
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gordana Šelo | -- | 5106 | 2022-11-07 10:57:30 |
Video Upload Options
Agro-food industrial residues (AFIRs) are generated in large quantities all over the world. The vast majority of these wastes are lignocellulosic wastes that are a source of value-added products. Technologies such as solid-state fermentation (SSF) for bioconversion of lignocellulosic waste, based on the production of a wide range of bioproducts, offer both economic and environmental benefits. The versatility of application and interest in applying the principles of the circular bioeconomy make SSF one of the valorization strategies for AFIRs that can have a significant impact on the environment of the wider community.
1. Introduction
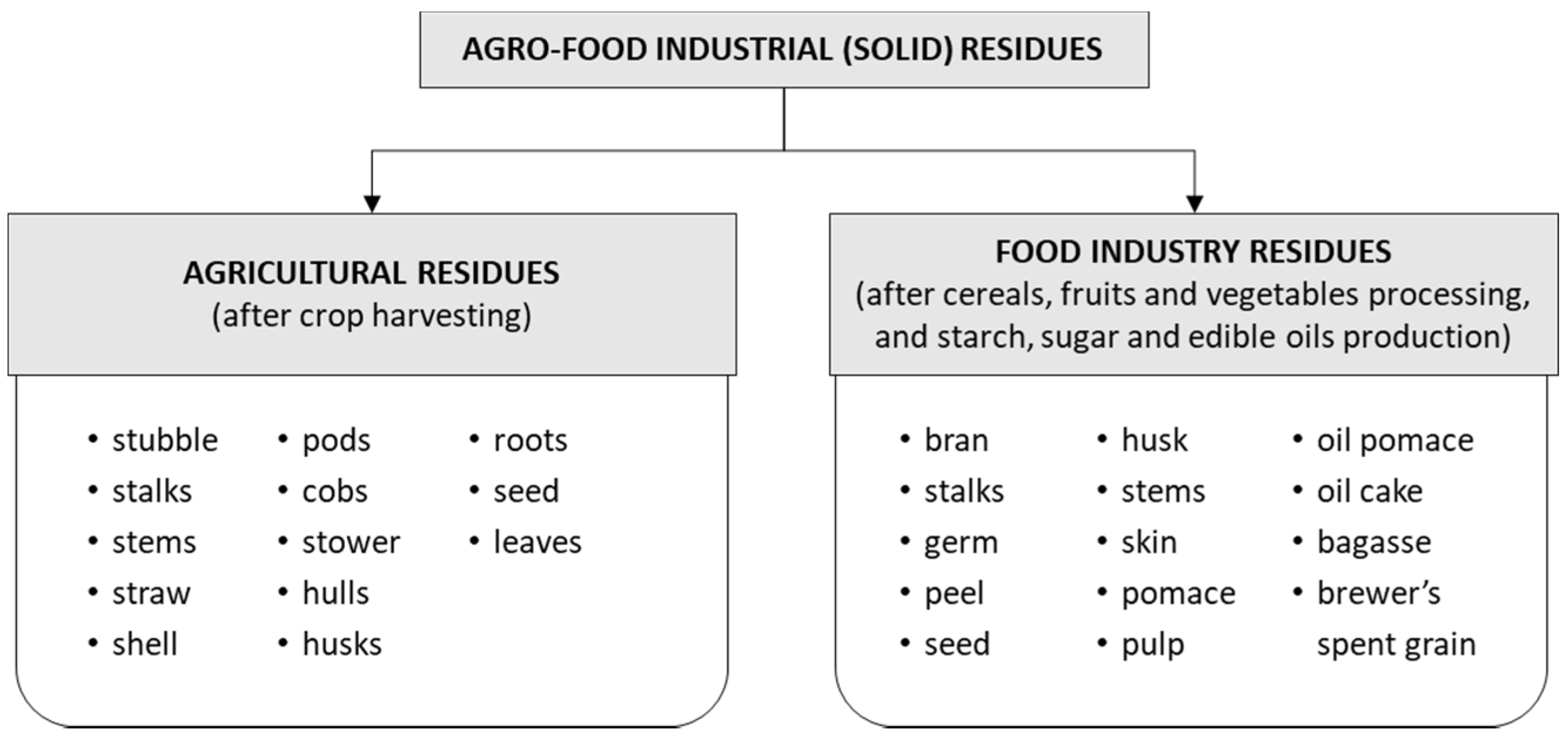
2. Agro-Food Industrial Residues
3. Solid-State Fermentation (SSF)
3.1. General
3.2. Substrates Used in SSF
3.3. Microorganisms Used in SSF
4. Enzyme Production by SSF
4.1. Lignocellulolytic Enzymes
4.2. Cellulolytic Enzymes
|
Enzymes |
Microorganism |
Substrate |
Reference |
|
|---|---|---|---|---|
|
Lignolytic |
laccase |
Trametes versicolor |
corn silage, brewers’ spent grain, barley husk |
|
|
Trametes pubescens |
banana skin |
[37] |
||
|
Pleurotus eryngii |
peach waste |
[59] |
||
|
Aspergillus flavus PUF5 |
dried ridge gourd peel |
[60] |
||
|
Ganoderma lucidum |
wheat bran |
[61] |
||
|
Lysinibacillus sp. |
wheat bran |
[62] |
||
|
manganese peroxidase lignin peroxidase |
Inonotus obliquus |
birch branch, beech branch, rice straw, wheat straw, wheat bran, sugarcane bagasse, cassava peel, peanut shell |
[52] |
|
|
Cellulolytic |
cellulase endoglucanase exoglucanase |
Trichoderma sp. |
corn cob, wheat bran |
[63] |
|
Penicillium roqueforti |
rice husk |
[64] |
||
|
Aspergilus fumigatus |
wheat straw |
[65] |
||
|
Thermoascus aurantiacus |
Jatropha deoiled seed cake |
[53] |
||
|
Aspergillus fumigatus |
wheat straw |
[66] |
||
|
Trichoderma viride Ganoderma lucidum |
corn stover |
[67] |
||
|
cellobiase |
Humicola insolens |
paddy straw, soybean pod husk, sugarcane bagasse, groundnut shells, corn stalks and pigeonpea pod husk |
[68] |
|
|
β-glucosidase |
Lichtheimia ramosa |
wheat bran, soy bran, corn cob, corn straw, rice peel, sugar cane bagasse |
[69] |
|
|
Thermoascus aurantiacus Aureobasidium pullulans |
wheat bran, soy bran, soy peel, corn cob, corn straw |
[70] |
||
|
Trichoderma viride Ganoderma lucidum |
corn stover |
[67] |
||
|
Hemicellulolytic |
xylanase |
Aspergillus oryzae |
wheat bran |
[34] |
|
Aspergillus tubingensis |
wheat straw, sorghum straw |
[71] |
||
|
Bacillus stearothermophilus |
wheat bran |
[72] |
||
|
Aspergillus niger |
rice straw |
[73] |
||
|
Aspergillus awamori |
tomato pomace |
[74] |
||
|
Thermomyces lanuginosus |
wheat bran |
[75] |
||
|
Humicola insolens |
paddy straw, soybean pod husk, sugarcane bagasse, groundnut shells, corn stalks and pigeonpea pod husk |
[68] |
||
4.3. Hemicellulolytic Enzymes
5. Production of Phenolic Compounds and Other Value-Added Compounds
|
Products |
Conditions |
Remarks |
Reference |
|---|---|---|---|
|
Total polyphenolic compounds from apple pomace |
Substrate: apple pomace, treated with inducers: copper sulphate (2 mM), veratryl alcohol (2 mM) and Tween-80 (0.1%); pH 4.5; autoclaved (121 °C, 30 min), moisture content 72% w/v. Microorganism: P. chrysosporium, inoculation with spore suspension (2.5 × 106 spores/g of solid). SSF: carried out in flasks, in controlled environment at 37 ± 1 °C for 14 days. Extraction (optimization):
After the extraction, sample mixture was centrifuged at 9268× g for 20 min to obtain the supernatant for further determination of total phenolic content (at 725 nm) and free radical scavenging activity (DPPH method at 517 nm). |
The phenol content was higher in the fermented apple pomace, and the antioxidant activity correlated with the increase in polyphenol content, with both values depending on the type of solvent, extraction temperature, extraction time, and method used. |
[87] |
|
Individual polyphenolic compound from grape pomace |
Substrate: corn silage, particle size 1.0–2.0 cm; autoclaved (121 °C, 20 min). Microorganism: T. versicolor TV-6, cultivated on PDA medium for 7 days at 27 °C; five mycelial plugs (diameter 1 cm) suspended in 10 cm3 of sterile water (inoculum). SSF: performed in laboratory jars at 27 °C for 5, 9, 13, and 20 days. Extraction: milled dry substrate after SSF was extracted by 50% ethanol with solid/liquid ratio 1:40, in a shaking-water bath at 80 °C by (200 rpm) for 120 min. After the extraction, samples were centrifuged for 10 min at 10,000× g in order to obtain liquid extracts for further UHPLC analysis of phenolic acids. |
After 20 days of corn silage treatment with T. versicolor, 10.4-, 3.4-, 3.0-, and 1.8-fold increments in extraction yield of syringic acid, vanillic acid, p-hydroxybenzoic acid, and caffeic acid, respectively, were reached. |
[56] |
|
Phenolic antioxidants from grape waste |
Substrate: grape waste, dehydrated at 60 °C/24 h, pulverized (30-mesh), stored at 22 °C. Microorganism: different fungal strains: A. niger GH1, PSH, Aa-20, ESH; Penicillium pinophilum ESH2, ESH3; Penicillium purpurogenum GH2; inoculation with 2 × 107 fungal spores per gram of solid support. SSF: performed in tray reactor at 30 °C/60 h. Assay: total antioxidant activity of the extracts was tested by two different free radical (DPPH· and ABTS·+) inhibitions; free gallic acid content was estimated by HPLC. |
The extracts of grape waste enhanced their free radical scavenging and preserved the capacity to avoid the lipid peroxidation after SSF. Gallic acid is not the only phenolic compound related to the free radical scavenging and antioxidant properties of the fermented samples. |
[88] |
|
Phenolic antioxidants from pomegranate peels |
Substrate: pomegranate peels, cleaned, dried at 60 °C/48 h, pulverized, stored at room temperature in black bags. Microorganism: A. niger GH1; inoculation with 2 × 107 spores/g of plant material, or substrate impregnated with culture broth. SSF: carried out in flasks at 30 °C for 96 h. Assay: tannins were analyzed using a spectrophometric method; concentration of gallic and ellagic acids was determined by HPLC. |
The ellagic acid was accumulated considerably in pomegranate peels after fungal fermentation, which demonstrated that the high level of hydrolysable tannins in pomegrante peel tannins are mainly ellagitannins. |
[89] |
|
Phenolic antioxidants from chokeberry pomace |
Substrate: chokeberry (cultivar “Nero”) pomace, dried < 40 °C, ground (0.5–1 mm), stored at 18 °C; moisturized (65%) with a nutrient solution (containing yeast extract and glucose), pH 5.5; autoclaved at 121 °C/30 min. Microorganism: A. niger ATCC-6275 and R. oligosporus ATCC-22959; inoculating cultures were produced by growing the strains on fresh PDA at 27 °C for 10 days, and spore inoculum was prepared by washing the agar surface with sterile distilled water. SSF: was carried out in in Erlenmeyer flasks at 30 °C for 12 days; substrate was inoculated with spore suspension 2 × 107 spores/g of solid. Extraction: in an ultrasonic bath for 30 min at 40 °C with solvent mixture (hydrochloric acid: methanol: water in the ratio 1: 80: 19). The mixtures were centrifuged (4000× g for 10 min); supernatants were filtered and evaporated under vacuum and then stored in methanol (4 °C) until analysis (total phenolics, flavonoids, and anthocyanins; individual phenolics; antioxidant activities). |
The extractable phenolics increased more than 1.7-fold during both fermentation processes, and a similar trend was observed for total flavonoids. The free radical scavenging ability of phenolic extracts were significantly enhanced during the SSFs. The amounts of flavonols and cinnamic acids increased while the concentrations of glycosylated anthocyanins decreased substantially. |
[90] |
|
Water-soluble phenolic antioxidants from cranberry pomace |
Substrate: freshly pressed cranberry pomace, vacuum-dried and stored in a refrigerator. Microorganism: Lentinus edodes was maintained on PDA slants and Petri plates at 4 °C and sub-cultured. The fungus was resuscitated by transferring onto a PDA plate and cultured at room temperature 20 days before use. SSF: carried out in in Erlenmeyer flasks at 28 °C for 25 days (cranberry pomace + calcium carbonate + water + ammonium nitrate or fish protein hydrolysate was autoclaved at 121 °C for 20 min and the vegetative mycelia from one PDA plate were inoculated into flasks). Extraction: distilled water or 95% ethanol was added to fungus–pomace flask and the culture was homogenized for 1 min and then centrifuged at 15,000× g at 4 °C for 20 min and then filtered. |
There was an increase in the extractable phenolic content. Both phenolics and antioxidant capacity correlated with the increase in the β-glucosidase activity, showing that the enzyme may play an important role in the release of phenolic aglycones from cranberry pomace and, therefore, increase the antioxidant capacity. |
[76] |
UAE—ultrasonic-assisted extraction; MAE—microwave-assisted extraction; PDA—potato dextrose agar; DPPH—1,1-diphenyl-2-picrylhydrazyl radical; ABTS·+—2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) radical; UHPLC—ultra-high-performance liquid chromatography; HPLC—high-performance liquid chromatography.
References
- EUR-Lex—52007DC0059—EN—EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:52007DC0059 (accessed on 14 March 2021).
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural Bioactive Compounds from Winery By-Products as Health Promoters: A Review. Int. J. Mol. Sci. 2014, 15, 15638–15678.
- Călinoiu, L.F.; Cătoi, A.-F.; Vodnar, D.C. Solid-State Yeast Fermented Wheat and Oat Bran as A Route for Delivery of Antioxidants. Antioxidants 2019, 8, 372.
- Sharma, S.K.; Bansal, S.; Mangal, M.; Dixit, A.K.; Gupta, R.K.; Mangal, A.K. Utilization of Food Processing By-Products as Dietary, Functional, and Novel Fiber: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1647–1661.
- Mussatto, S.I.; Ballesteros, L.F.; Martins, S.; Teixeira, J.A. Use of Agro-Industrial Wastes in Solid-State Fermentation Processes. Ind. Waste 2012, 274.
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food Waste: A Potential Bioresource for Extraction of Nutraceuticals and Bioactive Compounds. Bioresour. Bioprocess. 2017, 4, 18.
- Allikian, K.; Edgar, R.; Syed, R.; Zhang, S. Fundamentals of Fermentation Media. In Essentials in Fermentation Technology; Berenjian, A., Ed.; Learning Materials in Biosciences; Springer International Publishing: Cham, Switzerland, 2019; pp. 41–84. ISBN 978-3-030-16230-6.
- Mitchell, D.A.; Krieger, N. Solid-State Cultivation Bioreactors. In Essentials in Fermentation Technology; Berenjian, A., Ed.; Learning Materials in Biosciences; Springer International Publishing: Cham, Switzerland, 2019; pp. 105–133. ISBN 978-3-030-16230-6.
- Plácido, J.; Capareda, S. Ligninolytic Enzymes: A Biotechnological Alternative for Bioethanol Production. Bioresour. Bioprocess. 2015, 2, 23.
- Hildén, K.; Mäkelä, M.R. Role of Fungi in Wood Decay. In Reference Module in Life Sciences; Roitberg, B.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 2018, ISBN 978-0-12-809633-8.
- Filipe, D.; Fernandes, H.; Castro, C.; Peres, H.; Oliva-Teles, A.; Belo, I.; Salgado, J.M. Improved Lignocellulolytic Enzyme Production and Antioxidant Extraction Using Solid-State Fermentation of Olive Pomace Mixed with Winery Waste. Biofuels Bioprod. Biorefining-Biofpr 2020, 14, 78–91.
- Dulf, F.V.; Vodnar, D.C.; Toşa, M.I.; Dulf, E.-H. Simultaneous Enrichment of Grape Pomace with γ-Linolenic Acid and Carotenoids by Solid-State Fermentation with Zygomycetes Fungi and Antioxidant Potential of the Bioprocessed Substrates. Food Chem. 2020, 310, 125927.
- Sarsaiya, S.; Jain, A.; Kumar Awasthi, S.; Duan, Y.; Kumar Awasthi, M.; Shi, J. Microbial Dynamics for Lignocellulosic Waste Bioconversion and Its Importance with Modern Circular Economy, Challenges and Future Perspectives. Bioresour. Technol. 2019, 291, 121905.
- Olivero, G.; Turrini, F.; Vergassola, M.; Boggia, R.; Zunin, P.; Donno, D.; Beccaro, G.L.; Grilli, M.; Pittaluga, A. The 3Rs: Reduction and Refinement through a Multivariate Statistical Analysis Approach in a Behavioural Study to Unveil Anxiolytic Effects of Natural Extracts of Tilia Tomentosa. Biomed. Sci. Eng. 2019, 3.
- Pinela, J.; Omarini, A.B.; Stojković, D.; Barros, L.; Postemsky, P.D.; Calhelha, R.C.; Breccia, J.; Fernández-Lahore, M.; Soković, M.; Ferreira, I.C.F.R. Biotransformation of Rice and Sunflower Side-Streams by Dikaryotic and Monokaryotic Strains of Pleurotus Sapidus: Impact on Phenolic Profiles and Bioactive Properties. Food Res. Int. 2020, 132, 109094.
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-Industrial Wastes and Their Utilization Using Solid State Fermentation: A Review. Bioresour. Bioprocess. 2018, 5, 1.
- Rameshaiah, G.N.; Jagadish Reddy, M.L. Applications of Ligninolytic Enzymes from a White-Rot Fungus Trametes Versicolor. Univers. J. Environ. Res. Technol. 2015, 5, 1–7.
- Tan, Y.X.; Mok, W.K.; Lee, J.; Kim, J.; Chen, W.N. Solid State Fermentation of Brewers’ Spent Grains for Improved Nutritional Profile Using Bacillus Subtilis WX-17. Fermentation 2019, 5, 52.
- Tian, M.; Wai, A.; Guha, T.K.; Hausner, G.; Yuan, Q. Production of Endoglucanase and Xylanase Using Food Waste by Solid-State Fermentation. Waste Biomass Valorization 2018, 9, 2391–2398.
- Rodríguez Couto, S.; López, E.; Sanromán, M.Á. Utilisation of Grape Seeds for Laccase Production in Solid-State Fermentors. J. Food Eng. 2006, 74, 263–267.
- Behera, S.S.; Ray, R.C.; Das, U.; Panda, S.K.; Saranraj, P. Microorganisms in Fermentation. In Essentials in Fermentation Technology; Berenjian, A., Ed.; Learning Materials in Biosciences; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–39. ISBN 978-3-030-16230-6.
- Steudler, S.; Werner, A.; Walther, T. It Is the Mix that Matters: Substrate-Specific Enzyme Production from Filamentous Fungi and Bacteria Through Solid-State Fermentation. In Solid State Fermentation: Research and Industrial Applications; Steudler, S., Werner, A., Cheng, J.J., Eds.; Advances in Biochemical Engineering/Biotechnology; Springer International Publishing: Cham, Switzerland, 2019; pp. 51–81. ISBN 978-3-030-23675-5.
- Planinić, M.; Zelić, B.; Čubel, I.; Bucić-Kojić, A.; Tišma, M. Corn Forage Biological Pretreatment by Trametes Versicolor in a Tray Bioreactor. Waste Manag. Res. 2016, 34, 802–809.
- Mishra, V.; Jana, A.K. Sweet Sorghum Bagasse Pretreatment by Coriolus Versicolor in Mesh Tray Bioreactor for Selective Delignification and Improved Saccharification. Waste Biomass Valorization 2019, 10, 2689–2702.
- Tišma, M.; Planinić, M.; Bucić-Kojić, A.; Panjičko, M.; Zupančič, G.D.; Zelić, B. Corn Silage Fungal-Based Solid-State Pretreatment for Enhanced Biogas Production in Anaerobic Co-Digestion with Cow Manure. Bioresour. Technol. 2018, 253, 220–226.
- Pinheiro, V.E.; Michelin, M.; Vici, A.C.; de Almeida, P.Z.; de Moraes Polizeli, M.D. Trametes Versicolor Laccase Production Using Agricultural Wastes: A Comparative Study in Erlenmeyer Flasks, Bioreactor and Tray. Bioprocess Biosyst. Eng. 2020, 43, 507–514.
- Thomas, L.; Larroche, C.; Pandey, A. Current Developments in Solid-State Fermentation. Biochem. Eng. J. 2013, 81, 146–161.
- Rodriguez Couto, S. Exploitation of Biological Wastes for the Production of Value-Added Products under Solid-State Fermentation Conditions. Biotechnol. J. 2008, 3, 859–870.
- Singhania, R.R.; Patel, A.K.; Soccol, C.R.; Pandey, A. Recent Advances in Solid-State Fermentation. Biochem. Eng. J. 2009, 44, 13–18.
- Jain, A.; Morlok, C.K.; Henson, J.M. Comparison of Solid-State and Submerged-State Fermentation for the Bioprocessing of Switchgrass to Ethanol and Acetate by Clostridium Phytofermentans. Appl. Microbiol. Biotechnol. 2013, 97, 905–917.
- Tišma, M.; Žnidaršić-Plazl, P.; Šelo, G.; Tolj, I.; Šperanda, M.; Bucić-Kojić, A.; Planinić, M. Trametes Versicolor in Lignocellulose-Based Bioeconomy: State of the Art, Challenges and Opportunities. Bioresour. Technol. 2021, 124997.
- Farinas, C.S. Developments in Solid-State Fermentation for the Production of Biomass-Degrading Enzymes for the Bioenergy Sector. Renew. Sustain. Energy Rev. 2015, 52, 179–188.
- Soccol, C.R.; da Costa, E.S.F.; Letti, L.A.J.; Karp, S.G.; Woiciechowski, A.L.; de Souza Vandenberghe, L.P. Recent Developments and Innovations in Solid State Fermentation. Biotechnol. Res. Innov. 2017, 1, 52–71.
- Pirota, R.D.; Tonelotto, M.; da Silva Delabona, P.; Fonseca, R.F.; Paixão, D.A.; Baleeiro, F.C.; Neto, V.B.; Farinas, C.S. Enhancing Xylanases Production by a New Amazon Forest Strain of Aspergillus Oryzae Using Solid-State Fermentation under Controlled Operation Conditions. Ind. Crops Prod. 2013, 45, 465–471.
- Figueroa-Montero, A.; Esparza-Isunza, T.; Saucedo-Castañeda, G.; Huerta-Ochoa, S.; Gutiérrez-Rojas, M.; Favela-Torres, E. Improvement of Heat Removal in Solid-State Fermentation Tray Bioreactors by Forced Air Convection. J. Chem. Technol. Biotechnol. 2011, 86, 1321–1331.
- Mitchell, D.A.; von Meien, O.F.; Luz, L.F.L.; Berovič, M. Substrate, Air, and Thermodynamic Parameters for SSF Bioreactor Models. Solid-State Ferment. Bioreact. Fundam. Des. Oper. 2006, 265–278.
- Osma, J.F.; Toca Herrera, J.L.; Rodríguez Couto, S. Banana Skin: A Novel Waste for Laccase Production by Trametes Pubescens under Solid-State Conditions. Application to Synthetic Dye Decolouration. Dyes Pigments 2007, 75, 32–37.
- Dulf, F.V.; Vodnar, D.C.; Socaciu, C. Effects of Solid-State Fermentation with Two Filamentous Fungi on the Total Phenolic Contents, Flavonoids, Antioxidant Activities and Lipid Fractions of Plum Fruit (Prunus domestica L.) by-Products. Food Chem. 2016, 209, 27–36.
- Zhao, H.-M.; Guo, X.-N.; Zhu, K.-X. Impact of Solid State Fermentation on Nutritional, Physical and Flavor Properties of Wheat Bran. Food Chem. 2017, 217, 28–36.
- Martínez, O.; Sánchez, A.; Font, X.; Barrena, R. Valorization of Sugarcane Bagasse and Sugar Beet Molasses Using Kluyveromyces Marxianus for Producing Value-Added Aroma Compounds via Solid-State Fermentation. J. Clean. Prod. 2017, 158, 8–17.
- Liu, X.; Yu, X.; Zhang, T.; Wang, Z.; Xu, J.; Xia, J.; He, A.; Yan, Y.; Xu, J. Novel Two-Stage Solid-State Fermentation for Erythritol Production on Okara–Buckwheat Husk Medium. Bioresour. Technol. 2018, 266, 439–446.
- Srivastava, N.; Srivastava, M.; Ramteke, P.W.; Mishra, P.K. Chapter 23—Solid-State Fermentation Strategy for Microbial Metabolites Production: An Overview. In New and Future Developments in Microbial Biotechnology and Bioengineering; Gupta, V.K., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 345–354. ISBN 978-0-444-63504-4.
- López-Pérez, M.; Viniegra-González, G. Production of Protein and Metabolites by Yeast Grown in Solid State Fermentation: Present Status and Perspectives. J. Chem. Technol. Biotechnol. 2016, 91, 1224–1231.
- Saroj, P.; Manasa, P.; Narasimhulu, K. Characterization of Thermophilic Fungi Producing Extracellular Lignocellulolytic Enzymes for Lignocellulosic Hydrolysis under Solid-State Fermentation. Bioresour. Bioprocess. 2018, 5, 31.
- Ashok, A.; Doriya, K.; Rao, D.R.M.; Kumar, D.S. Design of Solid State Bioreactor for Industrial Applications: An Overview to Conventional Bioreactors. Biocatal. Agric. Biotechnol. 2017, 9, 11–18.
- Kovačić, D.; Kralik, D.; Rupčić, S.; Jovičić, D.; Spajić, R.; Tišma, M. Soybean Straw, Corn Stover and Sunflower Stalk as Possible Substrates for Biogas Production in Croatia: A Review. Chem. Biochem. Eng. Q. 2017, 31, 187–198.
- Singh, S.K.; Sczakas, G.; Soccol, C.R.; Pandey, A. Production of Enzymes by Solid-state Fermentation. In Current Developments in Solid-state Fermentation; Pandey, A., Soccol, C.R., Larroche, C., Eds.; Springer: New York, NY, USA, 2008; pp. 183–204. ISBN 978-0-387-75213-6.
- Abdel-Azeem, A.M.; Abdel-Azeem, M.A.; Abdul-Hadi, S.Y.; Darwish, A.G. Aspergillus: Biodiversity, Ecological Significances, and Industrial Applications. In Recent Advancement in White Biotechnology Through Fungi: Volume 1: Diversity and Enzymes Perspectives; Yadav, A.N., Mishra, S., Singh, S., Gupta, A., Eds.; Fungal Biology; Springer International Publishing: Cham, Switzerland, 2019; pp. 121–179. ISBN 978-3-030-10480-1.
- Senthivelan, T.; Kanagaraj, J.; Panda, R.C. Recent Trends in Fungal Laccase for Various Industrial Applications: An Eco-Friendly Approach—A Review. Biotechnol. Bioprocess Eng. 2016, 21, 19–38.
- Ozcirak Ergun, S.; Ozturk Urek, R. Production of Ligninolytic Enzymes by Solid State Fermentation Using Pleurotus Ostreatus. Ann. Agrar. Sci. 2017, 15, 273–277.
- Niladevi, K.N. Ligninolytic Enzymes. In Biotechnology for Agro-Industrial Residues Utilisation: Utilisation of Agro-Residues; nee’Nigam, P.S., Pandey, A., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 397–414. ISBN 978-1-4020-9942-7.
- Xu, X.; Lin, M.; Zang, Q.; Shi, S. Solid State Bioconversion of Lignocellulosic Residues by Inonotus Obliquus for Production of Cellulolytic Enzymes and Saccharification. Bioresour. Technol. 2018, 247, 88–95.
- Dave, B.R.; Sudhir, A.P.; Subramanian, R.B. Purification and Properties of an Endoglucanase from Thermoascus Aurantiacus. Biotechnol. Rep. Amst. Neth. 2015, 6, 85–90.
- Yeoman, C.J.; Han, Y.; Dodd, D.; Schroeder, C.M.; Mackie, R.I.; Cann, I.K.O. Thermostable Enzymes as Biocatalysts in the Biofuel Industry. Adv. Appl. Microbiol. 2010, 70, 1–55.
- Grassick, A.; Murray, P.G.; Thompson, R.; Collins, C.M.; Byrnes, L.; Birrane, G.; Higgins, T.M.; Tuohy, M.G. Three-Dimensional Structure of a Thermostable Native Cellobiohydrolase, CBH IB, and Molecular Characterization of the Cel7 Gene from the Filamentous Fungus, Talaromyces Emersonii. Eur. J. Biochem. 2004, 271, 4495–4506.
- Bucić-Kojić, A.; Šelo, G.; Zelić, B.; Planinić, M.; Tišma, M. Recovery of Phenolic Acid and Enzyme Production from Corn Silage Biologically Treated by Trametes Versicolor. Appl. Biochem. Biotechnol. 2017, 181, 948–960.
- Tišma, M.; Šalić, A.; Planinić, M.; Zelić, B.; Potočnik, M.; Šelo, G.; Bucić-Kojić, A. Production, Characterisation and Immobilization of Laccase for an Efficient Aniline-Based Dye Decolourization. J. Water Process Eng. 2020, 36, 101327.
- Tišma, M.; Jurić, A.; Bucić-Kojić, A.; Panjičko, M.; Planinić, M. Biovalorization of Brewers’ Spent Grain for the Production of Laccase and Polyphenols. J. Inst. Brew. 2018, 124, 182–186.
- Akpinar, M.; Ozturk Urek, R. Induction of Fungal Laccase Production under Solid State Bioprocessing of New Agroindustrial Waste and Its Application on Dye Decolorization. 3 Biotech 2017, 7, 98.
- Ghosh, P.; Ghosh, U. Bioconversion of Agro-Waste to Value-Added Product Through Solid-State Fermentation by a Potent Fungal Strain Aspergillus flavus PUF5. In Utilization and Management of Bioresources; Ghosh, S.K., Ed.; Springer: Singapore, 2018; pp. 291–299.
- Murugesan, K.; Nam, I.-H.; Kim, Y.-M.; Chang, Y.-S. Decolorization of Reactive Dyes by a Thermostable Laccase Produced by Ganoderma Lucidum in Solid State Culture. Enzyme Microb. Technol. 2007, 40, 1662–1672.
- Sharma, A.; Gupta, V.; Khan, M.; Balda, S.; Gupta, N.; Capalash, N.; Sharma, P. Flavonoid-Rich Agro-Industrial Residues for Enhanced Bacterial Laccase Production by Submerged and Solid-State Fermentation. 3 Biotech 2017, 7, 200.
- Pandey, S.; Srivastava, M.; Shahid, M.; Kumar, V.; Singh, A.; Trivedi, S.; Srivastava, Y.K. Trichoderma Species Cellulases Produced by Solid State Fermentation. J. Data Min. Genom. Proteom. 2015, 6, 170.
- Marques, G.L.; dos Santos Reis, N.; Silva, T.P.; Ferreira, M.L.O.; Aguiar-Oliveira, E.; de Oliveira, J.R.; Franco, M. Production and Characterisation of Xylanase and Endoglucanases Produced by Penicillium Roqueforti ATCC 10110 Through the Solid-State Fermentation of Rice Husk Residue. Waste Biomass Valorization 2018, 9, 2061–2069.
- Saqib, A.A.N.; Hassan, M.; Khan, N.F.; Baig, S. Thermostability of Crude Endoglucanase from Aspergillus Fumigatus Grown under Solid State Fermentation (SSF) and Submerged Fermentation (SmF). Process Biochem. 2010, 45, 641–646.
- Mahmood, R.T.; Asad, M.J.; Mehboob, N.; Mushtaq, M.; Gulfraz, M.; Asgher, M.; Minhas, N.M.; Hadri, S.H. Production, Purification, and Characterization of Exoglucanase by Aspergillus Fumigatus. Appl. Biochem. Biotechnol. 2013, 170, 895–908.
- Shahzadi, T.; Anwar, Z.; Iqbal, Z.; Anjum, A.; Aqil, T.; Bakhtawar Afzal, A.; Kamran, M.; Mehmood, S.; Irshad, M. Induced Production of Exoglucanase, and β-Glucosidase from Fungal Co-Culture of T. Viride and G. Lucidum. Adv. Biosci. Biotechnol. 2014, 5, 426–433.
- Singla, D.; Taggar, M.S. Production of Cellulases by Solid State Fermentation of Different Agricultural Residues Using Humicola Insolens MTCC 1433. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1409–1418.
- Garcia, N.F.L.; da Silva Santos, F.R.; Gonçalves, F.A.; da Paz, M.F.; Fonseca, G.G.; Leite, R.S.R. Production of β-Glucosidase on Solid-State Fermentation by Lichtheimia Ramosa in Agroindustrial Residues: Characterization and Catalytic Properties of the Enzymatic Extract. Electron. J. Biotechnol. 2015, 18, 314–319.
- Leite, R.S.R.; Alves-Prado, H.F.; Cabral, H.; Pagnocca, F.C.; Gomes, E.; Da-Silva, R. Production and Characteristics Comparison of Crude β-Glucosidases Produced by Microorganisms Thermoascus Aurantiacus e Aureobasidium Pullulans in Agricultural Wastes. Enzyme Microb. Technol. 2008, 43, 391–395.
- Pandya, J.J.; Gupte, A. Production of Xylanase under Solid-State Fermentation by Aspergillus Tubingensis JP-1 and Its Application. Bioprocess Biosyst. Eng. 2012, 35, 769–779.
- Dhiman, S.S.; Sharma, J.; Battan, B. Pretreatment Processing of Fabrics by Alkalothermophilic Xylanase from Bacillus Stearothermophilus SDX. Enzyme Microb. Technol. 2008, 43, 262–269.
- Park, Y.; Kang, S.; Lee, J.; Hong, S.; Kim, S. Xylanase Production in Solid State Fermentation by Aspergillus Niger Mutant Using Statistical Experimental Designs. Appl. Microbiol. Biotechnol. 2002, 58, 761–766.
- Umsza-Guez, M.A.; Díaz, A.B.; Ory, I.D.; Blandino, A.; Gomes, E.; Caro, I. Xylanase Production by Aspergillus Awamori under Solid State Fermentation Conditions on Tomato Pomace. Braz. J. Microbiol. 2011, 42, 1585–1597.
- Gaffney, M.; Doyle, S.; Murphy, R. Optimization of Xylanase Production by Thermomyces Lanuginosus in Solid State Fermentation. Biosci. Biotechnol. Biochem. 2009, 73, 2640–2644.
- Vattem, D.A.; Shetty, K. Ellagic Acid Production and Phenolic Antioxidant Activity in Cranberry Pomace (Vaccinium macrocarpon) Mediated by Lentinus Edodes Using a Solid-State System. Process Biochem. 2003, 39, 367–379.
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic Compounds: Natural Alternative in Inflammation Treatment. A Review. Cogent Food Agric. 2016, 2, 1131412.
- Tan, D.; Yin, J.; Chen, G.-Q. Production of Polyhydroxyalkanoates. In Current Developments in Biotechnology and Bioengineering; Pandey, A., Negi, S., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 655–692. ISBN 978-0-444-63662-1.
- Collins, T.; Gerday, C.; Feller, G. Xylanases, Xylanase Families and Extremophilic Xylanases. FEMS Microbiol. Rev. 2005, 29, 3–23.
- Gupta, V.; Garg, S.; Capalash, N.; Gupta, N.; Sharma, P. Production of Thermo-Alkali-Stable Laccase and Xylanase by Co-Culturing of Bacillus Sp. and B. Halodurans for Biobleaching of Kraft Pulp and Deinking of Waste Paper. Bioprocess Biosyst. Eng. 2015, 38, 947–956.
- David, A.; Singh Chauhan, P.; Kumar, A.; Angural, S.; Kumar, D.; Puri, N.; Gupta, N. Coproduction of Protease and Mannanase from Bacillus Nealsonii PN-11 in Solid State Fermentation and Their Combined Application as Detergent Additives. Int. J. Biol. Macromol. 2018, 108, 1176–1184.
- Helkar, P.B.; Sahoo, A.; Patil, N. Review: Food Industry By-Products Used as a Functional Food Ingredients. Int. J. Waste Resour. 2016, 6, 1–6.
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards Integral Utilization of Grape Pomace from Winemaking Process: A Review. Waste Manag. 2017, 68, 581–594.
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Chapter 2—Phenolic Compounds: Structure, Classification, and Antioxidant Power. In Bioactive Compounds; Campos, M.R.S., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 33–50. ISBN 978-0-12-814774-0.
- Antonic, B.; Jancikova, S.; Dordevic, D.; Tremlova, B. Apple Pomace as Food Fortification Ingredient: A Systematic Review and Meta-Analysis. J. Food Sci. 2020, 85, 2977–2985.
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Ferulic and P-Coumaric Acids Extraction by Alkaline Hydrolysis of Brewer’s Spent Grain. Ind. Crops Prod. 2007, 25, 231–237.
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Valéro, J.R. Solid-State Fermentation of Apple Pomace Using Phanerocheate Chrysosporium—Liberation and Extraction of Phenolic Antioxidants. Food Chem. 2011, 126, 1071–1080.
- Martínez-Ávila, G.C.; Aguilera-Carbó, A.F.; Rodríguez-Herrera, R.; Aguilar, C.N. Fungal Enhancement of the Antioxidant Properties of Grape Waste. Ann. Microbiol. 2012, 62, 923–930.
- Aguilar, C.N.; Aguilera-Carbo, A.; Robledo, A.; Ventura, J.; Belmares, R.; Martinez, D.; Rodríguez-Herrera, R.; Contreras, J. Production of Antioxidant Nutraceuticals by Solid-State Cultures of Pomegranate (Punica granatum) Peel and Creosote Bush (Larrea tridentata) Leaves. Food Technol. Biotechnol. 2008, 46, 218–222.
- Dulf, F.V.; Vodnar, D.C.; Dulf, E.-H.; Diaconeasa, Z.; Socaciu, C. Liberation and Recovery of Phenolic Antioxidants and Lipids in Chokeberry (Aronia melanocarpa) Pomace by Solid-State Bioprocessing Using Aspergillus Niger and Rhizopus Oligosporus Strains. LWT 2018, 87, 241–249.




