, based on accurate clinical pathophysiology and the identification of biomarkers, rather than on interventions[4]. An international neonatal consortium[5] recently emphasized the concept that a definition of BPD should be clinically meaningful and strongly associated with the subsequent development of respiratory problems. The long-term effects of BPD are not all reached by 36 weeks after conception, making this age a less important milestone for the purposes of BPD stratification.
For this purpose, Isayama et al.[6] explored different timepoints to better diagnose BPD and predict respiratory outcomes, identifying the need for oxygen at 40 weeks PMA as the best predictor for serious respiratory morbidity, leaving an open field to the debate not only on which clinical data but also on which timepoint to be used.
Jensen et al.[7] recently reviewed the 18 existing definitions of BPD with a view to identify the most appropriate one for predicting childhood morbidity through 18–26 months. They found that the best definition (correctly predicting death or severe respiratory morbidity in 81% of cases) classified disease severity on ventilatory support at 36 weeks PMA alone, regardless of any supplemental oxygen use.
As Thebaud et al.[8] explained, a definition’s predictive value can be improved by means of better objective measurements and biomarkers of lung injury and BPD, and by incorporating antenatal factors, such as intrauterine growth restriction (IUGR), a mother’s hypertension or smoking, and male sex of her offspring. A single definition is unlikely to be able to address all the needs of neonatologists and pediatricians, caregivers, pharmacists, parents, and industries, nor is it likely to point towards significant endpoints. However, a more appropriate general definition would provide a better description of patients with BPD and enable more targeted preventive therapies.
2. Pathology
In infants with BPD, the pathological findings[9] include fewer secondary septa and alveoli, and sites of emphysema, meaning a smaller alveolar surface area, which is crucial to proper gas exchange. Angiogenesis is impaired with dysmorphic vessels and capillaries. Thickening of the muscle layer of the arterioles prompts an increase in vascular resistance and pulmonary hypertension. More fibrotic tissue is formed, with a widening and thickening of the interstitial spaces (Figure 1). This picture differs slightly from earlier descriptions (before surfactants became available), such as Northway’s [10] report of injury, inflammation and fibrosis caused by volutrauma or barotrauma, and oxygen toxicity. Today’s “new” BPD is the result of less severe injury in very immature lungs[11], but very little is known about the pathology of this “new BPD”—partly because fewer autopsies are being performed.
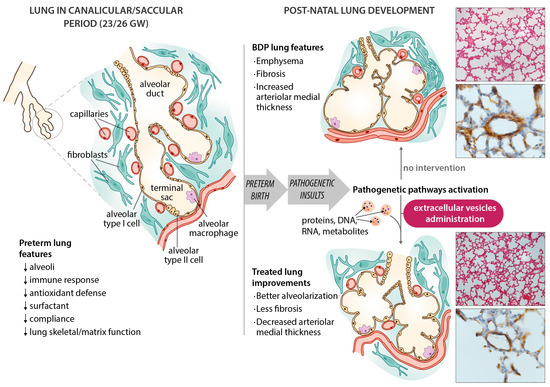
Figure 1. Schematic diagram of bronchopulmonary dysplasia (BPD) pathogenesis, with the main features of the disease in the preterm lung. Microphotographs show improvements in an experimental animal model (60% hyperoxia-induced BPD in rat pups) after administering extracellular vesicles (EVs). Representative lung sections from hyperoxia-induced BPD (top right), and EV-treated animals (bottom right), showing effects of hyperoxia and EVs on alveolarization (upper side of microphotographs; stained with hematoxylin and eosin, scale bars 150 µm) and vessels less than 100 µm in diameter (lower side of the microphotographs; stained with anti-α-smooth muscle actin antibodies, scale bars 23.8 µm). Hyperoxia-induced changes in alveolarization (lower total number of alveoli; larger alveolar volume) and vascularization (increased medial thickness index) are minimized by the intratracheal administration of MSC-EVs.
Angiogenesis and vessel branching reportedly drive alveolar growth in the lung, so the dysmorphic vascular architecture and angiogenesis described in BPD patients are important. Vascular channels have been described in infants who died of BPD. These vessels run through lobular sites normally occupied by veins towards the pulmonary arteries, then to the vasa vasorum of the pulmonary plexus and bronchial venous plexus[12]. These changes presumably provide the anatomical grounds for pulmonary hypertension in BPD, but these shunts may also serve as a “pop-up” valve to reduce the severity of pulmonary hypertension.
More collaboration between neonatologists and pathologists, a greater awareness of the importance of conducting autopsies on deceased BPD patients, high-resolution imaging, and increasingly reliable animal models could help us to shed more light on the still dark side of BPD.
3. Pathogenesis
A better understanding of how the premature infant’s lung develops and of the pathogenesis of BPD could drive the search for novel treatments. Lung growth appears to be an intricate, highly orchestrated process involving multiple cell lines, and is guided by numerous different signaling pathways, which could all be disrupted by factors implicated in the onset of BPD[13].
At birth, most preterm newborns are in the saccular and canalicular phases of lung morphogenesis (assuming a viability limit at 24 weeks PMA). There have occasionally been reports of survivors born at 22 WG coping with breathing even during the canalicular phase of lung development[14].
Given the cellular architecture of an underdeveloped lung, low gestational age is the most significant determinant correlating with the onset of BPD. The premature lung has several features that predispose it to BPD, in addition to the well-known lack of surfactant. It also has: less-developed skeletal airway structures (extracellular matrix, collagen or elastin); less-developed antioxidant mechanisms; a lower compliance; and inadequate fluid clearance[15]. All these features make the lung more vulnerable, but many other factors contribute to causing BPD in such vulnerable tissue. BPD has a multifactorial etiology, with prenatal and postnatal mechanisms causing inflammation and injury. The consequent disruption of the lung’s development also involves an aberrant repair mechanism.
References
- Jobe, A.H.; Bancalari, E. Bronchopulmonary dysplasia. In Proceedings of the American Journal of Respiratory and Critical Care Medicine; American Thoracic Society: New York, NY, USA, 2001; Volume 163, pp. 1723–1729. [Google Scholar] [CrossRef]
- Walsh, M.C.; Wilson-Costello, D.; Zadell, A.; Newman, N.; Fanaroff, A. Safety, reliability, and validity of a physiologic definition of bronchopulmonary dysplasia. J. Perinatol. 2003, 23, 451–456.
- Higgins, R.D.; Jobe, A.H.; Koso-Thomas, M.; Bancalari, E.; Viscardi, R.M.; Hartert, T.V.; Ryan, R.M.; Kallapur, S.G.; Steinhorn, R.H.; Konduri, G.G.; et al. Bronchopulmonary dysplasia: Executive summary of a workshop. J. Pediatr. 2018, 197, 300–308.
- Ibrahim, J.; Bhandari, V. The definition of bronchopulmonary dysplasia: An evolving dilemma. Pediatr. Res. 2018.
- Steinhorn, R.; Davis, J.M.; Göpel, W.; Jobe, A.; Abman, S.; Laughon, M.; Bancalari, E.; Aschner, J.; Ballard, R.; Greenough, A.; et al. Chronic pulmonary insufficiency of prematurity: Developing optimal endpoints for drug development. J. Pediatr. 2017, 191, e1-21.
- Isayama, T.; Lee, S.K.; Yang, J.; Lee, D.; Daspal, S.; Dunn, M.; Shah, P.S. Revisiting the definition of bronchopulmonary dysplasia effect of changing panoply of respiratory support for preterm neonates. JAMA Pediatr. 2017, 171, 271–279.
- Jensen, E.A.; Dysart, K.; Gantz, M.G.; McDonald, S.; Bamat, N.A.; Keszler, M.; Kirpalani, H.; Laughon, M.M.; Poindexter, B.B.; Duncan, A.F.; et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants an evidence-based approach. Am. J. Respir. Crit. Care Med. 2019, 200, 751–759.
- Bernard Thébaud; Kara N. Goss; Matthew Laughon; Jeffrey A. Whitsett; Steven H. Abman; Robin H. Steinhorn; Judy L. Aschner; Peter G. Davis; Sharon A. McGrath-Morrow; Roger F. Soll; et al.Alan H. Jobe Bronchopulmonary dysplasia. Nature Reviews Disease Primers 2019, 5, 1-23, 10.1038/s41572-019-0127-7.
- Jacqueline J. Coalson; Pathology of Bronchopulmonary Dysplasia. Seminars in Perinatology 2006, 30, 179-184, 10.1053/j.semperi.2006.05.004.
- William H. Northway; Robert C. Rosan; David Y. Porter; Pulmonary Disease Following Respirator Therapy of Hyaline-Membrane Disease. New England Journal of Medicine 1967, 276, 357-368, 10.1056/nejm196702162760701.
- Cristina M Alvira; Rory E. Morty; Can We Understand the Pathobiology of Bronchopulmonary Dysplasia?. The Journal of Pediatrics 2017, 190, 27-37, 10.1016/j.jpeds.2017.08.041.
- Csaba Galambos; Sunder Sims-Lucas; Steven H. Abman; Histologic Evidence of Intrapulmonary Anastomoses by Three-Dimensional Reconstruction in Severe Bronchopulmonary Dysplasia. Annals of the American Thoracic Society 2013, 10, 474-481, 10.1513/annalsats.201305-124oc.
- David E. Surate Solaligue; José Alberto Rodríguez-Castillo; Katrin Ahlbrecht; Rory Edward Morty; Recent advances in our understanding of the mechanisms of late lung development and bronchopulmonary dysplasia. American Journal of Physiology-Lung Cellular and Molecular Physiology 2017, 313, L1101-L1153, 10.1152/ajplung.00343.2017.
- Marilee C. Allen; Pamela K. Donohue; Amy E. Dusman; The Limit of Viability -- Neonatal Outcome of Infants Born at 22 to 25 Weeks' Gestation. New England Journal of Medicine 1993, 329, 1597-1601, 10.1056/nejm199311253292201.
- Eugenio Baraldi; Marco Filippone; Chronic Lung Disease after Premature Birth. New England Journal of Medicine 2007, 357, 1946-1955, 10.1056/nejmra067279.




