
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Florence VORSPAN | -- | 2254 | 2022-11-07 09:29:39 | | | |
| 2 | Jessie Wu | Meta information modification | 2254 | 2022-11-08 04:41:15 | | | | |
| 3 | Jessie Wu | + 5 word(s) | 2259 | 2022-11-08 04:48:04 | | | | |
| 4 | Jessie Wu | + 2 word(s) | 2261 | 2022-11-08 04:49:21 | | | | |
| 5 | Jessie Wu | Meta information modification | 2261 | 2022-11-08 04:50:35 | | |
Video Upload Options
Alcohol use disorders (AUD) is defined by the loss of control over alcohol intake and chronic, compulsive, heavy alcohol use despite adverse consequences. Among patients seeking treatment for AUD, the proportion of patients at treatment entry endorsing the criteria for pharmacological dependence was 63% for tolerance and 14% for withdrawal. Alcohol withdrawal (AW) syndrome is the combination of signs and symptoms occurring as soon as three to six hours after the last intake of alcohol in subjects with pharmacological dependence. The classical symptoms are tremor, perspiration, anxiety and adrenergic signs (hypertension, tachycardia). Untreated AW can lead to specific complications: delirium tremens (DT) and seizure. Several indirect complications of the adrenergic syndrome may also occur during an untreated AW syndrome as dehydration, cardiac failure or renal failure. Mortality reaches 8% in patients with AW syndrome hospitalized in intensive care units, because of any or the combination of those multiple organs complications. AW is still considered as a dangerous complication of undetected AUD during any surgery or medical inpatient treatment.
1. Alcohol Withdrawal as an Oxidative Stress Challenge for the Brain
1.1. Epilepsy, Delirium Tremens as Alcohol Withdrawal Neurological Complications: The Role of Oxidative Stress
1.2. Alcohol Withdrawal as a Vulnerability Period for Wernicke’s Encephalopathy: The Role of Oxidative Stress
1.2.1. Wernicke’s Encephalopathy Occurrence
1.2.2. Wernicke’s Encephalopathy Diagnosis
1.2.3. Systematic Thiamine Supplementation in Alcohol Use Disorder Patients
1.2.4. Wernicke’s Encephalopathy and Alcohol Withdrawal
1.2.5. Wernicke’s Encephalopathy and Oxidative Stress
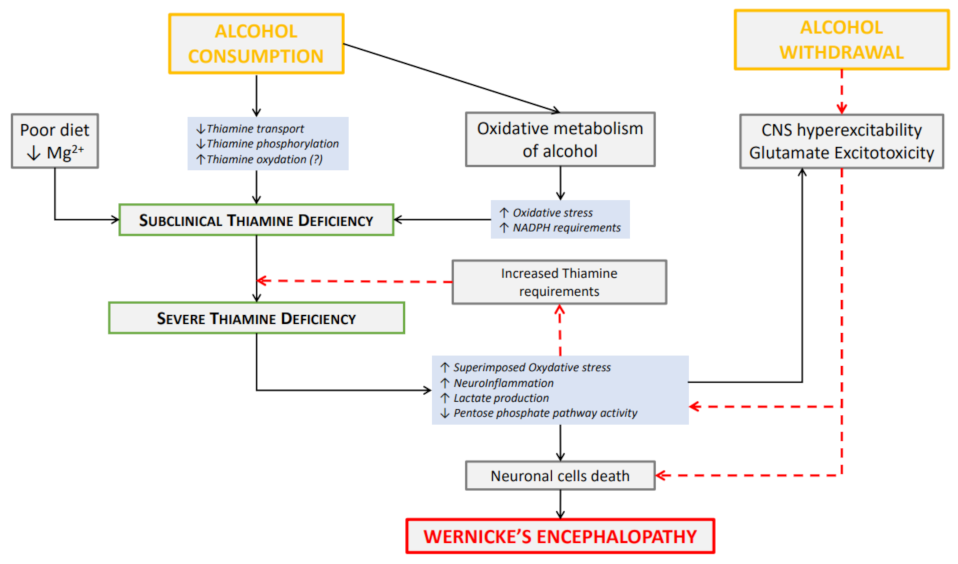
2. What Makes the Alcohol Withdrawal Period at High Risk for Oxidative Brain Damage?
References
- Becker, H.C.; Mulholland, P.J. Neurochemical mechanisms of alcohol withdrawal. Handb. Clin. Neurol. 2014, 125, 133–156.
- Jung, M.E.; Metzger, D.B. Alcohol Withdrawal and Brain Injuries: Beyond Classical Mechanisms. Molecules 2010, 15, 4984–5011.
- Brousse, G.; Arnaud, B.; Vorspan, F.; Richard, D.; Dissard, A.; Dubois, M.; Pic, D.; Geneste, J.; Xavier, L.; Authier, N.; et al. Alteration of Glutamate/GABA Balance During Acute Alcohol Withdrawal in Emergency Department: A Prospective Analysis. Alcohol Alcohol. 2012, 47, 501–508.
- N’Gouemo, P. Voltage-Sensitive Calcium Channels in the Brain: Relevance to Alcohol Intoxication and Withdrawal. Handb. Exp. Pharm. 2018, 248, 263–280.
- Huang, M.-C.; Chen, C.-H.; Peng, F.-C.; Tang, S.-H.; Chen, C.-C. Alterations in Oxidative Stress Status During Early Alcohol Withdrawal in Alcoholic Patients. J. Med. Assoc. 2009, 108, 560–569.
- Jung, M.E.; Yan, L.-J.; Forster, M.J.; Simpkins, J.W. Ethanol withdrawal provokes mitochondrial injury in an estrogen preventable manner. J. Bioenerg. Biomembr. 2008, 40, 35–44.
- Lebourgeois, S.; González-Marín, M.C.; Jeanblanc, J.; Naassila, M.; Vilpoux, C. Effect of N-acetylcysteine on motivation, seeking and relapse to ethanol self-administration: NAC reduces ethanol seeking and relapse in rats. Addict. Biol. 2018, 23, 643–652.
- Lebourgeois, S.; González-Marín, M.C.; Antol, J.; Naassila, M.; Vilpoux, C. Evaluation of N-acetylcysteine on ethanol self-administration in ethanol-dependent rats. Neuropharmacology 2019, 150, 112–120.
- Quintanilla, M.E.; Ezquer, F.; Morales, P.; Ezquer, M.; Olivares, B.; Santapau, D.; Herrera-Marschitz, M.; Israel, Y. N-Acetylcysteine and Acetylsalicylic Acid Inhibit Alcohol Consumption by Different Mechanisms: Combined Protection. Front. Behav. Neurosci. 2020, 14, 122.
- Parsons, A.L.M.; Bucknor, E.M.V.; Castroflorio, E.; Soares, T.R.; Oliver, P.L.; Rial, D. The Interconnected Mechanisms of Oxidative Stress and Neuroinflammation in Epilepsy. Antioxidants 2022, 11, 157.
- Lee, S.H.; Lee, M.; Ko, D.G.; Choi, B.Y.; Suh, S.W. The Role of NADPH Oxidase in Neuronal Death and Neurogenesis after Acute Neurological Disorders. Antioxidants 2021, 10, 739.
- Huang, M.-C.; Chen, C.-C.; Pan, C.-H.; Chen, C.-H. Comparison of Oxidative DNA Damage Between Alcohol-Dependent Patients with and Without Delirium Tremens. Alcohol. Clin. Exp. Res. 2014, 38, 2523–2528.
- Abdou, E.; Hazell, A.S. Thiamine Deficiency: An Update of Pathophysiologic Mechanisms and Future Therapeutic Considerations. Neurochem. Res. 2015, 40, 353–361.
- Arts, N.J.M.; Walvoort, S.J.; Kessels, R.P. Korsakoff’s syndrome: A critical review. Neuropsychiatr. Dis. Treat. 2017, 13, 2875–2890.
- Arts, N.J.M.; Pitel, A.-L.; Kessels, R.P.C. The contribution of mamillary body damage to Wernicke’s encephalopathy and Korsakoff’s syndrome. Handb. Clin. Neurol. 2021, 180, 455–475.
- Chandrakumar, A.; Bhardwaj, A.; W’t Jong, G.W. Review of thiamine deficiency disorders: Wernicke encephalopathy and Korsakoff psychosis. J. Basic. Clin. Physiol. Pharm. 2019, 30, 153–162.
- Coulbault, L.; Ritz, L.; Vabret, F.; Lannuzel, C.; Boudehent, C.; Nowoczyn, M.; Beaunieux, H.; Pitel, A.L. Thiamine and phosphate esters concentrations in whole blood and serum of patients with alcohol use disorder: A relation with cognitive deficits. Nutr. Neurosci. 2021, 24, 530–541.
- Ota, Y.; Capizzano, A.A.; Moritani, T.; Naganawa, S.; Kurokawa, R.; Srinivasan, A. Comprehensive review of Wernicke encephalopathy: Pathophysiology, clinical symptoms and imaging findings. Jpn. J. Radiol. 2020, 38, 809–820.
- Thomson, A.D.; Marshall, E.J. The natural history and pathophysiology of Wernicke’s Encephalopathy and Korsakoff’s Psychosis. Alcohol Alcohol. 2006, 41, 151–158.
- Thomson, A.D.; Marshall, E.J. The treatment of patients at risk of developing wernicke’s encephalopathy in the community. Alcohol Alcohol. 2006, 41, 159–167.
- Smith, T.J.; Johnson, C.R.; Koshy, R.; Hess, S.Y.; Qureshi, U.A.; Mynak, M.L.; Fischer, P.R. Thiamine deficiency disorders: A clinical perspective. Ann. N. Y. Acad. Sci. 2021, 1498, 9–28.
- Gomes, F.; Bergeron, G.; Bourassa, M.W.; Fischer, P.R. Thiamine deficiency unrelated to alcohol consumption in high-income countries: A literature review. Ann. N. Y. Acad. Sci. 2021, 1498, 46–56.
- Oudman, E.; Wijnia, J.W.; Oey, M.J.; van Dam, M.; Postma, A. Wernicke-Korsakoff syndrome despite no alcohol abuse: A summary of systematic reports. J. Neurol. Sci. 2021, 426, 117482.
- Scalzo, S.J.; Bowden, S.C.; Ambrose, M.L.; Whelan, G.; Cook, M.J. Wernicke-Korsakoff syndrome not related to alcohol use: A systematic review. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1362–1368.
- Wijnia, J.W.; Oudman, E.; van Gool, W.A.; Wierdsma, A.I.; Bresser, E.L.; Bakker, J.; van de Wiel, A.; Mulder, C.L. Severe Infections are Common in Thiamine Deficiency and May be Related to Cognitive Outcomes: A Cohort Study of 68 Patients With Wernicke-Korsakoff Syndrome. Psychosomatics 2016, 57, 624–633.
- Caine, D.; Halliday, G.M.; Kril, J.J.; Harper, C.G. Operational criteria for the classification of chronic alcoholics: Identification of Wernicke’s encephalopathy. J. Neurol. Neurosurg. Psychiatry 1997, 62, 51–60.
- Société Française d’Alcoologie. Mésusage de l’alcool: Dépistage, diagnostic et traitement. Recommandation de bonne pratique. Alcoologie Addictologie 2015, 37, 5–84.
- Whitfield, K.C.; Bourassa, M.W.; Adamolekun, B.; Bergeron, G.; Bettendorff, L.; Brown, K.H.; Cox, L.; Fattal-Valevski, A.; Fischer, P.R.; Frank, E.L.; et al. Thiamine deficiency disorders: Diagnosis, prevalence, and a roadmap for global control programs. Ann. N. Y. Acad. Sci. 2018, 1430, 3–43.
- Jones, K.S.; Parkington, D.A.; Cox, L.J.; Koulman, A. Erythrocyte transketolase activity coefficient (ETKAC) assay protocol for the assessment of thiamine status. Ann. N. Y. Acad. Sci. 2021, 1498, 77–84.
- Ganapathy, V.; Smith, S.B.; Prasad, P.D. SLC19: The folate/thiamine transporter family. Pflügers Arch. 2004, 447, 641–646.
- Lockman, P.R.; McAfee, J.H.; Geldenhuys, W.J.; Allen, D.D. Cation Transport Specificity at the Blood-Brain Barrier. Neurochem. Res. 2004, 29, 2245–2250.
- Thomson, A.D.; Guerrini, I.; Marshall, E.J. The Evolution and Treatment of Korsakoff’s Syndrome: Out of Sight, Out of Mind? Neuropsychol. Rev. 2012, 22, 81–92.
- Galvin, R.; Bråthen, G.; Ivashynka, A.; Hillbom, M.; Tanasescu, R.; Leone, M.A. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy: EFNS guidelines for Wernicke encephalopathy. Eur. J. Neurol. 2010, 17, 1408–1418.
- Pruckner, N.; Baumgartner, J.; Hinterbuchinger, B.; Glahn, A.; Vyssoki, S.; Vyssoki, B. Thiamine Substitution in Alcohol Use Disorder: A Narrative Review of Medical Guidelines. Eur. Addict. Res. 2019, 25, 103–110.
- Haber, P.S.; Riordan, B.C.; Winter, D.T.; Barrett, L.; Saunders, J.; Hides, L.; Gullo, M.; Manning, V.; Day, C.A.; Bonomo, Y.; et al. New Australian guidelines for the treatment of alcohol problems: An overview of recommendations. Med. J. Aust. 2021, 215, S3–S32.
- Vetreno, R.P.; Ramos, R.L.; Anzalone, S.; Savage, L.M. Brain and behavioral pathology in an animal model of Wernicke’s encephalopathy and Wernicke-Korsakoff Syndrome. Brain Res. 2012, 1436, 178–192.
- Vedder, L.C.; Hall, J.M.; Jabrouin, K.R.; Savage, L.M. Interactions Between Chronic Ethanol Consumption and Thiamine Deficiency on Neural Plasticity, Spatial Memory, and Cognitive Flexibility. Alcohol. Clin. Exp. Res. 2015, 39, 2143–2153.
- Ciccia, R.M.; Langlais, P.J. An examination of the synergistic interaction of ethanol and thiamine deficiency in the development of neurological signs and long-term cognitive and memory impairments. Alcohol. Clin. Exp. Res. 2000, 24, 622–634.
- Homewood, J.; Bond, N.W.; Mackenzie, A. The effects of single and repeated episodes of thiamin deficiency on memory in alcohol-consuming rats. Alcohol 1997, 14, 81–91.
- Palencia, G.; Teixeira, F.; Ortiz, A.; Perez, R.; Rios, C.; Sotelo, J. Detrimental effects of malnutrition on the damage induced by alcoholism: A study of animal models that simulate chronic alcoholism and malnutrition of large human groups. J. Stud. Alcohol 1994, 55, 113–120.
- Hazell, A.S. Astrocytes are a major target in thiamine deficiency and Wernicke’s encephalopathy. Neurochem. Int. 2009, 55, 129–135.
- Hazell, A.S.; Sheedy, D.; Oanea, R.; Aghourian, M.; Sun, S.; Jung, J.Y.; Wang, D.; Wang, C. Loss of astrocytic glutamate transporters in Wernicke encephalopathy. Glia 2010, 58, 148–156.
- Jhala, S.S.; Hazell, A.S. Modeling neurodegenerative disease pathophysiology in thiamine deficiency: Consequences of impaired oxidative metabolism. Neurochem. Int. 2011, 58, 248–260.
- Carvalho, F.; Pereira, S.; Pires, R.; Ferraz, V.; Romanosilva, M.; Oliveirasilva, I.; Ribeiro, A. Thiamine deficiency decreases glutamate uptake in the prefrontal cortex and impairs spatial memory performance in a water maze test. Pharmacol. Biochem. Behav. 2006, 83, 481–489.
- McLean, C.; Tapsell, L.; Grafenauer, S.; McMahon, A. Systematic review of nutritional interventions for people admitted to hospital for alcohol withdrawal. Nutr. Diet. 2020, 77, 76–89.
- Clergue-Duval, V.; Azuar, J.; Fonsart, J.; Delage, C.; Rollet, D.; Amami, J.; Frapsauce, A.; Gautron, M.-A.; Hispard, E.; Bellivier, F.; et al. Ascorbic Acid Deficiency Prevalence and Associated Cognitive Impairment in Alcohol Detoxification Inpatients: A Pilot Study. Antioxidants 2021, 10, 1892.
- Gautron, M.-A.; Questel, F.; Lejoyeux, M.; Bellivier, F.; Vorspan, F. Nutritional Status During Inpatient Alcohol Detoxification. Alcohol Alcohol. 2018, 53, 64–70.
- Girre, C.; Hispard, E.; Therond, P.; Guedj, S.; Bourdon, R.; Dally, S. Effect of Abstinence from Alcohol on the Depression of Glutathione Peroxidase Activity and Selenium and Vitamin E Levels in Chronic Alcoholic Patients. Alcohol. Clin. Exp. Res. 1990, 14, 909–912.
- O’Brien, N.L.; Quadri, G.; Lightley, I.; Sharp, S.I.; Guerrini, I.; Smith, I.; Heydtmann, M.; Morgan, M.Y.; Thomson, A.D.; Bass, N.J.; et al. SLC19A1 Genetic Variation Leads to Altered Thiamine Diphosphate Transport: Implications for the Risk of Developing Wernicke-Korsakoff’s Syndrome. Alcohol Alcohol. 2022, 57, 581–588.
- Airagnes, G.; Ducoutumany, G.; Laffy-Beaufils, B.; Le Faou, A.-L.; Limosin, F. Alcohol withdrawal syndrome management: Is there anything new? Rev. Med. Interne 2019, 40, 373–379.
- Maguire, D.; Burns, A.; Talwar, D.; Catchpole, A.; Stefanowicz, F.; Ross, D.P.; Galloway, P.; Ireland, A.; Robson, G.; Adamson, M.; et al. Randomised trial of intravenous thiamine and/or magnesium sulphate administration on erythrocyte transketolase activity, lactate concentrations and alcohol withdrawal scores. Sci. Rep. 2022, 12, 6941.
- Sarai, M.; Tejani, A.M.; Chan, A.H.W.; Kuo, I.F.; Li, J. Magnesium for alcohol withdrawal. Cochrane Database Syst. Rev. 2013, CD008358.
- Mahajan, V.R.; Elvig, S.K.; Vendruscolo, L.F.; Koob, G.F.; Darcey, V.L.; King, M.T.; Kranzler, H.R.; Volkow, N.D.; Wiers, C.E. Nutritional Ketosis as a Potential Treatment for Alcohol Use Disorder. Front. Psychiatry 2021, 12, 781668.
- Shi, Z.; Xie, Y.; Ren, H.; He, B.; Wang, M.; Wan, J.; Yuan, T.; Yao, X.; Su, H. Fish oil treatment reduces chronic alcohol exposure induced synaptic changes. Addict. Biol. 2019, 24, 577–589.




